Abstract
Wear particle-induced periprosthetic osteolysis remains the principal cause of aseptic loosening of orthopaedic implants. Monocytes/macrophages phagocytose wear particles and release cytokines that induce inflammatory response. This response promotes osteoclast differentiation and osteolysis. The precise mechanisms by which wear particles are recognized and induce the accumulation of inflammatory cells in the periprosthetic tissue have not been fully elucidated. Recent studies have shown that toll-like receptors (TLRs) contribute to the cellular interaction with wear particles. Wear particles are recognized by monocytes/macrophages through TLRs coupled with the adaptor protein MyD88. After the initial interaction, wear particles induce both local and systemic migration of monocytes/macrophages to the periprosthetic region. The cellular migration is mediated through chemokines including interleukin-8, macrophage chemotactic protein-1, and macrophage inhibitory protein-1 in the periprosthetic tissues. Interfering with chemokine-receptor axis can inhibit cellular migration and inflammatory response. This paper highlights recent advances in TLR, and chemokine participated in the pathogenesis of aseptic loosening. A comprehensive understanding of the recognition and migration mechanism is critical to the development of measures that prevent wear particle-induced aseptic loosening of orthopaedic implants.
1. Introduction
Total joint replacement (TJR) by the implantation of indwelling prostheses is an effective operation in terms of relieving pain and restoring function. The common long-term complication of TJR is loosening of an artificial joint that requires revision surgery [1–3]. Kurtz et al. have shown that total hip and total knee revisions will increase by 137% and 601%, respectively, from 2005 to 2030 in the United States [4]. In most cases, aseptic loosening is responsible for revision total joint replacement. It has been reported that aseptic loosening accounts for 70% of hip revisions and 44% of knee revisions [5, 6].
The dominant theory about the causes of aseptic loosening is the particle disease theory [7–9]. Particles can be generated as a result of wear. The concentration of wear particles is directly related to the amount of osteolysis. There are a great number of wear particles in the periprosthetic membranebetween bone and prosthesis. These wear particles which are biologically active and indigestible can initiate an innate inflammatory reaction [10–12]. Actually, wear particles alter the function of numerous cells including monocytes, macrophages, fibroblasts, osteoblasts, osteoclasts, and mesenchymal stem cells (MSCs). Macrophages have been accepted to be the key target of wear particles. Wear particles can induce the proliferation, differentiation, and activation of macrophages [13, 14]. Upon activation, macrophages secrete a series of inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), IL-1β, IL-6, and IL-8. These inflammatory mediators can induce osteoclast differentiation or inhibit osteoblast differentiation, leading to periprosthetic bone resorption [15, 16]. Moreover, some cytokines can attract and recruit large numbers of cells including macrophages, osteoclasts, and lymphocytes to the local site. These recruited cells then produce more cytokines, and a progress perpetuates a cycle of inflammatory response [17–19]. Apart from macrophages, osteoblasts and fibroblasts can also phagocytose particles, which significantly increase the expression of TNF-α, IL-6, and RANKL [20, 21]. It has recently been shown that MSCs participate in wear particle-induced aseptic loosening. MSCs are identified around the joint replacement and contribute to maintainingosseous tissue integrity [22]. Wear particles can induce the production of inflammatory cytokine of MSCs on the one hand, while inhibit osteogenic activity on the other hand, resulting in osteolysis in periprosthetic region [23, 24].
Although numerous studies have demonstrated the events underlying periprosthetic inflammation and osteolysis, there are still more questions. First, the mechanisms of the initial cellular interaction with wear particles and the subsequent inflammatory mediator production remain unknown. Second, wear particles induce not only a local response but also a systemic reaction. The mechanism of cellular migration induced by wear particles needs further clarification. In 2007, Takagi et al. first reported that toll-like receptors (TLRs) were detected in the tissues around aseptically loosened implants [25]. TLR-deficient mice displayed decreased osteolysis. There is increasing evidence that TLRs play a critical role in initiating cellular interaction with particles and the subsequent inflammatory cascade [26, 27]. Lassus et al. reported that chemokines participated in the cellular migration in response to wear particles [28, 29]. On the basis of these findings, this paper will discuss the role of TLRs in the recognition of wear particles and the role of chemokines in the cellular migration induced by wear particles, respectively. We believe that this could provide valuable insight into the design of preventive and therapeutic strategies in the future.
2. TLRs and Wear Particle-Induced Aseptic Loosening
2.1. TLRs and Ligands
TLRs belong to a class of pattern recognition receptors that enable the innate immune system to distinguish self- from nonself-structure. Thirteen TLRs have been identified in mammals since 1997. TLR1, TLR2, TLR4, TLR5, TLR6, TLR10, and TLR11 are expressed on the cell surface, whereas TLR3, TLR7, TLR8, and TLR9 are expressed intracellularly on endosomal membranes [30]. They are type I transmembrane proteins composed of an intracellular toll/interleukin-1 receptor (TIR) domain and leucine-rich repeat motifs in the extracellular domain. Leucine-rich repeats recognize danger signals, while TIRs recruit adaptor proteins and mediate downstream signaling [31, 32]. TLRs can recognize a myriad of stimuli including pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs are exogenous molecules derived from bacteria, virus, and fungi. DAMPs include endogenous intracellular molecules and extracellular matrix. The intracellular molecules, such as heat-shock protein (HSP) and high mobility group protein 1 (HMGB1), are released into the extracellular milieu by necrotic cells and activated leukocytes. The extracellular matrix includes biglycan, tenascin-C, and hyaluronic acid which are released upon injury and noninfectiousinflammatory response. The PAMPs and DAMPs identified for each TLR member are listed in Table 1 [30, 33].
