Abstract
Objective
To investigate the stem cell-like characteristics of human periodontal ligament (PDL) stromal cells outgrown from orthodontically extracted premolars and to evaluate the potential for myogenic differentiation.
Methods
PDL stromal cells were obtained from extracted premolars by using the outgrowth method. Cell morphological features, self-replication capability, and the presence of cell-surface markers, along with osteogenic, adipogenic, and chondrogenic differentiation, were confirmed. In addition, myogenic differentiation was induced by the use of 5-aza-2'-deoxycytidine (5-Aza) for DNA demethylation.
Results
PDL stromal cells showed growth patterns and morphological features similar to those of fibroblasts. In contrast, the proliferation rates of premolar PDL stromal cells were similar to those of bone marrow and adipogenic stem cells. PDL stromal cells expressed surface markers of human mesenchymal stem cells (i.e., CD90 and CD105), but not those of hematopoietic stem cells (i.e., CD31 and CD34). PDL stromal cells were differentiated into osteogenic, adipogenic, and chondrogenic lineages. Myotube structures were induced in PDL stromal cells after 5-Aza pretreatment, but not in the absence of 5-Aza pretreatment.
Conclusions
PDL stromal cells isolated from extracted premolars can potentially be a good source of postnatal stem cells for oromaxillofacial regeneration in bone and muscle.
Keywords: Human periodontal ligament stem cell, Myogenic differentiation, Cell biology, Molecular biology, Soft tissue
INTRODUCTION
Stem cells are undifferentiated cells characterized by the capacity for self-proliferation (i.e., stem cell renewal) as well as pluripotency (i.e., the ability to differentiate into multiple tissues and organs). Gronthos et al.1 and Seo et al.2 have reported the isolation of dental pulp (DP) stem cells and periodontal ligament (PDL) stem cells from extracted human third molars. Unlike stem cells derived from other organs, dental stem cells are relatively easy to access and are associated with fewer ethical concerns than most other types of stem cells.3
Stem cells isolated from the third molars (so called "wisdom teeth") have been the most widely used dental cells in stem cell research.1-4 However, due to the high incidence of impaction of the third molar, it is difficult to isolate fresh stem cells in most clinical settings without surgical contamination. Multiple premolars are frequently extracted to relieve malocclusions in various age groups. Because premolars can be extracted relatively easily without tooth damage, both DP and PDL cells can be isolated from a single donor tooth, which substantially increases the number of extractable primary cell populations per tooth. In most instances, premolars can be easily extracted as a completely intact structure and are in occlusion withholding occlusal forces during oral functions such as mastication. The appropriate level of mechanical stretch of mesenchymal stem cells (MSCs) serves as a positive modulator of proliferation, differentiation, and extracellular matrix production.5 Therefore, cells extracted from intact occluding premolars may serve as strong candidates for regenerative procedures.
Two different methods may be used to isolate cells from teeth. One method is the outgrowth technique, in which tissues are attached to the bottom of the culture dish, and stromal cells grow out from the boundary of the tissue on the dish surface. The second method is the enzyme digestion technique, in which minced tissues are digested into a single-cell suspension with various enzymes. The enzyme digestion technique appears to preferentially extract PDL stem cells, which have more proliferative capacity. On the other hand, the outgrowth technique appears to preferentially extract stromal cells, which have lesser proliferative capacity, indicative of a more fibroblast-like phenotype that does not effectively differentiate into osteogenic, adipogenic, or chondrogenic lineages.6,7 Recently, plastic-adherent fibroblast-like stromal cells that were expanded from tissues have been investigated for their potential putative cellular therapeutic properties in a variety of clinical indications.8
With the growth of adult and esthetic dentistry, the regeneration of soft as well as hard dental tissues has been of great interest to many clinicians. Although orthognathic surgery and bone augmentation have been successful to treat the most difficult types of skeletal discrepancies in the field of orthodontics, the most favorable procedural outcomes also require the regeneration of soft tissues, including the masticatory muscle, nerves, and vessels, following these procedures. In addition to these conventional surgical interventions, the potential application of dental stem cells for the regeneration of periodontal hard tissues such as the cementum, bones, and teeth, both in vitro and in vivo, have been reported often in recent years.9-13 However, compared to advancements in hard tissue regeneration by using PDL stem cells, few studies have been published concerning the potential for the application of stem cells in the regeneration of soft tissues, especially skeletal muscle.14
Therefore, the objective of this study was to investigate the characteristics of human PDL stromal cells isolated by the outgrowth method and to compare the PDL stromal cells to stem cells derived from other tissues. Subsequently, we evaluated the potential for myogenic differentiation with the use of PDL stromal cells in the potent ial application of soft tissue regeneration in clinical orthodontic settings.
