Abstract
Objective
A growing body of evidence indicates that the protein deacetylase SirT1 affects chondrocyte biology and survival. This report aims to evaluate in-vivo effects of SirT1 on cartilage biology in 9 month-old mice 129/J murine strains.
Methods
Heterozygous (SirT1+/−) and wild type (WT; SirT1+/+) mice were systematically compared for musculoskeletal features, scored for OA severity and chondrocyte viability in articular cartilage at 1 month and 9 months post-natally. Sections of femoro-tibial joints were stained for type II collagen, aggrecan and active caspase 3. Articular murine chondrocytes were isolated and immunoblotted for SirT1 and active caspase 3.
Results
Phenotypic observations show that at the age of 1 month SirT1+/− mice were smaller than the wild type and presented a significant reduction in full-length SirT1 (FLSirT1; 110kDa) protein levels. Nine month-old SirT1+/− and WT mice did not express FLSirT1, however immunoblot assays of 9M articular cartilage-derived protein extracts revealed the inactive cleaved variant of SirT1 (75SirT1; 75kDa) was decreased in the SirT1+/− compared to WT mice. Nine month-old SirT1+/− mice possessed enhanced OA and enhanced levels of active caspase 3, compared to age-matched WT mice.
Conclusion
The data suggest that the presence of 75SirT1 may prolong viability of articular chondrocytes in adult 9 month-old mice.
Keywords: Osteoarthritis, SirT1, apoptosis, Cathepsin B
INTRODUCTION
SirT1 has been recently shown to impact cartilage biology and chondrocyte survival (1–4). Several reports support that the full-length SirT1 (FLSirT1) enhances chondrocyte survival through PTP1B inhibition or mitochondrial-related pathways (2,3). In fact, enhanced chondrocyte apoptosis has been attributed to cartilage degeneration in osteoarthritis (OA) and Rheumatoid Arthritis (RA) (5). It appears that the presence of proinflammatory cytokines as TNFα and IL1β, may elicit enhanced chondrocyte death and increased levels of secreted catabolic enzymes (5,6). Our recent data shows that chondrocytes exposed to TNFα undergo Cathepsin B-mediated cleavage of the nuclear full-length SirT1 (FLSirT1; 110kDa) to generate a stable but enzymatically inactive 75kDa fragment of SirT1 (75SirT1), which is partly exported to the cytoplasm (7,4). In the cytoplasm, 75SirT1 colocalizes with the mitochondrial membrane and associates with Cytochrome C (CytoC), preventing chondrocyte apoptosis (4).
It is widely held that aging is a known risk factor for OA development (8). Given our previous data, age-related systemic inflammation (9) could induce the formation of 75SirT1 from its w.t. FLSirT1 form. We therefore attempt to monitor age-related affects on FLSirT1 and their attributes in cartilage homeostasis and chondrocyte viability.
To date, total body knock out mice for SirT1 (null; SirT1−/−) do not survive longer than 1 month (1M) post-natally (10), and are thus inappropriate for testing OA development in aged mice. McBurney and colleagues reported that SirT1 null embryos of the 129/J strain possess abnormal phenotypic features and are likely not to survive postnatally (10). During embryogenesis and post-natal stages, SirT1 null mice are significantly smaller as compared to WT littermates, implying that SirT1 may be involved in early and late developmental stages. Here we propose to test our hypothesis by a systematic comparison of adult 9 month-old mice;(9M) and young (1M) heterozygous (SirT1 +/−) and WT 129/J mice.
METHODS
Animal Protocols
Experimental procedures involving 129/J mice were carried out in accordance with NIH Committees for animal use and care (ARAC guidelines). Mice were grown in 12 hour light/dark cycles and received unlimited food and water for the duration of their maintenance. 129/J heterozygous (SirT1 +/−, n = 6 for each experiment), and wild-type (SirT1 +/+, n = 6 for each experiment) littermates were generated from SirT1 +/− matings. Genomic DNA from mice tail fragment was periodically sampled for genotyping via PCR using the Extract-N-Amp Tissue PCR kit (Sigma-Aldrich, Saint Louis, MO). PCR primer sequences for SirT1 amplification were (Forward) 5′-TTCACATTGCATGTGTGTGTG-3’ and (Reverse) 5′-TAGCCTGCGTAGTGTTGGTG-3'.