Table 1.
Toll-like receptors and their corresponding ligands.
| TLR | Exogenous ligands (PAMPs) | Endogenous ligands (DAMPs) |
|---|---|---|
| TLR1 | Triacyl lipopeptide Soluble factors |
β-defensin 3 |
| TLR2 (dimerization with TLR1or 6) |
Lipoglycans (mycobacterium) Lipoteichoic acids (gram-positive bacteria) Peptidoglycan (gram-positive bacteria) Zymosan (yeast) |
HSP 60, HSP70, gp96, β-defensin 3, HMGB1, surfactant proteins, HMGB1-nucleosome complexes, serum amyloid A, eosinophil-derived neurotoxin, antiphospholipid antibodies, cardiac myosin, PAUF, CEP, monosodium urate crystals, Biglycan, versican, hyaluronic acid fragments |
| TLR3 | dsRNA | mRNA |
| TLR4 | LPS (gram-negative bacteria) Mannan (Candida) Envelope protein (virus) Hsp70 (exogenous) |
HMGB1, surfactant proteins, β-defensin 2, HSP60, HSP70, HSP72, HSP22, Gp96, S100A8, S100A9, neutrophil elastase, antiphospholipid antibodies, lactoferrin, serum amyloid A, oxidized LDL, saturated fatty acids, resistin, PAUF, monosodium urate crystals, Biglycan, fibronectin EDA, fibrinogen, tenascin C, Heparin sulphate fragments, Hyaluronic acid fragments |
| TLR5 | Flagellin (gram-negative bacteria) | Undetermined |
| TLR6 | Diacylpolypeptide Lipoteichoic acid (gram-positive bacteria) |
Undetermined |
| TLR7/8 | ss RNA (virus) | Antiphospholipid antibodies, cardiac myosin, ss RNA, |
| TLR9 | CpG motif (bacteria, virus) | IgG-chromatin complexes, mitochondrial DNA |
| TLR10 | Diacylated peptide? | Immunostimulatory CpG motifs |
| TLR11 | Profilin-like molecule | Undetermined |
2.2. TLR Signaling and Negative Regulators
Ligand-TLR interactions trigger the recruitment of adaptor proteins through the cytoplasmic TIR domain. So far, five TIR-containing adaptors have been identified: myeloid differentiation primary response gene 88 (MyD88), TIR domain-containing adaptor protein (TIRAP)/Mal, TIR domain-containing adaptor protein-inducing IFN-β (TRIF)/TICAM1, TRIF-related adaptor molecule (TRAM)/TICAM2, and Sterile-α and HEAT-armadillo motifs containing protein (SARM) [34, 35]. Depending onthe usage of adaptor molecules, the signaling pathways activated by TLR are divided into MyD88-dependent and MyD88-independent pathways. MyD88 is the main adaptor shared by TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, and TLR11. TIRAP/Mal is involved in theMyD88-dependent pathwayvia TLR2 and TLR4. The MyD88-dependent pathways lead to the activation of nuclear factor (NF)-κB and activating protein-1 (AP-1), which is responsible for the production of proinflammatory cytokines such as TNF-α, IL-1, and IL-12. MyD88-independent pathways are mediated by TRIF or TRAM. TRIF is an important adaptor which is used by TLR3. TRAM can link the TIR domain of TLR4 with TRIF. The MyD88-independent pathways lead to the activation of NF-κB, AP-1, and interferon-regulatory factors (IRFs). The activation of transcription factors finally triggers the production of cytokines including TNF-α, IL-1β, IL-6, and IFN-α/β/γ.
TLR signaling mediatesinflammatoryresponses which are important for host defense. However, inappropriate TLR signaling may be responsible for the pathogenesis of autoimmune diseases, chronic inflammatory diseases, and aseptic inflammatory diseases [36]. To avoid harmful and excessive inflammatory responses, the immune system has developed multiple mechanisms to control TLR signaling. Many negative regulators have been reported. According to the regulatory mechanisms, these negative regulators can be classified into three groups [37]: (1) negative regulators for degradation of signal proteins: TAG, SARM, IRF4, TIPE2, NLRX1, NLRC5, TANK, MSK1, MSK2, TAK1, SHP1, SHP-2, A20, CYLD, USP4, and DUBA; (2) negative regulators for degradation of signal proteins: PDLIM2, Trim30α, and TRIM38; (3) negative regulators for transcriptional control: ATF3, IkBNS, Bcl-3, Nurr1, Ah receptor, Zc3h12a, TTP, miR-155, miR-146a, and miR-21.
2.3. TLRs and Aseptic Loosening
TLRs have been observed on variety of cells including monocytes/macrophages, lymphocytes, fibroblasts, osteoblasts, and osteoclasts. It hasrecently been reported that TLRs were found in periprosthetictissues of patients with aseptic loosening. The TLR-positive cells are dominantly monocyte/macrophages [25, 38, 39]. Moreover, both PAMPs and DAMPs have been indicated in the activation of TLRs in aseptic loosening [40, 41]. These findings suggested that TLRs may play an important role in the pathogenesis of aseptic loosening. We will now mainly discuss TLR1/2 and TLR4 which have attracted more attention than other TLRs.