MATERIALS AND METHODS
Isolation and culture of PDL stromal cells
Intact premolars were collected from 3 female adults (age range, 18 to 22.1 years) at the Department of Orthodontics, Gangnam Severance Dental Hospital, under the approved guidelines set by the Institutional Review Board. The PDL stromal cells were obtained from PDL tissues and prepared in alpha-modified Eagle medium (αMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin and 100 µg/mL streptomycin using a previously described outgrowth method.7 All studies were performed using the cells from passage 3. For comparison, PDL stromal cells from impacted third molars were collected using the same method. Human dermal fibroblasts (HDFs; Lonza, Gaithersburg, MD, USA), human bone marrow-derived stem cells (BMSCs; Lonza) and adipose-derived stem cells (ADSCs; Lonza) were also cultured under the same conditions.
Proliferation rate of PDL cells and other MSCs
The proliferation rates of the PDL stromal cells from functional premolars, human BMSCs, and ADSCs were compared. Cells were seeded at a concentration of 4 × 106 cells per well with αMEM or conditioned medium (CM) with 10 µL WST assay reagent (EZ-Cytox Cell Viability Assay Kit; Daeil Lab Service, Seoul, Korea) and incubated for 2 hours. Optical density was measured at 450 nm.
Fluorescence-activated cell sorting (FACS) analysis
The mesenchymal markers CD90 and CD105, as well as hematopoietic and endothelial markers, CD34 and CD31, respectively (eBioscience, San Diego, CA, USA), were used. Control cells were cultured in the absence of primary antibody. All data were acquired with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) and analyzed with the FCSExpress V3 software (De Novo Software, Thornhill, Canada).
Evaluation of multipotency
Osteogenesis was induced by incubating the stem cells in 2 mL αMEM supplemented with, 10 nM dexamethasone, 1 mg/mL β-glycerophosphate, and 50 µg/mL ascorbic acid for 14 days and stained with 20 mg/mL Alizarin Red S (Sigma Chemicals, St. Louis, MO, USA) solution. Adipogenesis was induced by incubating the stem cells in adipogenic differentiation medium (STEMPRO® Adipogenesis Differentiation Kit, Invitrogen) for 14 days, followed by staining with 3 mg/mL Oil Red O solution (Sigma Chemicals). Chondrogenesis was induced by incubating a 10-µL droplet of stem cells (1.6 × 107 viable cells/mL) supplemented with 1 mL of warmed STEMPRO Chondrogenic Differentiation media (Invitrogen) supplemented with 10 ng/mL of transforming growth factor β1 (Human TGF-β1; R&D Systems Inc., Minneapolis, MN, USA), followed by Alcian blue staining.
In vitro myogenesis
Myogenesis was induced in the presence or absence of 24-hours pretreatment with 2 µM 5-aza-2'-deoxycytidine (5-Aza; Wako Pure Chemicals, Osaka, Japan), a DNA demethylation agent.12,15 Subsequently, myogenesis-inducing Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% hydrocortisone, 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin was added. The stem cell medium was changed every 2 - 3 days for a 28-day-period.
Immunofluorescence staining of myogenic cells
Cells were fixed in cold methanol for 2 min and blocked with 0.3% bovine serum albumin (BSA) for 1 hour at room temperature. The cells were then incubated with monoclonal mouse anti-human desmin (1:100; DakoCytomation, Glostrup, Denmark) overnight at 4℃ in a humidified chamber. The cells were washed and then incubated with the secondary antibody, rabbit anti-mouse FITC-conjugated IgG (Invitrogen Life Technologies). The nuclei were counterstained with DAPI (Vector Laboratories Inc., Burlingame, CA, USA) and evaluated under a confocal microscope (LSM710; Carl Zeiss Microscopy GmbH, Carl Zeiss Inc., Jena, Germany).
