Abstract
Objectives
Identification of HIV infection in exposed infants facilitates early therapy, which may limit viral reservoirs that maintain HIV infection under HAART.
Methods
The dynamics of the resting CD4+ T-cell latent HIV reservoir was determined over the first 2 years of life in 17 HIV-infected infants initiating lopinavir/ritonavir-based HAART at a median age of 8.1 weeks and achieving adequate suppression of plasma viral load by 24 weeks.
Results
The resting CD4+ T-cell latent HIV reservoir was detected in 12 of 14 (86%) infants tested at 24 weeks of HAART [median frequency 1.88 infectious units per million (IUPM); range <0.22 to 81.7), and remained measurable (median IUPM =0.32; range < 0.22 to 3.25) in six of 10 (60%) children retested at 96 weeks. The reservoir declined, from 24 to 96 weeks of HAART, at an estimated mean rate of 0.028 log10 IUPM/month, corresponding to a half-life of 11 months (95% confidence interval 6–30 months]. A strong relationship was found between the frequency of latently infected CD4+T cells at 96 weeks of HAART and time to first undetectable plasma viral load (Spearman r =0.91, P <0.001).
Conclusion
Although the resting CD4+ T-cell latent reservoir remains detectable over the first 2 years of HAART in a substantial proportion of infants, its size is associated with time to first undetectable viral load. To minimize HIV reservoirs in infants, rapid curtailment of viremia may limit HIV reservoirs and should be a therapeutic goal of early HAART in infants.
Keywords: early HAART, infants, latent reservoir dynamics
Introduction
Nearly 370 000 new HIV infections occur annually in children [1]. Although HAART during recently acquired HIV infection in adults and adolescents is not yet standard [2], it is recommended for all HIV- infected infants because of the survival benefit conferred [3]. In infants, early HAART is possible, as those infected in utero can be identified within a few days of life, and those with intrapartum HIV transmission by 2–6 weeks of age [3]. Initiation of HAART soon after infection in infants may minimize the size of the long-lived reservoir for HIV in resting memory CD4+ T cells, as the memory CD4+ T-cell compartment is under development during infancy [4].
The reservoir for HIV in resting memory CD4+ T cells is established when naive CD4+ T cells, upon antigen exposure, differentiate into memory cells, including those directed toward HIV [5,6]. On average, the size of the replication-competent HIV reservoir is approximately one in a million resting memory CD4+ T cells in children [7,8], and adults treated during chronic HIV infection [9,10]. The estimated half-life of the latent reservoir in adults treated during chronic infection ranges between 6 and 44 months [9–11] with an estimated time to eradication with continuous HAART of 70 years. However, initiation of HAART during acute HIV infection in adults diminishes the size of the resting CD4+ T-cell HIV reservoir such that it is rare to recover replication-competent virus from peripheral blood mononuclear cells after 6 months to 1 year of HAART. In such treated adults, the size of the replication-competent reservoir quickly falls (within 6 months to 1 year of therapy) below the level of 0.07 infectious units per million (IUPM) CD4+ T cells and estimates of decay rate are no longer possible with currently available methods [10].
Data on the size and decay of replication-competent resting CD4+ T-cell HIV reservoirs in infants have not been reported, but may affect refinement of therapeutic strategies for this population. In this study, we measured the size and decay of the resting CD4+ T-cell HIV reservoir during the first 2 years of HAART in infants initiating lopinavir/ritonavir (LPV/r)-based HAART between 2 weeks and 6 months of age [12,13]. We report on the subset of infants who achieved adequate suppression of plasma HIV RNA within 24 weeks of treatment initiation, and maintained suppression for up to 2 years, thereby permitting assessment of the dynamics of the latent reservoir during HAART.
Participants and methods
Twenty-four of the 31 HIV-infected infants enrolled in a phase 1/2 dose-finding study of LPV/r-based HAART [300/75 mg/m2 per dose; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) study P1030 [12,13]] were eligible for this substudy. Seven infants were excluded from study for living outside of the United States, which precluded overnight shipment of blood samples for real-time processing of latent viral cultures. To be included in the latent reservoir analyses over the 96 weeks of study, infants must have achieved plasma HIV RNA level less than 400 copies/ml or at least a 2 log10 copies/ml decline from their pretreatment level by week 24 while on HAART.