TLR1 and TLR2 are expressed on the cell surface and canform heterodimer [33]. They participate in the recognition of extracellular microbial pathogenic components including lipoprotein/lipopeptides, peptidoglycan, and atypical LPS [34]. When mouse femur was inserted with stainless steel rod and titanium particles, the expressions of TLR1 and TLR2 were found in peri-implant and bone tissues. RAW 264.7 cells expressed both TLR1 and TLR2. However, only TLR1 was increased when cultured with titanium particles [42]. TLR2 was detected on monocytes/macrophages in aseptic synovial-like membranes from loose implants [43]. Hirayama et al. found that the expression of TLR2 was markedly increased after stimulation with LPS-coated titanium particles. On the contrary, other TLRs such as TLR4, TLR5, and TLR9 were decreased, suggesting a self-protective mechanism after stimulation with LPS-coated titanium particles [26]. Using a murine calvarial model of particle-induced osteolysis, Greenfield et al. found the TLR2−/− mice displayed more limited osteolysis than wild mice. TLR2−/− macrophages secreted reduced TNF-α when challenged with titanium particles. These in vitro and in vivo data strongly support the critical role of TLR1/2 in aseptic loosening of implants [41].
TLR4 is the membrane receptor that can recognize LPS, mannan, glycoinositolphospholipids, envelope proteins, or some self-proteins including HSP60 and HSP70 [44, 45]. As a receptor for LPS, TLR4 has received the most attention in aseptic loosening. There was a significant increase of TLR4 in the tissue around loosened replacement implants [25, 43, 46, 47]. The mutation of TLR4 resulted in inhibited inflammatory response and osteolysis when exposed to wear particles [48]. TLR4−/− mice displayed decreased osteolysis. These findings indicated that TLR4 played a key role in the pathogenesis of aseptic loosening. Monocytes/macrophages are equipped with TLRs and HSPs. Hao et al. found that UHMWPE particle upregulated the expressions of TLR4 and HSP60 on monocytes. HSP60 can bind to TLR4, leading to the production of inflammatory cytokines such as IL-1 β, IL-6, and TNF-α [40]. In this scenario, TLR4 played a critical role because interfering with TLR4 resulted in reduced cytokine production [40]. Like UHMWPE particle, titanium particle exposure can also elicit cytokine production and osteolysis. This phenomenon resulted from the engagement of TLR4 and wear particleswithadherentLPS. [48]. LPS can be detected in the tissues around aseptically loosened implants, which contributed to the inflammatory responses induced by wear particles [49]. However, it is still unclear whether endotoxin is required for the biological response to that wear particles. People found wear particles with LPS decreased the mRNA expression of TLR4 compared to wear particles without LPS. This can be explained by a self-protective mechanism. LPS-coated wear particles can be easily identified by macrophages via TLR4. After the initiation of response, TLR4 was downregulated to prevent excessive harmful host response [26]. The reduced expression of TLR4 mRNA was also found in RAW 264.7 cells or rat macrophages stimulated with titanium particles in vitro [25, 42]. It seemed that auto- or paracrine inflammatory cytokine downregulated the expression of TLR to avoid damage caused by excessive inflammatory responses [42]. Interestingly, TLR4−/− macrophages showed similar levels of TNF-α compared to wild-type macrophages when challenged with wear particles. The unexpected results may be caused by the cells used in the experiments because a variety of cells expressed TLR4. Beidelschies et al. found that, in macrophages that lack TLR4 and TLR2, the section of TNF-α was completely neutralized when stimulated with wear particles. However, osteolysis in vivo was only partly inhibited. They supposed that early inflammatory response induced by particles was TLR dependent, while later osteolysis was just partially TLR dependent.
Ligand-TLR binding induces rearrangements of TIR domains and recruitment of adaptors (MyD88, TRIF, and TIRAP), triggering the activation of NF-κB. Pearl et al. found that the inflammatory responses induced by PMMA particles can be decreased using a MyD88 inhibitor. Similar results were found in MyD88−/− macrophages stimulated with PMMA [27]. These findings strongly supported that particles can be recognized through TLR, partly dependent on MyD88 signaling pathway. Maitra et al. reported that UHMWPE particles activated TLR1/TLR2, leading to an inflammatory program mediated by NF-κB-signaling pathway [50]. It has been reported that the p38 and JNK signaling pathways can mediate wear particle-induced osteoclast differentiation in vitro. However, the relationship between specific TLRs and downstream p38 and JNK-signaling pathways remains unknown in wear particle-induced aseptic loosening. It is hypothesized that wear particles can be recognized by TLR, triggering downstream signaling pathway including AP-1 and NF-κB, leading to inflammatory responses and osteolysis (Figure 1).
Figure 1.
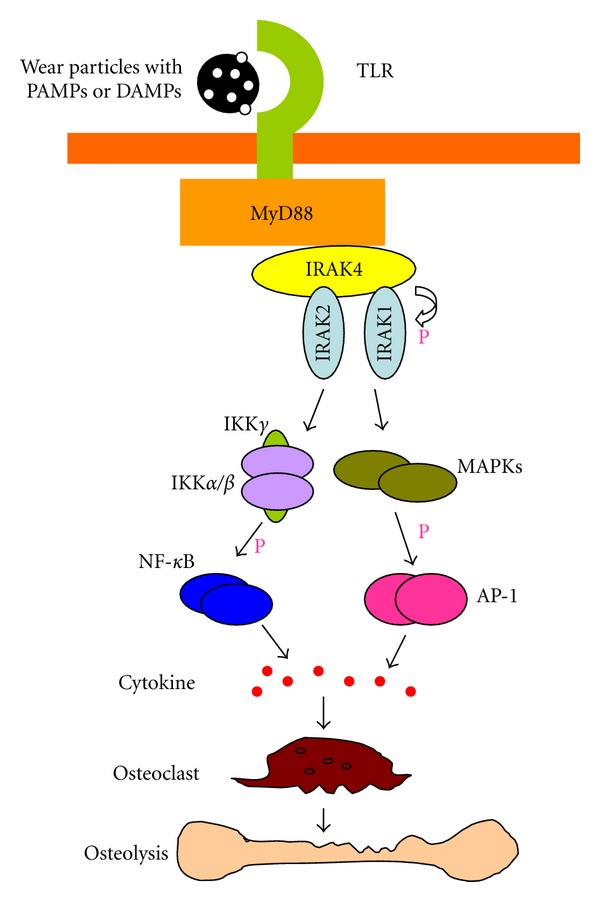
Wear particles induced TLR signal pathway. TLRs recognize wear particles with adherent PAMPs or DAMPs via MyD88. The binding of TLR and MyD88 phosphorylates IRAK4 which in turn phosphorylates IRAK1. The activation of AP-1 and NF-κB leads inflammatory cytokine production and osteoclast differentiation which contribute to osteolysis.