Statistical analysis
Data analysis was performed with one-way analysis of variance by using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Significant differences between each cell group were identified with Tukey post-test analysis. The significance level was set at p < 0.05.
RESULTS
Primary cells outgrown from PDL tissues
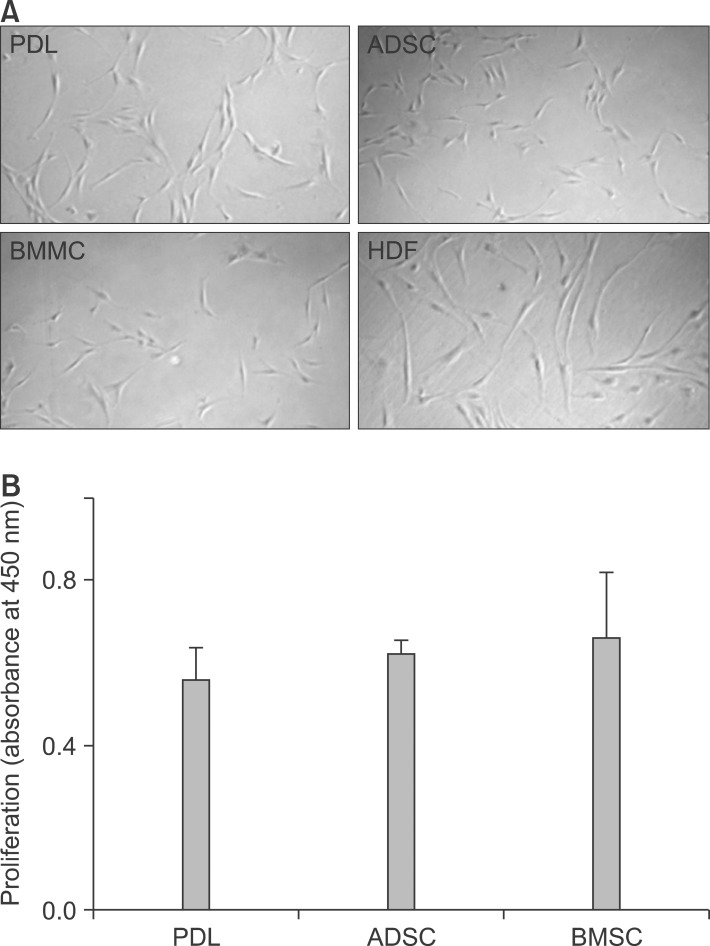
After 7 days, the attached PDL stromal cells began growing out from the tissue as a monolayer, forming colonies by approximately 14 days. The cells successfully attached to the dish surface, demonstrating a uniform growth pattern with morphological features similar to those of spindle-like fibroblasts, BMSCs, ADSCs, and HDFs (Figure 1A).
Figure 1.
Proliferation of periodontal ligament (PDL) stromal cells compared to that of other cell types. A, The morphological features of PDL stromal cells were similar to those of spindle-like fibroblasts (×40). B, Proliferation rates of PDL stromal cells were similar to the rates for ADSCs and BMMCs. ADSC, adipogenic stem cell; BMMC, bone marrow mesenchymal stem cell; HDF, human dermal fibroblast.
Proliferation rate of PDL stromal cells and stem cells from other sources
The proliferation rate of PDL stromal cells from the intact premolars (PDL4) was similar to that of BMSCs and ADSCs (Figure 1B).