Additional analyses of the latent reservoir were performed at weeks 48 and 96 if HIV RNA was maintained less than 400 copies/ml while still taking study treatment. Study participants who had transient viremia or ‘blips’ were not excluded from the analysis. Seventeen of the 24 infants eligible for this substudy fulfilled these criteria for analyses of the latent reservoir at week 24, and underwent repeat analyses at weeks 48 and 96, as long as virologic control was maintained.
As previously published from the parent trial, infants had intensive LPV pharmacokinetic evaluations with area under the curve calculated at study week 2 [12,13]. The substudy was approved by the clinical trials performance sites where samples were collected and by the Johns Hopkins University School of Medicine Institutional Review Board.
Laboratory methods
HIV RNA assays
Evaluations of plasma viral load (Amplicor HIV-1 Monitor test, version 1.5; Roche Molecular Systems, Branchburg, New Jersey, USA; lower limit of quantification of 400 copies/ml) were done frequently over the course of the study (study entry, weeks 2, 4, 8, 12, 16, 20, 24, 32, 36, 40 and 48 and then every 12 weeks thereafter) [12,13]. In the parent study, all infants were followed until the last enrolled infant completed 48 weeks of study treatment. Among infants meeting the eligibility criteria for the present study at week 24, all but one remained on study treatment and maintained virologic suppression through at least 96 weeks of follow-up; that infant had completed 88 weeks of treatment when the study ended and had the final HIV RNA measurement at that time.
Quantification of the latent reservoir
The size of the replication-competent resting CD4+ T-cell reservoir was determined using endpoint-limiting dilution cultures, as previously described [14–16]. Whole blood was collected at three time points (24, 48 and 96 weeks) during HAART for quantitative evaluation of the resting CD4+ T-cell latent reservoir. Correct assessment of the reservoir size can be measured only in patients responding to HAART [7,10,17], that is, those having achieved either undetectable plasma viral loads at the time of analysis or significantly reduced viremia from baseline, demonstrating potent inhibition of virus replication [18]. The blood volumes used (median 2.9 ml) were determined by what could be drawn feasibly from infants for the study. Pretherapy latent reservoir studies were not performed due to limitations on the allowable blood volume to be drawn from neonates, and the inability to quantify stably integrated HIV with the coculture methods used when virus replication is not controlled. The lower limit of detection of the assay used for this study was 0.22 IUPM resting CD4+ T cells, corresponding to coculture of at least 5.25 million of purified resting CD4+ T cells. In our previous studies, most children initiating HAART during chronic HIV infection have a detectable resting CD4+ T-cell latent reservoir (median frequency of 0.8 IUPM; a measurement derived from coculture of approximately 2.25 million resting CD4+ T cells) [7].
Statistical analysis
Differences in pretreatment viral loads, CD4+ and CD8+ T-cell counts and frequencies of latently infected CD4+T cells were compared between infants meeting vs. not meeting the eligibility criteria, and between infants initiating HAART at less than 6 weeks of age vs. between 6 weeks and 6 months of age using Wilcoxon’s rank-sum and Fisher’s exact tests. A linear mixed effects model was used to assess the decay of latently infected cells (expressed as log10 IUPM) between 24 and 96 weeks of HAART [19]. Spearman’s correlation of coefficient was used to evaluate associations between reservoir size and other parameters.
Results
Baseline characteristics
Table 1 summarizes the demographic, immunologic and virologic characteristics of the 17 infants included and the seven excluded from the analysis of the latent reservoir at week 24. The seven infants were excluded for the following reasons: one infant had no sample obtained at week 24 and subsequently developed virologic failure by week 32; five infants were excluded for suboptimal plasma HIV RNA suppression by week 24; and one infant expired before 24 weeks of study treatment. Antiretroviral prophylaxis was heterogeneous for the group (Table 1). The median CD4 percentage at start of HAART was 34 and 35% for infants who were included and excluded from the substudy of the dynamics of the resting CD4+ T-cell latent reservoir, respectively. However, the seven infants excluded from the analysis had lower pretreatment HIV RNA levels (median 4.7 vs. 6.1 log10 copies/ml; P =0.004) and a longer duration of antiretroviral chemoprophylaxis (median 7.4 vs. 3.7 weeks; P =0.036) (Table 1). All except one infant received treatment with lamivudine combined with either zidovudine or stavudine in addition to the LPV/r.
Table 1.