3. Chemokines and Wear Particle-Induced Aseptic Loosening
3.1. Chemokines and Chemokine Receptors
Chemokines are a group of small proteins with a crucial role in leukocyte migration and activation. These molecules can also affect cytokine secretion, apoptosis, phagocytosis, angiogenesis, and collagen production [51, 52]. The chemokine family can be classified into four groupsas CXC, CC, CX3C, and XC, based on the number and spacing of conserved cysteines. CXC, CC, and CX3C chemokines contain four conserved cysteines, whereas XC chemokines contain two conserved cysteines. In the CXC and CX3C chemokines, one (CXC) or three (CX3C) amino acids are inserted between the first two of fourcysteines. In the CC chemokines, the first two cysteines are adjacent. About 50 chemokines have been identified in humans. Most chemokines are members of CC or CXC groups, while others belong to XC or CX3C groups [53, 54].
The effects of chemokines are mediated by a family of G protein-coupled receptor on the target cell surface [55]. These chemokine receptors are also classified into four groups as CXCR, CCR, CX3R and XCR, based on the chemokine group they bind. About 25 chemokine receptors have been identified in human. The relationship between chemokines and chemokine receptors is complex because chemokine receptors bind different chemokines and vice versa.
3.2. Chemokines in Particle-Induced Cellular Migration
In the early time, it was assumed that wear particles induced a localized response. In brief, wear particles stimulated resident cells (including macrophages, osteoblast) to produce cytokines such as TNF-α, IL-1α, IL-1β, and IL-6, resulting in local osteolysis. It has recently been shown that wear particles can induce chemokine expression in macrophages, fibroblasts, and osteoblasts, indicating a cellular migration mechanism in aseptic loosening [56–58]. Monocytes/macrophages accumulated in the periprosthetic tissue were mainly polarized M1 macrophages. Rao et al. hypothesized that monocyte/macrophage progenitors may be attracted to the local microenvironment in response to wear particles, and then differentiate into M1 phenotype [10]. More recently, Ren and Gibon both demonstrated that wear particles induced significant chemotaxis of macrophages in vivo [29, 59, 60]. These findings strongly support the mechanism of cellular migration to the site around the implants, which contributed to pathogenesis of aseptic loosening (Figure 2). In the following article we will focus on the specific chemokines involved in the cellular migration in response to wear particles.
Figure 2.
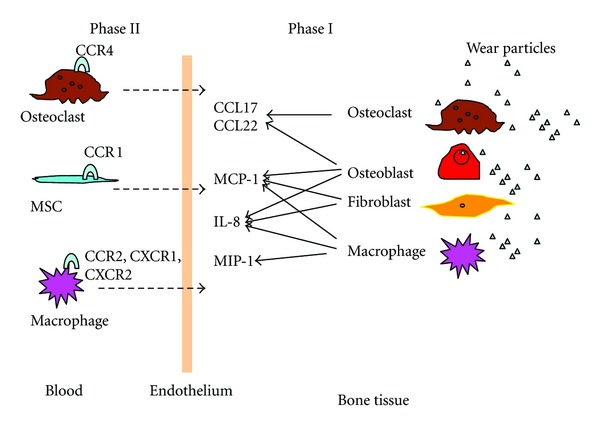
Wear particles induced chemokine expression and cellular migration.
IL-8, also known as CXCL8, is a member of the CXC chemokine family [61]. IL-8 is mainly produced by monocytes/macrophages. The coupled receptors for IL-8 are CXCR1 and CXCR2, which are located on the surface of macrophages, endothelial cells, mast cells, and epithelial cells [62]. The binding of IL-8 to receptors can trigger a series of biological effects including the activation and recruitment of neutrophils and macrophages [63]. Many reports have shown that IL-8 was upregulated in periprosthetic tissues [28, 64, 65]. These findings indicated that IL-8 can be a marker of aseptic loosening. Actually, wear particles can stimulate the production of IL-8 by MG-63 and primary osteoblasts in vitro [66–70]. Kaufman et al. reported that primary human macrophages produced high level of IL-8 upon the stimulation of TiAlV particles [70]. UHMWPE and CoCr particles can also stimulate primary human macrophages to produce IL-8, although mildly compared to TiA1V particles. The latest research has shown that titanium particles can increase the production of IL-8 by MSCs [24]. The increased production of IL-8 can attract the migration of macrophages and osteoclasts to the site around implants, leading to osteolysis.
