Abstract
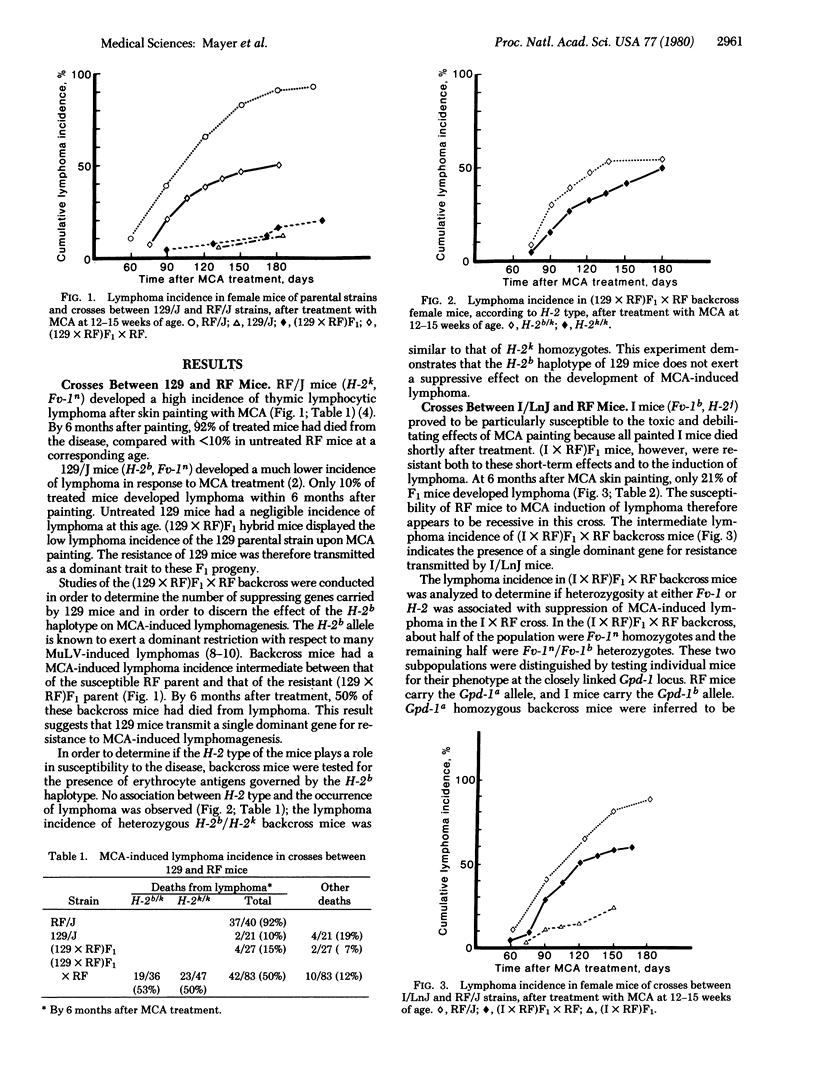
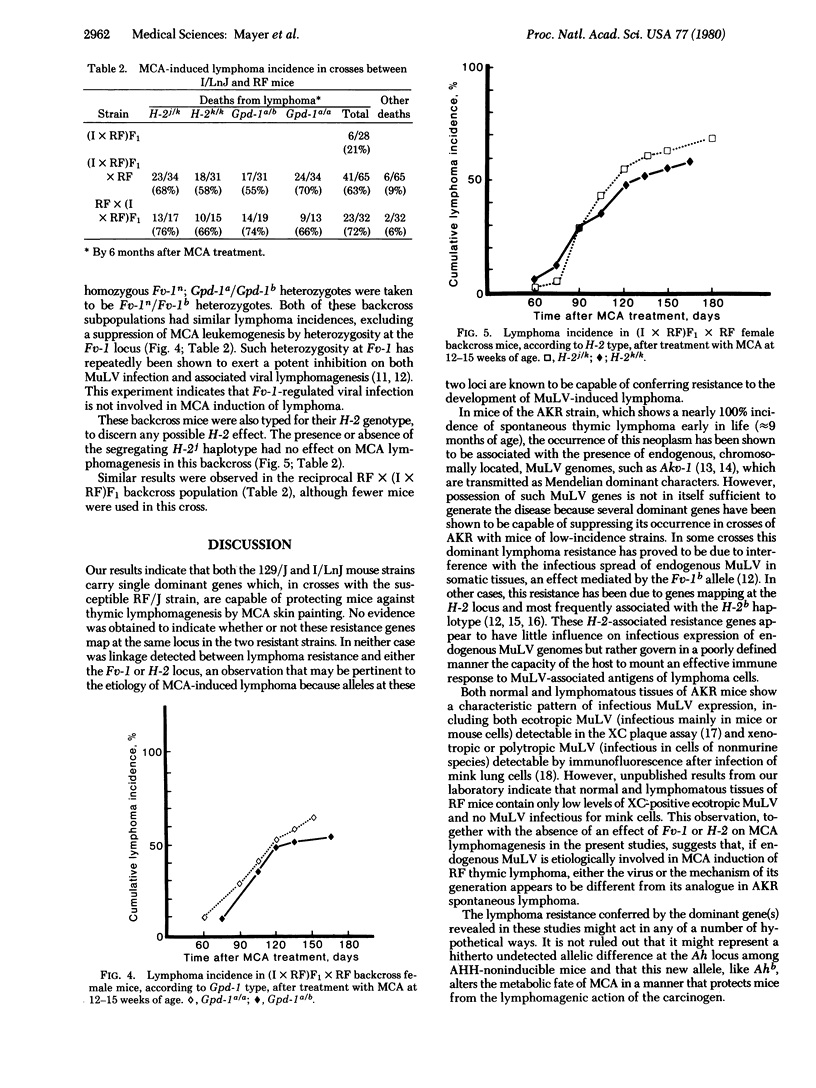
Mice of the RF/J strain are highly susceptible to induction of thymic lymphoma by skin painting with 3-methylcholanthrene (MCA), whereas mice of the 129/J and I/LnJ strains are resistant. Resistance was the dominant trait in F1 mice of crosses of RF with each resistant strain. Analysis of the lymphoma incidence in MCA-painted backcross populations indicated segregation of a single dominant gene for resistance in both crosses. None of these strains show inducibility of the aryl hydrocarbon hydroxylase enzyme system, a phenotype attributed to the dominant Ahb gene which is also known to influence susceptibility to MCA-induced lymphoma. The occurrence of the disease in these backcrosses was independent of the hosts' phenotype at either the H-2 or Fv-1 locus, both of which have shown an influence on susceptibility to murine leukemia virus-associated lymphoma in other experimental systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chattopadhyay S. K., Rowe W. P., Teich N. M., Lowy D. R. Definitive evidence that the murine C-type virus inducing locus Akv-1 is viral genetic material. Proc Natl Acad Sci U S A. 1975 Mar;72(3):906–910. doi: 10.1073/pnas.72.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Reynals M. L., Cook C. Resistance to skin tumorigenesis by 3-methylcholanthrene in mice susceptible to leukemia. J Natl Cancer Inst. 1974 Mar;52(3):1001–1003. doi: 10.1093/jnci/52.3.1001. [DOI] [PubMed] [Google Scholar]
- Duran-Reynals M. L., Lilly F., Bosch A., Blank K. J. The genetic basis of susceptibility to leukemia induction in mice by 3-methylcholanthrene applied percutaneously. J Exp Med. 1978 Feb 1;147(2):459–469. doi: 10.1084/jem.147.