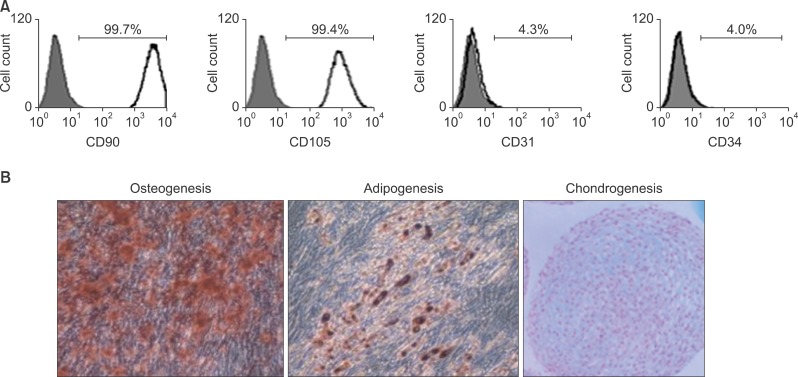
FACS analysis
PDL stromal cells tested positive for the MSC surface markers CD90 and CD105, but tested negative for the hematopoietic stem cell markers CD31 and CD34 (Figure 2A).
Figure 2.
Multipotency of periodontal ligament (PDL) stromal cells. A, PDL stromal cells displayed high expression levels of the mesenchymal stem cell surface markers CD90 and CD105, but very low expression levels of the hematopoietic stem cell surface markers CD31 and CD34. Gray solid fill, isotype; black line, marker of interest. B, Calcified nodules, lipid cluster formation, and chondrogenic pellet formation were noted after osteogenic, adipogenic, and chondrogenic induction, respectively.
Differentiation of PDL cells
The formation of calcified nodules, lipid clusters, and chondrogenic pellets was confirmed after osteogenic, adipogenic, and chondrogenic induction of PDL stromal cells (Figure 2B).
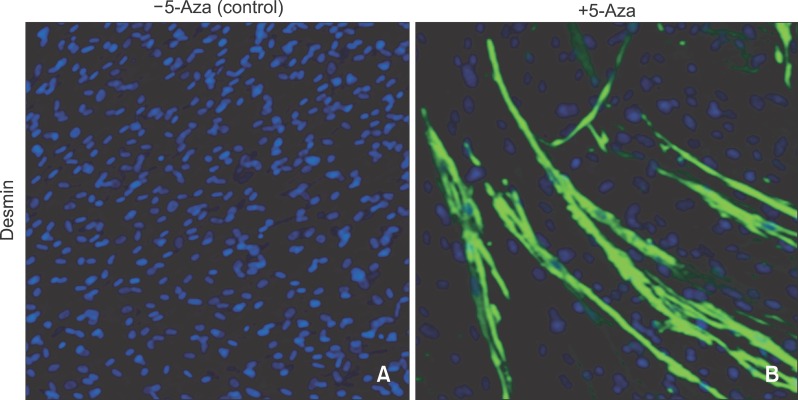
Myogenic differentiation
After 28 days of myogenic induction following 5-Aza pretreatment, myotube-like structures were observed in PDL stromal cells. The tube-like structures expressed desmin, a subunit of intermediate filaments in skeletal muscle, demonstrating the differentiation potential of PDL stromal cells into skeletal myocytes. In contrast, myotube-like structures and desmin expression were not observed in the control cells incubated in myogenesis-inducing medium in the absence of 5-Aza pretreatment (Figure 3).
Figure 3.
Myotube formation and desmin expression of periodontal ligament (PDL) stromal cells. Control cells cultured in the absence of 5-aza-2'-deoxycytidine (5-Aza) treatment, A and cells pretreated with 5-Aza for DNA demethylation, B. Myotube-like structures that were positive for desmin (B, green) were only observed in B. Cell nucleus counterstained with DAPI (blue).
DISCUSSION
We isolated PDL stromal cells from healthy premolars extracted for orthodontic purposes and confirmed that these cells possess a high survival rate, high proliferation rate, and multipotency. In addition, these cells were induced with 5-Aza to form muscle-like structures in vitro.
According to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, MSCs must possess the following minimal criteria.16 First, MSCs must adhere to plastic when maintained in standard culture conditions with tissue culture dishes. Second, MSCs must express the markers CD105, CD73, and CD90, as measured by flow cytometry, but should not express CD45, CD34, CD14, CD11b, CD79a, CD19, or HLA class II. Third, the cells must be capable of differentiating into osteoblasts, adipocytes, and chondrocytes under standard in vitro differentiation conditions. Our results showed that PDL stromal cells from adult premolars displayed the characteristics of MSCs. During orthodontic treatment, multiple premolars are frequently extracted and discarded as medical waste. It is noteworthy that extracted premolars are a good source of postnatal stem cells, which can be applied in various clinical situations.