Patient characteristics.
| Total analyzed (N =17) (71%) | Total nonanalyzed (N =7) (29%) | P-value | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 9 (53) | 3 (43) | 1.00 |
| Female | 8 (47) | 4 (57) | |
| Race, n (%) | |||
| White | 0 (0) | 2 (29) | |
| African–American | 13 (76) | 4 (57) | 0.090 |
| Hispanic | 4 (24) | 1 (14) | |
| Antiretroviral chemoprophylaxis (n) | 13 | 7 | |
| Median duration, weeks (IQR) | 3.7 (3.4, 6.1) | 7.4 (4.9, 10.1) | 0.036 |
| Infant prophylactic regimen, n (%) | 0.066 | ||
| None | 4 (24) | 0 (0) | |
| Zidovudine only | 10 (59) | 4 (57) | |
| Zidovudine with nevirapine | 2 (12) | 0 (0) | |
| Zidovudine with lamivudine | 0 (0) | 1 (14) | |
| Zidovudine with lamivudine and nevirapine | 1 (6) | 0 (0) | |
| Zidovudine with lamivudine and nelfinavir | 0 (0) | 2 (29) | |
| CDC disease classification at start of HAART, n (%) | |||
| N | 13 (76) | 4 (57) | 0.17 |
| A | 2 (12) | 0 (0) | |
| B | 2 (12) | 1 (14) | |
| C | 0 (0) | 2 (29) | |
| Age at start of HAART | |||
| Median, weeks | 8.1 | 15.4 | 0.11 |
| IQR | 5.7–11.0 | 5.9–23.4 | |
| CD4+ cell count before HAART | |||
| Median, % | 34 | 35 | |
| IQR | 29–41 | 18–49 | 0.85 |
| Pre-HAART plasma HIV-1 RNA | |||
| Median (HIV-1-RNA), log10 copies/ml | 6.1 | 4.7 | 0.004 |
| IQR | 5.7–6.5 | 4.4–5.4 | |
| Nucleoside reverse transcriptase inhibitors used with lopinavir/ritonavir, n (%) | 0.36 | ||
| Zidovidune with lamivudine | 9 (53) | 3 (43) | |
| Stavudine with lamivudine | 7 (41) | 2 (29) | |
| Stavudine with abacavir | 1 (6) | 1 (14) | |
| Zidovudine with didanosine | 0 (0) | 1 (14) | |
CDC, Centers for Disease Control; IQR, interquartile range.
Extent of viral load suppression by cohort
The median time to first undetectable viral load (i.e. time to <400 copies/ml) with HAART for the 17 infants was 17 weeks [interquartile range (IQR) 11.7, 24.9]. Eleven of the 17 (65%) infants had suppressed plasma HIV RNA to less than 400 copies/ml by week 24, the time of first analysis of the latent reservoir. The remaining six infants had not reached plasma HIV RNA levels less than 400 copies/ml (range 455–1043 copies/ml) by week 24, but had greater than a 2 log10 copies/ml (range 2.83–4.30 log10 copies/ml) decrease in plasma viral load relative to pretreatment levels, which supported an adequate response to HAART. Following suppression to less than 400 copies/ml, ‘blips’ were observed in nine of the 17 infants through 96 weeks when the latent reservoir was last sampled. The median number of ‘blips’ for these nine infants was 2 with most (72.7%; eight of 11) under 1000 copies/ml (median HIV RNA viral load of 688 copies/ml; range 410–5167).