MCP-1, also known as CCL2, was identified based on its ability to chemoattract monocytes in vitro [71, 72]. Further studies showed that MCP-1 can also attract memory T cells, natural killer cells, and macrophagesto the sites of inflammation through the activation of CCR2 or CCR4 [73, 74]. It has been demonstrated that MCP-1 can be produced by endothelial cells, osteoblasts, fibroblasts, monocytes, and macrophages [75, 76]. Wear particles can increase the expression of MCP-1 in primary human macrophages, MG-63 cells, and fibroblasts, resulting in the recruitment of monocytes/macrophages [57, 58, 70, 77]. Increased expression of MCP-1 was also displayed in tissues from patients with failed arthroplasties, indicating that MCP-1 may be a potential marker of osteolysis [57, 78]. Huang et al. showed that PMMA or UHMWPE particles increased MCP-1 expression in RAW 264.7 cells. Supernatant from particle-stimulated RAW 264.7 cells displayed increased chemotacticresponse inTHP-1 cells, which can be mitigated by neutralizing antibody to MCP-1 [79]. Interestingly, blocking CCR2 receptor reduced PMMA-induced THP-1 cell migration, while has no effect on UHMWPE-induced THP-1 cell migration. Since most studies mentioned above are operated in vitro, it is impossible to understand whether the cells responding to wear particles are motivated locally or systemically. To clarify this issue, Gibon et al. injected MCP-1 into femur in a murine femoral implant model. They found that MCP-1 recruited exogenous RAW 264.7 cells to the femur upon the stimulation of UHMWPE particles. Blocking the interaction of MCP-1/CCR2 resulted in decreased migration of RAW 264.7 cells. MCP-1 also recruited primary murine macrophages into femur upon the stimulation of UHMWPE particles. Moreover, the recruitment of primary macrophages was lower when CCR2-deficient macrophages were injected [29]. These findings indicated that wear particle-induced MCP-1 expression was critical to the migration of macrophages and subsequent inflammatory responses. The interruption of MCP-1/CCR2 axis may be a useful strategy to inhibit osteolysis.
MIP-1 includes MIP-1α (CCL3) and MIP-1β (CCL4). It is mainly produced by lymphocytes, monocytes, macrophages, fibroblasts, and epithelial cells [80]. MIP-1α played an important role in the migration of T cells, B cells, monocytes, dendritic cells, neutrophils, and natural killer cells [81]. The biological effects of MIP-1α were mediated through their engagement with CCR1, CCR4, and CCR5 [51]. Like MCP-1, the expression of MIP-1α was found in the periprosthetic tissues. Titanium and PMMA particles upregulated the production of MIP-1α in primary human monocytes/macrophages, leading to increased migration of human monocyte. Neutralizing antibody to MIP-1α mitigated the wear particle-induced migration [57]. These findings demonstrated that cellular migration mediated by MIP-1α was important in wear particle-stimulated inflammatory responses. However, in another research, MIP-1α seemed not to play a role in the chemotaxis function of wear particle-challenged RAW 264.7 cells. First, RAW 264.7 cells produced similar levels of MIP-1α when stimulated with or without wear particles. Second, although MIP-1α possessed potent chemotactic ability for macrophages, neutralizing antibody to MIP-1α failed to inhibit the migration of THP-1 cells in the culture stimulated with particles [79]. These results are complicated and have not been explained by now. One possible explanation may be the specific culture condition or neutralizing antibody used in the experiments. Surprisingly, the supernatant from PMMA-challenged RAW 264.7 cells significantly chemoattracted human MSCs, which can be inhibited by neutralizing antibody to MIP-1α. This result indicated that particle-stimulated MIP-1α release was responsible for the migration of MSCs [79]. CCR1 was one of the receptors for MIP-1α. Huang et al. found that neutralizing antibody to CCR1 failed to affect the migration of MSCs in culture with wear particles in vitro. On the contrary, using a murine model, Gibon et al. have recently shown that UHWMPE particles induced increased migration and osteoblast differentiation of MSCs in vivo, which can be neutralized by antagonist to CCR1 [82]. Since CCR1 can bind to a variety of ligands including MIP-1α, MCP-3, and RANTES, these findings were not sufficient to support the chemotaxis ability of MIP-1α. The specific chemokines participated in the migration of MSCs to the site around implants that need further investigation.
CCL17 and CCL22 are the two recognized ligands for the chemokine receptor CCR4. They are known to be mainly produced by cell lineages closely related to osteoclasts such as dendritic cells. CCL22 has been shown to be expressed by activated macrophages and mature dendritic cells, whereas CCL17 has been shown to be secreted by keratinocytes and endothelial cells. Titanium particles increased the expressions of CCL17 and CCL22 in osteoclasts and hFOB cells. Moreover, the expression of CCR4 was upregulated when osteoclast precursors were stimulated with titanium particles. These results implied a role for CCL17 and CCL22 in the chemotaxis of CCR4 expressing osteoclast progenitors to the site around implants [83].