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORER P. A., MIKULSKA Z. B. The antibody response to tumor inoculation; improved methods of antibody detection. Cancer Res. 1954 Oct;14(9):651–655. [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri R. E., Ratrie H., Whitmire C. E. Evidence of a genetic relationship between susceptibility to 3-methyl-cholanthrene-induced subcutaneous tumors and inducibility of aryl hydrocarbon hydroxylase. J Natl Cancer Inst. 1973 Jul;51(1):197–200. doi: 10.1093/jnci/51.1.197. [DOI] [PubMed] [Google Scholar]
- LILLY F., BOYSE E. A., OLD L. J. GENETIC BASIS OF SUSCEPTIBILITY TO VIRAL LEUKAEMOGENESIS. Lancet. 1964 Dec 5;2(7371):1207–1209. doi: 10.1016/s0140-6736(64)91043-8. [DOI] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Lilly F. The inheritance of susceptibility to the Gross leukemia virus in mice. Genetics. 1966 Mar;53(3):529–539. doi: 10.1093/genetics/53.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. The role of genetics in Gross virus leukemogenesis. Bibl Haematol. 1970;(36):213–220. doi: 10.1159/000391710. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Sato H. Genetic mapping of the Fv-1 lcous of the mouse. Science. 1973 May 11;180(4086):640–641. doi: 10.1126/science.180.4086.640. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle F. H., Shows T. B., Roderick T. H. Autosomal control of an electrophoretic variant of glucose-6-phosphate dehydrogenase in the mouse (Mus musculus). Genetics. 1968 Apr;58(4):599–606. doi: 10.1093/genetics/58.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant J. R., Snell G. D. The H-2 locus and viral leukemogenesis as studied in congenic strains of mice. J Natl Cancer Inst. 1968 Aug;41(2):597–604. [PubMed] [Google Scholar]
- Thomas P. E., Kouri R. E., Hutton J. J. The genetics of aryl hydrocarbon hydroxylase induction in mice: a single gene difference between C57BL-6J and DBA-2J. Biochem Genet. 1972 Apr;6(2):157–168. doi: 10.1007/BF00486400. [DOI] [PubMed] [Google Scholar]