Frequency of resting CD4+ T-cell infection and early HAART in infants
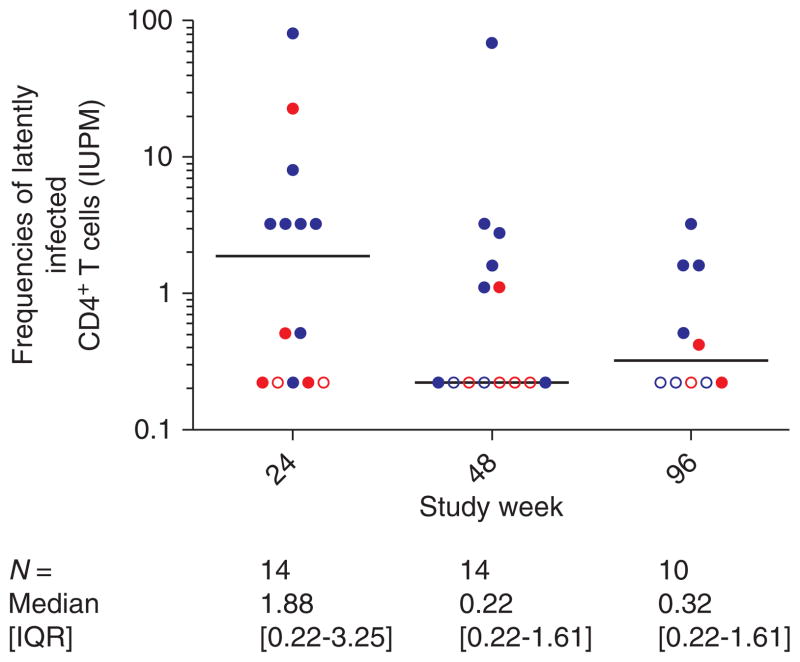
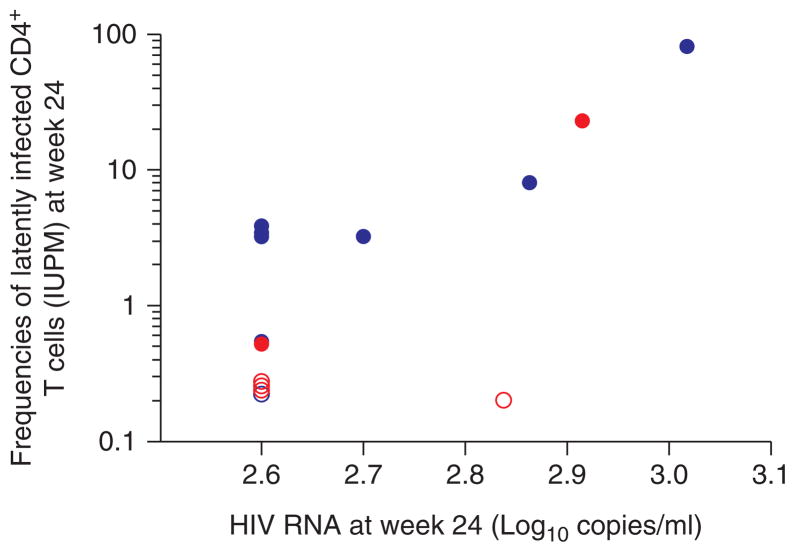
At the first study time point at week 24, 14 of 17 (82%) infants had samples available for analysis of resting CD4+ T-cell latent reservoir, six of whom were treated before 6 weeks of age. After 24 weeks of HAART, replication-competent virus was recovered from most (86% of the 14 infants). The frequency of resting CD4+ T-cell infection was variable [median IUPM =1.88 (IQR 0.22–3.25; range <0.22 to 81.7), with two (14%) of the 14 infants (both treated before 6 weeks of age) having undetectable levels (i.e. IUPM <0.22; Fig. 1) [12,13]. The infants initiating HAART by age 6 weeks tended to have lower frequencies of latently infected resting CD4+ T cells (median IUPM =0.22) by 24 weeks of HAART compared with the those starting HAART after age 6 weeks (median age 10.5 weeks; median IUPM 3.25; P =0.071; Fig. 1). A strong association existed between the frequency of latently infected CD4+ T cells and plasma viral load at week 24 (Spearman r =0.62; P =0.018; Fig. 2) [12,13]. Infants who still had detectable plasma HIV RNA at week 24, even though substantially lower than pretreatment levels, had higher frequencies of latently infected CD4+ T cells compared with the 10 infants who achieved undetectable plasma viral load by week 24 (median IUPM 8.08 vs. 0.51, respectively; P =0.053), although this did not reach statistical significance. Time to reach virologic suppression to plasma viral load less than 400 copies/ml was 11.7 weeks in the seven infants starting HAART by 6 weeks of age and 18.4 weeks in the 10 infants starting HAART after 6 weeks of age (P =0.057). Area under the LPV concentration time curve at 2 weeks of treatment [12,13] was not correlated with time to virologic suppression (r =−0.42; P =0.13) nor with IUPM at week 24 (r =0.21; P =0.54).
Fig. 1. Frequencies of latently infected resting CD4+ T cells in infectious units per million (IUPM) at 24, 48 and 96 weeks of study treatment in infants initiating lopinavir/ritonavir HAART at less than 6 months of age.
Each circle represents a single measurement per participant. At week 24, all infants with plasma HIV-1 RNA <400 or >2-log drop in plasma viral load below pretreatment levels were included. Measurements on infants initiating HAART at less than or greater than 6 weeks of age [12,13] are shown in red and blue, respectively. Latent reservoir measurements at weeks 48 and 96 are restricted to those infants who maintained suppression (apart from blips) through to week 96 of HAART. IUPM measurements that were below the limit of detection of the assay (IUPM <0.22) are indicated by open symbols. A horizontal line indicates median size of the latent reservoir at each study time point.