4. Conclusions and Perspective
Aseptic loosening is the common cause of the failure of TJR, and the mechanisms underlying it appear complex and multifaceted. A large number of scholars have focused on the biological activity of wear particles. Macrophages recognize wear particles and release proinflammatory mediators, leading to osteoclast activation and osteolysis. Although it has been accepted that the interaction between wear particles and macrophages is critical, little is known about how wear particles are recognized and activate macrophages in the early inflammatory response. In vivo and in vitro studies have supplied strong evidence that wear particles can activate macrophages through TLRs. TLRs are evolutionarily conserved pattern recognition receptors in sensing exogenous PAMPs and endogenous DAMPs. Both PAMPs and DAMPs are responsible for the activation of TLRs in aseptic loosening. DAMPs/PAMPs-TLR interaction may be a novel mechanism of aseptic loosening. Although the relationship between TLRs and chemokines have not been clarified, it seems possible that the activation of TLR may promote macrophages to express different chemokines. In brief, the pathogenesis of implant-associated aseptic loosening includes a series of events: macrophages response to wear particles acting as PAMPs/DAMPs via various TLRs, especially TLR4 and TLR2. These TLRs then interact with adaptor protein MyD88, finally triggering the activation of NF-κB and production of inflammatory cytokines. The cytokines induced by wear particles include chemokines such as IL-8, MCP-1, and MIP-1. These chemokines bind to specific G-protein-linked transmembrane receptors and activate intracellular signaling pathways, leading to recruit more macrophages, MSCs, neutrophils, and osteoclasts to the site of injury. The accumulation of cells further facilitates propagation of inflammatory and subsequent osteolytic events.
Given the fact that wear particles are recognized via TLRs, effective strategies can be designed to block the event. For example, MyD88 interfering may block TLR downstream signaling pathway and then prevent wear particle-induced periprosthetic osteolysis. Another promising approach involves inhibiting recruitment of macrophages and other cells to the inflammatory site. Pharmacologic intervention targeted at chemokine-receptor axis may provide the means to mitigate the response to wear particles. Indeed, in vivo study has shown disruption of MCP-1 ligand-receptor axis can inhibit wear particle-induced migration of macrophages and osteolysis. In general, understanding the mechanisms of wear particle-induced cellular activation and migration will provide insight into the prevention and treatment of prosthetic aseptic loosening. The future of research needs to focus on some areas: the specific TLRs which are activated by exposure to different types of wear particles, the downstream signaling pathway mediated by TLR, the specific chemokine-receptor axis participating in wear particle-induced cellular migration, the exact role of cell recruited by wear particles in aseptic loosening.
References
- 1.Farber A, Chin R, Song Y, Huie P, Goodman S. Chronic antigen-specific immune-system activation may potentially be involved in the loosening of cemented acetabular components. Journal of Biomedical Materials Research. 2001;55(3):433–441. doi: 10.1002/1097-4636(20010605)55:3<433::aid-jbm1033>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Koulouvaris P, Ly K, Ivashkiv LB, et al. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. Journal of Orthopaedic Research. 2008;26(1):106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 3.Chiu R, Ting M, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. Journal of Biomedical Materials Research Part A. 2009;89(1):242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Journal of Bone and Joint Surgery Series A. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthopaedica Scandinavica. 2000;71(2):111–121. doi: 10.1080/000164700317413067. [DOI] [PubMed] [Google Scholar]
- 6.Robertsson O, Knutson K, Lewold S, Lidgren L. The Swedish Knee Arthroplasty Register 1975–1997: an update with special emphasis on 41,223 knees operated on in 1988–1997. Acta Orthopaedica Scandinavica. 2001;72(5):503–513. doi: 10.1080/000164701753532853. [DOI] [PubMed] [Google Scholar]
- 7.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Goodman S, Aspenberg P, Song Y, et al. Tissue ingrowth and differentiation in the bone-harvest chamber in the presence of cobalt-chromium-alloy and high-density-polyethylene particles. Journal of Bone and Joint Surgery Series A. 1995;77(7):1025–1035. doi: 10.2106/00004623-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Maloney WJ, Smith RL. Periprosthetic osteolysis in total hip arthroplasty: the role of particulate wear debris. Instructional Course Lectures. 1996;45:171–182. [PubMed] [Google Scholar]
- 10.Rao AJ, Gibon E, Ma T, Yao Z, Smith RL, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomaterialia. 2012;8(7):2815–2823. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witt JD, Swann M. Metal wear and tissue response in failed titanium alloy total hip replacements. Journal of Bone and Joint Surgery Series B. 1991;73(4):559–563. doi: 10.1302/0301-620X.73B4.2071635. [DOI] [PubMed] [Google Scholar]
- 12.Wooley PH, Schwarz EM. Aseptic loosening. Gene Therapy. 2004;11(4):402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 13.Purdue PE. Alternative macrophage activation in periprosthetic osteolysis. Autoimmunity. 2008;41(3):212–217. doi: 10.1080/08916930701694626. [DOI] [PubMed] [Google Scholar]
- 14.Miyanishi K, Trindade MCD, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. Journal of Bone and Mineral Research. 2003;18(9):1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- 15.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS Journal. 2006;2(2):102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz SM, Gonzales JB. Inflammatory response to implant particulates in a macrophage/osteoblast coculture model. Calcified Tissue International. 1996;59(5):392–396. doi: 10.1007/s002239900145. [DOI] [PubMed] [Google Scholar]
- 17.St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. Journal of Orthopaedic Research. 2010;28(11):1418–1424. doi: 10.1002/jor.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck RT, Illingworth KD, Saleh KJ. Review of periprosthetic osteolysis in total joint arthroplasty: an emphasis on host factors and future directions. Journal of Orthopaedic Research. 2012;30(4):541–546. doi: 10.1002/jor.21554. [DOI] [PubMed] [Google Scholar]