Previous studies have reported that stem cells obtained from the outgrowth method may be inferior in quality when compared to the cells from the enzyme digestion method with respect to multipotency and effectiveness of differentiation.6,7 However, from the clinician's point of view, the enzyme digestion method is more time consuming and more expensive due to the use of multiple enzymes. In addition, the use of enzymes derived from different species may have cross-reaction issues further limiting clinical applications in humans.7 Therefore, we used the outgrowth method following standard protocols. Our results suggest that PDL stromal cells derived from this method have similar proliferation rates as MSCs derived from other tissues. Moreover, the multipotency to differentiate into osteoblast, adipocyte and chondroblast lineages is similar to that previously reported for enzyme-digested PDL stem cells.14
Unlike the substantial number of hard tissue-regeneration strategies based on the application of PDL cells, the potential for skeletal muscle regeneration using PDL stem cells has been scarcely reported.14 Bone and muscle differentiation is modulated in a complementary manner; muscle progenitor cells frequently differentiate into osteoblasts by the suppression of muscle differentiation factors.17 MSCs possess the potential for osteocyte differentiation; therefore, myogenesis of DP stem cells is ineffective in standard myogenic medium.12 However, the treatment of stem cells with 5-Aza prior to myogenic induction has been reported to cause successful differentiation of mouse DP stem cells into myotubes.12 Pretreatment with 5-Aza results in demethylation of the DNA of genes encoding muscle-specific transcriptional factors, which under other circumstances is inhibited by the methylation of the transcriptional regulatory region.12 In a manner similar to that for DP stem cells, we were able to induce the differentiation of human PDL stromal cells into myotubes by pretreatment of the cells with 5-Aza, but not in its absence.
Many clinical orthodontists are aware of the importance of soft tissues in oromaxillofacial regeneration. Masticatory muscle volume and function are often critically important for diagnosis, treatment efficiency, positive outcomes, and long-term prognosis. The results of this study indicate that PDL stromal cells isolated from orthodontically extracted premolars may be a good source of postnatal stem cells for oromaxillofacial regeneration of hard tissues, as well as soft tissues, including the facial muscles.
CONCLUSION
PDL stromal cells from extracted premolars displayed characteristics of MSCs.
PDL stromal cells differentiated into myogenic lineages following pretreatment with 5-Aza, a DNA demethylation agent.
PDL stromal cells may be a good source of stem cells for oromaxillofacial regeneration, including the facial muscles.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 3.Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149–160. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Spector M. In vitro response of the bone marrow-derived mesenchymal stem cells seeded in a type-I collagen-glycosaminoglycan scaffold for skin wound repair under the mechanical loading condition. Mol Cell Biomech. 2009;6:217–227. [PubMed] [Google Scholar]
- 6.Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Iwasaki K, Feghali KE, Komaki M, Ishikawa I, Izumi Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch Oral Biol. 2011;56:380–388. doi: 10.1016/j.archoralbio.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14:516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 9.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 10.Fang D, Seo BM, Liu Y, Sonoyama W, Yamaza T, Zhang C, et al. Transplantation of mesenchymal stem cells is an optimal approach for plastic surgery. Stem Cells. 2007;25:1021–1028. doi: 10.1634/stemcells.2006-0576. [DOI] [PubMed] [Google Scholar]
- 11.Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyuz S, Ayhan S, et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010;133:95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuka R, Nozaki T, Uemura Y, Matsuoka Y, Sasaki Y, Shinohara M, et al. 5-Aza-2'-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol. 2010;55:350–357. doi: 10.1016/j.archoralbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med. 2008;6:35. doi: 10.1186/1479-5876-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS. Plasticity of stem cells derived from adult periodontal ligament. Regen Med. 2009;4:809–821. doi: 10.2217/rme.09.55. [DOI] [PubMed] [Google Scholar]
- 15.Shiota M, Heike T, Haruyama M, Baba S, Tsuchiya A, Fujino H, et al. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp Cell Res. 2007;313:1008–1023. doi: 10.1016/j.yexcr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32:395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129:2987–2995. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]