Fig. 2. Correlation between frequency of latently infected resting CD4+ T cells (log-scale) and HIV RNA level at week 24.
Measurements on infants initiating HAART at less than or greater than 6 weeks of age [12,13] are shown in red and blue, respectively. Measurements that were below the limit of detection of the assay (IUPM <0.22) are indicated by open symbols.
Longitudinal analysis of the dynamics of the latent reservoir after up to 2 years of HAART was performed on 14 of 17 infants (Fig. 1), 11 of whom also had measurements at 24 weeks of HAART. Three of 17 infants were unevaluable between 24 and 48 weeks of HAART due to virologic failure between 24 and 48 weeks of study treatment (n =2), and withdrawal from study before the follow-up study visit at week 48 (n =1). The median frequency of resting CD4+ T-cell infection decreased after 48 weeks of HAART and was 0.22 IUPM in the 14 evaluable infants, 43% of whom had levels below the limit of detection of the assay (i.e. IUPM <0.22) (Fig. 1). At week 96, 10 infants also had samples available for repeat analysis. The median frequency of latently infected resting CD4+ T cells carrying replication-competent HIV among these 10 infants was 0.32 which was a decreased from 0.66 IUPM at week 48, with 40% having an undetectable level (i.e. IUPM <0.22). Thus, after 96 weeks of suppressive therapy, 60% of the infants still had quantifiable levels of resting CD4+ T-cell infection at or above 0.22 IUPM.
The association between the frequency of resting CD4+ T-cell infection and percentages before and following effective HAART in infants was also examined. The median absolute changes in CD4 percentages from start of HAART to weeks 24, 48 and 96 were 0, 4 and 8%, respectively. The frequency of resting CD4+ T-cell infection at weeks 24, 48 and 96 was not correlated with percentages of CD4+ T cells at start of HAART, although, at week 24, became inversely correlated with CD4 percentage (r =−0.59; P =0.041). At weeks 48 and 96 of HAART, the inverse correlation between frequencies of latently infected CD4+ T cells and CD4 percentage was not maintained. Associations between the percentage of CD8+ T cells and the frequency of resting CD4+ T-cell infection was observed at weeks 24 (r =0.62; P =0.025) and 48 (r =0.58; P =0.049) of HAART. However, this association was not seen at 96 weeks of HAART.
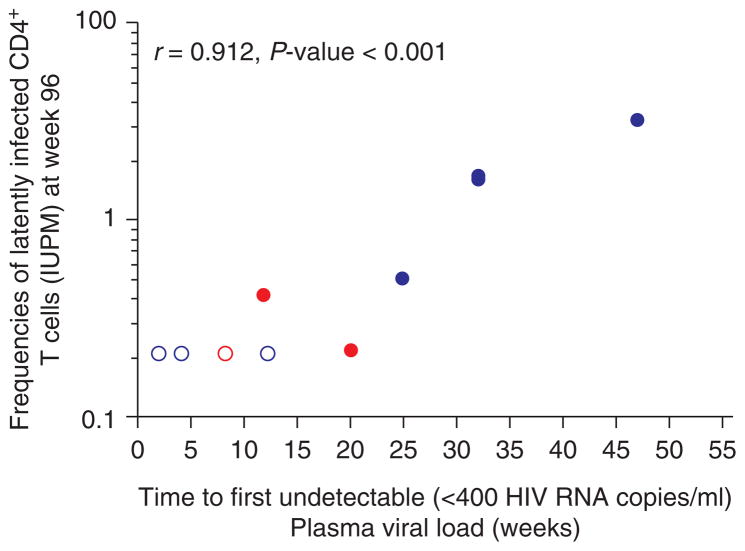
In this study, we found that time to first virologic suppression to less than 400 copies/ml was correlated with the frequency of resting CD4+ T-cell infection at week 24 (r =0.72; P =0.004) and remained highly correlated through 96 weeks of HAART (r =0.91; P <0.001; Fig. 3) [12,13]. In a mixed effects model in which undetectable values (i.e. IUPM 0.22) were conservatively replaced by the smallest detectable level as reported by others [9,20], the frequency of resting CD4+ T-cell infection decreased between 24 and 96 weeks of HAART at an estimated mean rate of 0.028 log10 IUPM/month (95% CI 0.010–0.047; P =0.004), corresponding to a half-life of 11 months (95% CI 6–30 months). Even with hypothetical replacement of the undetectable levels with a value of 0.11 IUPM in the mixed effects model, the decay estimates were similar and the estimated mean decay rate was 0.034 log10 IUPM/month (95% CI 0.014–0.055; P =0.002), corresponding to a half-life of 9 months (95% CI 6–22 months).