- 19.Bauer TW. Particles and periimplant bone resorption. Clinical Orthopaedics and Related Research. 2002;(405):138–143. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Koreny T, Tunyogi-Csapó M, Gál I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis and Rheumatism. 2006;54(10):3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 21.Pioletti DP, Kottelat A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials. 2004;25(27):5803–5808. doi: 10.1016/j.biomaterials.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Okafor CC, Haleem-Smith H, Laqueriere P, Manner PA, Tuan RS. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. Journal of Orthopaedic Research. 2006;24(3):461–473. doi: 10.1002/jor.20075. [DOI] [PubMed] [Google Scholar]
- 23.Wang ML, Nesti LJ, Tuli R, et al. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. Journal of Orthopaedic Research. 2002;20(6):1175–1184. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 24.Haleem-Smith H, Argintar E, Bush C, et al. Biological responses of human mesenchymal stem cells to titanium wear debris particles. Journal of Orthopaedic Research. 2012;30(6):853–863. doi: 10.1002/jor.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi M, Tamaki Y, Hasegawa H, et al. Toll-like receptors in the interface membrane around loosening total hip replacement implants. Journal of Biomedical Materials Research Part A. 2007;81(4):1017–1026. doi: 10.1002/jbm.a.31235. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama T, Tamaki Y, Takakubo Y, et al. Toll-like receptors and their adaptors are regulated in macrophages after phagocytosis of lipopolysaccharide-coated titanium particles. Journal of Orthopaedic Research. 2011;29(7):984–992. doi: 10.1002/jor.21369. [DOI] [PubMed] [Google Scholar]
- 27.Pearl JI, Ma T, Irani AR, et al. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32(24):5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lassus J, Waris V, Xu JW, et al. Increased interleukin-8 (IL-8) expression is related to aseptic loosening of total hip replacement. Archives of Orthopaedic and Trauma Surgery. 2000;120(5-6):328–332. doi: 10.1007/s004020050475. [DOI] [PubMed] [Google Scholar]
- 29.Mao X, Pan X, Peng X, Cheng T, Zhang X. Inhibition of titanium particle-induced inflammation by the proteasome inhibitor bortezomib in murine macrophage-like RAW 264.7 cells. Inflammation. 2012;35(4):1411–1418. doi: 10.1007/s10753-012-9454-5. [DOI] [PubMed] [Google Scholar]
- 30.Takagi M. Toll-like receptor—a potent driving force behind rheumatoid arthritis. Journal of Clinical and Experimental Hematopathology. 2011;51(2):77–92. doi: 10.3960/jslrt.51.77. [DOI] [PubMed] [Google Scholar]
- 31.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/Cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 32.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes and Infection. 2004;6(15):1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Pope RM, Huang QQ. The role of Toll-like receptors in rheumatoid arthritis. Current Rheumatology Reports. 2009;11(5):357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Akira S. Toll-like receptors in innate immunity. International Immunology. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 36.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nature Reviews Immunology. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends in Immunology. 2012;33(9):449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Pajarinen J, Cenni E, Savarino L, et al. Profile of toll-like receptor-positive cells in septic and aseptic loosening of total hip arthroplasty implants. Journal of Biomedical Materials Research Part A. 2010;94(1):84–92. doi: 10.1002/jbm.a.32674. [DOI] [PubMed] [Google Scholar]
- 39.Lähdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis—a potential to respond against danger signals? Journal of Orthopaedic Research. 2010;28(2):184–190. doi: 10.1002/jor.20979. [DOI] [PubMed] [Google Scholar]
- 40.Hao HN, Zheng B, Nasser S, et al. The roles of monocytic heat shock protein 60 and Toll-like receptors in the regional inflammation response to wear debris particles. Journal of Biomedical Materials Research Part A. 2010;92(4):1373–1381. doi: 10.1002/jbm.a.32474. [DOI] [PubMed] [Google Scholar]
- 41.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate toll-like receptors. The Journal of Biological Chemistry. 2010;285(42):32378–32384. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pajarinen J, Mackiewicz Z, Pöllänen R, et al. Titanium particles modulate expression of Toll-like receptor proteins. Journal of Biomedical Materials Research Part A. 2010;92(4):1528–1537. doi: 10.1002/jbm.a.32495. [DOI] [PubMed] [Google Scholar]
- 43.Tamaki Y, Takakubo Y, Goto K, et al. Increased expression of toll-like receptors in aseptic loose periprosthetic tissues and septic synovial membranes around total hip implants. Journal of Rheumatology. 2009;36(3):598–608. doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 44.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. The Journal of Immunology. 2000;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 46.Hou L, Sasaki H, Stashenko P. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infection and Immunity. 2000;68(8):4681–4687. doi: 10.1128/iai.68.8.4681-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang L, Jung JY, Wang EW, et al. Pseudomonas aeruginosa lipopolysaccharide induces osteoclastogenesis through a toll-like receptor 4 mediated pathway in vitro and in vivo. Laryngoscope. 2007;117(5):841–847. doi: 10.1097/MLG.0b013e318033783a. [DOI] [PubMed] [Google Scholar]
- 48.Bi Y, Seabold JM, Kaar SG, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. Journal of Bone and Mineral Research. 2001;16(11):2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 49.Nalepka JL, Lee MJ, Kraay MJ, et al. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clinical Orthopaedics and Related Research. 2006;(451):229–235. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 50.Maitra R, Clement CC, Scharf B, et al. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Molecular Immunology. 2009;47(2-3):175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rollins BJ. Chemokines. Blood. 1997;90(3):909–928. [PubMed] [Google Scholar]