Fig. 3. Correlation between frequency of latently infected resting CD4+ T cells at 96 weeks of HAART (log-scale) and time to reach undetectable plasma viral load of less than 400 copies/ml.
Measurements on infants initiating HAART at less than or greater than 6 weeks of age [12,13] are shown in red and blue, respectively. Measurements that were below the limit of detection of the assay (IUPM <0.22) are indicated by open symbols.
Discussion
Limiting reservoirs for HIV has relevance for ongoing residual viremia during long-term HAART [11,21] and potential for eradication of infected cells that maintain HIV infection despite HAART [22]. This is the first study, to our knowledge, to characterize size and decay of the resting CD4+ T-cell latent HIV reservoir when treatment is instituted within the first 6 months of life in HIV-infected infants. We found that despite initiating HAART, as early as a median 8.1 weeks of age, replication-competent virus was recovered for up to 2 years of continuous HAART in a substantial proportion (60%) of early-treated infants, although infants initiating HAART before 6 weeks of age tended to have low frequencies (IUPM at or below 0.22) of resting CD4+ T-cell infection by 24 weeks of HAART. It is important to note that in early-treated infants, the frequency of resting CD4+ T-cell infection was quite variable even after 6 months of HAART, and long after the less stable pool of resting cells bearing unintegrated virus (estimated half-life of 5 days) [17] would persist. Nevertheless, the replication-competent latent reservoir in resting CD4+ T cells continued to decline between 24 and 96 weeks of HAART, at a mean estimated half-life of 11 months to reach a median frequency of resting CD4+ T-cell infection of 0.32 IUPM resting CD4+T cells by 96 weeks of HAART. Together, these findings fit with the mathematical model recently proposed by Archin et al. [20] with respect to ongoing decay of resting cell infection in the first few years of HAART in early-treated, HIV-infected adults. Although we cannot fully exclude a dilutional effect of CD4+ T-cell increase as a potential reason for the observed lowering of frequencies of resting CD4+ T-cell infection during HAART, we did not find an inverse correlation between changes in CD4+ T-cell percentages and resting CD4+ T-cell infection frequencies at 48 and 96 weeks of HAART. In mathematical models of decay of resting CD4+ T-cell infection, the potential for a dilutional effect from immune reconstitution or contribution from homeostatic proliferation is usually not imputed [20]. Our findings also support the notion that the resting CD4+ T-cell latent reservoir is indeed heterogeneous in its composition with respect to turnover [10]. The reduced variability in resting CD4+ T-cell-infected cell frequencies observed in the infants treated by age 6 weeks supports the notion that the more dynamic pool of latently infected resting CD4+ T cells may be restricted if infants are treated very early (before 6 weeks of age).
Slower clearance of plasma viremia during HAART in infants in our study was strongly associated with higher frequencies of latently infected resting CD4+ T cells, even after 96 weeks of therapy. With effective HAART, a biphasic reduction in plasma viremia occurs in adults due to inhibition of new infections of susceptible target cells and decay of preexisting infected cells [18]. HIV-infected infants also exhibit similar biphasic decay in plasma viral load during HAART [23–25]. However, individual differences in time to reach undetectable viremia were observed in this study of early HAART in infants that was irrespective of pretreatment viral load and may reflect the amount and types of infected cells present before therapy is instituted [26], although additional factors such as genetic, immunologic and virologic factors may also influence decay in plasma viremia. The cells contributing to viremia during the second phase of viral load decay, when observed, during HAART, originates from virus production from cells with longer half-lives, which may include the two populations of latently infected CD4+ T cells [18,27]. Our observation of significant correlation between time to first undetectable viral load, plasma viremia and frequencies of latently infected CD4+ T cells at week 24 supports an interaction between latently infected CD4+ T cells and plasma viral load decay in the first 6 months of HAART. However, the clinical relevance of a shorter time to reach undetectable viral load levels after the start of HAART (12 weeks compared with 12–24 weeks) is not yet defined [28]. Our finding that shorter time to suppression of viremia was correlated with the frequency of the infected resting CD4+ T-cell pool at 96 weeks of HAART may, however, provide a more globally accessible clinical parameter to guide future studies aimed at restricting this reservoir, if confirmed in other studies. Although the contribution of low levels of ongoing replication to slower decay of plasma viremia during HAART cannot be fully excluded in this study, this seems less likely as no correlation was found between LPV concentrations and time to undetectable plasma viral load. In addition, we reported previously on the lack of sequence divergence, in the latent reservoir over 96 weeks of HAART in this cohort, which is less likely to occur in the presence of ongoing virus replication [15]. A correlation between infectious HIV recovery from resting CD4+ T cells and residual viremia was also recently reported in adult patients on HAART [29]. Nevertheless, attempts to shorten the time to reach undetectable levels in infants, for example, with antiretroviral drug regimens that contain integrase inhibitors that are known to hasten clearance of plasma viremia [30], warrants further study in this population, especially with recent emphasis on strategies to clear viral reservoirs in infected individuals.