- 52.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annual Review of Pharmacology and Toxicology. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 53.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 54.Pease JE, Williams TJ. Chemokines and their receptors in allergic disease. Journal of Allergy and Clinical Immunology. 2006;118(2):305–318. doi: 10.1016/j.jaci.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annual Review of Immunology. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 56.Lind M, Trindade MCD, Schurman DJ, Goodman SB, Smith RL. Monocyte migration inhibitory factor synthesis and gene expression in particle-activated macrophages. Cytokine. 2000;12(7):909–913. doi: 10.1006/cyto.1999.0647. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima Y, Sun DH, Trindade MCD, et al. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. Journal of Bone and Joint Surgery Series B. 1999;81(1):155–162. doi: 10.1302/0301-620x.81b1.8884. [DOI] [PubMed] [Google Scholar]
- 58.Yaszay B, Trindade MCD, Lind M, Goodman SB, Smith RL. Fibroblast expression of C-C chemokines in response to orthopaedic biomaterial particle challenge in vitro. Journal of Orthopaedic Research. 2001;19(5):970–976. doi: 10.1016/S0736-0266(01)00003-1. [DOI] [PubMed] [Google Scholar]
- 59.Ren PG, Huang Z, Ma T, Biswal S, Smith RL, Goodman SB. Surveillance of systemic trafficking of macrophages induced by UHMWPE particles in nude mice by noninvasive imaging. Journal of Biomedical Materials Research Part A. 2010;94(3):706–711. doi: 10.1002/jbm.a.32744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB. Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis. Clinical Orthopaedics and Related Research. 2011;469(1):113–122. doi: 10.1007/s11999-010-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remick DG. Interleukin-8. Critical Care Medicine. 2005;33(12, supplement):S466–S467. doi: 10.1097/01.ccm.0000186783.34908.18. [DOI] [PubMed] [Google Scholar]
- 62.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. The Journal of Biological Chemistry. 2004;279(23):24372–24386. doi: 10.1074/jbc.M401364200. [DOI] [PubMed] [Google Scholar]
- 63.Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clinical Cancer Research. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 64.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. Journal of Arthroplasty. 1995;10(4):498–506. doi: 10.1016/s0883-5403(05)80152-4. [DOI] [PubMed] [Google Scholar]
- 65.Sabokbar A, Rushton N. Role of inflammatory mediators and adhesion molecules in the pathogenesis of aseptic loosening in total hip arthroplasties. Journal of Arthroplasty. 1995;10(6):810–816. doi: 10.1016/s0883-5403(05)80080-4. [DOI] [PubMed] [Google Scholar]
- 66.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Titanium particles induce the immediate early stress responsive chemokines IL-8 and MCP-1 in osteoblasts. Journal of Orthopaedic Research. 2002;20(3):490–498. doi: 10.1016/S0736-0266(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 67.Fritz EA, Jacobs JJ, Glant TT, Roebuck KA. Chemokine IL-8 induction by particulate wear debris in osteoblasts is mediated by NF-κB. Journal of Orthopaedic Research. 2005;23(6):1249–1257. doi: 10.1016/j.orthres.2005.03.013.1100230603. [DOI] [PubMed] [Google Scholar]
- 68.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Chemokine gene activation in human bone marrow-derived osteoblasts following exposure to particulate wear debris. Journal of Biomedical Materials Research Part A. 2006;77(1):192–201. doi: 10.1002/jbm.a.30609. [DOI] [PubMed] [Google Scholar]
- 69.Lochner K, Fritsche A, Jonitz A, et al. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. International Journal of Molecular Medicine. 2011;28(6):1055–1063. doi: 10.3892/ijmm.2011.778. [DOI] [PubMed] [Google Scholar]
- 70.Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. Journal of Biomedical Materials Research Part A. 2008;84(2):464–474. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 71.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 72.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. The Journal of Experimental Medicine. 1989;169(4):1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circulation Research. 2004;95(9):858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 74.Balkwill F. Cancer and the chemokine network. Nature Reviews Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 75.Lu Y, Xiao G, Galson DL, et al. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. International Journal of Cancer. 2007;121(4):724–733. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. The Journal of Biological Chemistry. 2007;282(45):33098–33106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- 77.Trindade MCD, Schurman DJ, Maloney WJ, Goodman SB, Smith RL. G-protein activity requirement for polymethylmethacrylate and titanium particle-induced fibroblast interleukin-6 and monocyte chemoattractant protein-1 release in vitro. Journal of Biomedical Materials Research. 2000;51(3):360–368. doi: 10.1002/1097-4636(20000905)51:3<360::aid-jbm9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 78.Dasa V, Kramer JM, Gaffen SL, Kirkwood KL, Mihalko WM. Is monocyte chemotactic protein 1 elevated in aseptic loosening of TKA?: a pilot study. Clinical Orthopaedics and Related Research. 2012;470(7):1879–1884. doi: 10.1007/s11999-011-2191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Z, Ma T, Ren PG, Smith RL, Goodman SB. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. Journal of Biomedical Materials Research Part A. 2010;94(4):1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramos CDL, Canetti C, Souto JT, et al. MIP-1α[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-α and LTB4. Journal of Leukocyte Biology. 2005;78(1):167–177. doi: 10.1189/jlb.0404237. [DOI] [PubMed] [Google Scholar]
- 81.Maurer M, Von Stebut E. Macrophage inflammatory protein-1. International Journal of Biochemistry and Cell Biology. 2004;36(10):1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Gibon E, Ma T, Ren P-G, et al. Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. Journal of Orthopaedic Research. 2012;30(4):547–553. doi: 10.1002/jor.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cadosch D, Gautschi OP, Chan E, Simmen HP, Filgueira L. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. Journal of Biomedical Materials Research Part A. 2010;92(2):475–483. doi: 10.1002/jbm.a.32390. [DOI] [PubMed] [Google Scholar]