This study has several limitations. The restriction on the amount of blood that can be drawn from neonates and young infants over the time course of the study limited testing at earlier time points and constrained the detection limit of the culture assay to 0.22 IUPM, although this is the first study to comprehensively assess the replication-competent HIV reservoir in infants. As such, how far below the level of 0.22 IUPM (the ‘undetectable reservoir’ in this study) was achieved with early therapy in the 40% of infants studied at week 96 is unknown and requires further study with more sensitive approaches, including assessment of proviral burden. Limited cell numbers precluded concomitant analyses of decay in proviral DNA. Nevertheless, estimates of IUPM provide information on the clinically relevant proviral genomes with capacity to replicate and fuel rebound viremia if therapy is stopped. An additional limitation of the study is the lack of information on timing of infection as to whether infants were infected in utero or peripartum, which may have influenced the size of the reservoir, with potentially larger reservoirs in in-utero-infected infants. In some infants, even after 6 months of HAART, the frequency of latently infected cells was substantially higher than one per million resting CD4+ T cells, and as high as 82 IUPM. Nevertheless, important insights into the establishment and decay features of this cellular reservoir for HIV in infants initiating early HAART were obtained from this study, which may provide a new therapeutic target (time to undetectable viral load) with newer treatment strategies for this population.
In conclusion, despite early treatment in HIV-infected infants, the latent reservoir for HIV in resting CD4+ T cells is established and retains replication-competent virus at relatively high frequencies through the first 2 years of life. Attention to shortening the time to reach undetectable viral load levels, a marker of reduction in cells that contribute to second-phase decay, may minimize viral reservoirs in HIV-infected infants, and has potential to serve as a biomarker of reservoir size. These findings have implications for designing early therapeutic strategies for infants, including those aimed at virus eradication.
Acknowledgments
The authors wish to thank the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1030 study team (Kimberly Hudgens, MSHCA, MBA; Barbara Heckman, BS; Rohan Hazra, MD; Marisol Martinez-Tristani, MD; Mary Elizabeth Smith, MD; Jorge Pinto, MD, DSc; Lynette Purdue, PharmD; Renee Browning, RN, MSN; Adam Manzella, MA; and John Rodman, PharmD) for their invaluable contributions to the performance of the study. The authors also wish to thank the patients and families who participated in this study, and Abbott Laboratories for donation of the study drug. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases (NIAID) cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT group. Support of the sites was provided by the NIAID and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
The study on latent reservoirs was supported by NIAID grants R01 A1062446 and RO1HD057784–04 awarded to D.P., and an IMPAACT Subspecialty grant (U01-AI-068632 and UM1-AI-068632).
Overall support for the IMPAACT was provided by the NIAID (U01 AI068632), the Eunice Kennedy Shriver NICHD and the National Institute of Mental Health (NIMH) (AI068632).
Footnotes
Conflicts of interest
E.V.C. has served as a consultant to GlaxoSmithKline Bristol-Meyers Squibb and Johnson & Johnson. E.G.C. has had consultancies with Pfizer and Bristol-Meyers Squibb, and has owned stock+/− stock options in Abbott Labs, GlaxoSmithKline, Merck Inc., Bristol-Meyers Squibb and Johnsonn & Johnson, Novartis, Roche, and Sanofi. M.D.H. received grant support from Roche, honoraria or consultancies with Abbott Labs, Boehringer Ingelheim, Bristol-Meyers Squibb, Chiron, Medicines Development, Roche, Pfizer, Tibotec and Vironyx. K.L. has served as a consultant to Tibotec and Johnson and Johnson. D.P. has served as a consultant to Glaxo-SmithKlein. R.Y. has served on the Speaker’s Bureau for Merck Inc. and GlaxoSmithKline.
All other authors have no conflicts
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) [[Accessed 23 November 2010].];UNAIDS Report in the Global AIDS Epidemic. 2010 Dec; http://www.unaids.org.in/Publications_ReportonTheGlobalAIDSEpidemic2010.pdf.
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [[Accessed 27 March 2012].]. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 3. [[Accessed 11 August 2011]];Guidelines for the use of antiretroviral agents in pediatric HIV infection. http://aidsinfo.nih.gov/Contentfiles/lvguidelines/recommendations_only_Ped_2011.pdf.
- 4.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 6.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 7.Persaud D, Pierson T, Ruff C, Finzi D, Chadwick KR, Margolick JB, et al. A stable latent reservoir for HIV-1 in resting CD4(+) T lymphocytes in infected children. J Clin Invest. 2000;105:995–1003. doi: 10.1172/JCI9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruff CT, Ray SC, Kwon P, Zinn R, Pendleton A, Hutton N, et al. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J Virol. 2002;76:9481–9492. doi: 10.1128/JVI.76.18.9481-9492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 10.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 11.Ramratnam B, Mittler JE, Zhang L, Boden D, Hurley A, Fang F, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged antiretroviral therapy. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick EG, Capparelli EV, Yogev R, Pinto JA, Robbins B, Rodman JH, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–255. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick EG, Pinto J, Yogev R, Alvero CG, Hughes MD, Palumbo P, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J. 2009;28:215–219. doi: 10.1097/INF.0b013e31818cc053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 15.Persaud D, Palumbo P, Ziemniak C, Chen J, Ray SC, Hughes M, et al. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195:1402–1410. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 16.Persaud D, Ray SC, Kajdas J, Ahonkhai A, Siberry GK, Ferguson K, et al. Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:381–390. doi: 10.1089/aid.2006.0175. [DOI] [PubMed] [Google Scholar]
- 17.Blankson JN, Finzi D, Pierson TC, Sabundayo BP, Chadwick K, Margolick JB, et al. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:1636–1642. doi: 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 18.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 19.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 20.Archin N, Vaidya N, Kuruc J, Gay C, Kearney M, Cohen M, et al. Immediate antivrial therapy restricts resting CD4+ infection but does not accelerate the decay in latent infection [abstract 152]. 19th Conference on Retroviruses and Opportunistic Infections; Seattle. 2012. [Google Scholar]
- 21.Chun TW, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, et al. Relationship Between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–138. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 23.Luzuriaga K, Wu H, McManus M, Britto P, Borkowsky W, Burchett S, et al. Dynamics of human immunodeficiency virus type 1 replication in vertically infected infants. J Virol. 1999;73 :362–367. doi: 10.1128/jvi.73.1.362-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melvin AJ, Rodrigo AG, Mohan KM, Lewis PA, Manns-Arcuino L, Coombs RW, et al. HIV-1 dynamics in children. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:468–473. doi: 10.1097/00042560-199904150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo P, Wu H, Chadwick E, Ruan P, Luzuriaga K, Rodman J, et al. Virologic response to potent antiretroviral therapy and modeling of HIV dynamics in early pediatric infection. J Infect Dis. 2007;196:23–29. doi: 10.1086/518508. [DOI] [PubMed] [Google Scholar]
- 26.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hlavacek WS, Stilianakis NI, Notermans DW, Danner SA, Perelson AS. Influence of follicular dendritic cells on decay of HIV during antiretroviral therapy. Proc Natl Acad Sci U S A. 2000;97:10966–10971. doi: 10.1073/pnas.190065897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyle G, Demasi R, Hill A. Does earlier HIV RNA suppression provide long-term benefits? AIDS. 2010;24:1591–1593. doi: 10.1097/QAD.0b013e32833ad933. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi R, Bosch R, Aga E, Bedison M, Bastow B, Schmitz J, et al. Correlation between infectious HIV-1 recovery from resting CD4+ memory cells and residual viremia in patients on ART: results from ACTG A5173 [abstract]. 19th Conference on Retroviruses and Opportunistic Infections; 2012; Seattle. [Google Scholar]
- 30.Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]