Following euthanasia, articular cartilage was isolated and either fixed for histology or further processed for chondrocyte isolation according to Gosset et al., (2008) (12). Freshly isolated chondrocytes were processed to obtain protein extracts . Protein extracts (n=3) were supplemented with 10µg/mL of the Cathepsin B inhibitor ALLN (Calbiochem, Germany) and complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). They were then run on a 4–12% polyacrylamide gradient gel (Bio-Rad Laboratories, Israel) and blotted on a Polyvinylidene fluoride (PVDF) membrane. Following blocking, membranes were incubated with primary and secondary antibodies (Sigma-Aldrich, Saint Louis, MO). Primary antibodies used were active caspase 3 (Cell Signaling, MA), Cathepsin B and β-actin (Santa Cruz, CA). The N-terminally reactive SirT1 antibody (denoted as N-SirT1) was purchased from Millipore, MA and detected both FLSirT1 and the N-terminally intact 75SirT1 fragment (7).
For in-vitro assays w.t. murine SirT1 (mSirT1) was stably expressed in human OA-derived chondrocytes (n=3), in accordance with the procedure described in Dvir-Ginzberg et al., (2008) (1). The retroviral expression plasmids, pHanPuro-mSirT1 was a kind gift of Dr. Vittorio Sartorelli, NIAMS, NIH. Chondrocyte lines expressing mSirT1 were treated with TNFα 50 ng/mL (Peprotec, Israel) and 10 µg/mL ALLN (Calbiochem, Germany), for 24h. Following induction, protein extracts were obtained and immunoblot assays were carried out, as previously indicated.
Immunohistochemistry (IHC)
The knee joints were fixed in 4% paraformaldehyde, decalcified, dehydrated using a graded series of ethanol washes, and embedded in paraffin. Frontal and lateral sections of 5 µm were pre-digested with hyaluronidase (Sigma-Aldrich, Saint Louis, MO) in 10mM Tris-HCl, pH 7.5, for 30 min at 37°C, then incubated overnight at room temperature with primary antibodies for cleaved caspase 3 (Cell Signaling, MA), aggrecan or type II collagen (Santa Cruz, CA) in Tris-buffered saline (TBS, pH 7.4) containing 0.1% BSA. The presence of cleaved caspase 3 denotes end-stage apoptotic events. To further test early apoptotic cell death the M30 CytoDEATH fluorescent kit was used with fixed tissue samples (Roche Applied Science), according to the manufacturers guide. Using this kit, cell nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Saint Louis, MO) at a final concentration of 1 µg/mL in PBS Briefly, the antibody used in the kit recognizes an epitope of cytokeratin 18, which is presented after cleavage by caspases during early apoptotic events.
Additional sections were stained with Safranin O (Sigma-Aldrich, Saint Louis, MO), according to the manufacturers instructions. Following staining, slides were visualized under a Leica DMR Microscope (Leica Microsystems, IL). Representative sections were captured and analyzed for intensity using Image J software, or scored with the OARSI semi-quantitative scoring for mice (11). Following Image J software analyses, chondrocytes were considered positive for cleaved caspase 3, if they displayed an intensity 10-fold greater the background of the IHC section
Statistical Analysis and OA scoring
Students T-test was carried out to determine the differences between two equivalent treatments within a group. Error bars indicate the standard deviation around the mean value of data point. The severity of OA was scored on the medial tibial part of the knee joint, according to the OARSI semi-quantitative scoring for mice (11). Briefly, Safranin O- stained cartilage sections (medial tibial plateau and medial femoral plateau) are ranked according to loss of Safranin-O staining intensity, level of cartilage fibrillation, and erosions extending into calcified cartilage (11). Two representative sections of 6 different mice (n=6) within a genotype were blindly assessed by four different readers for OA severity and statistically analyzed, as indicated above.
RESULTS AND DISCUSSION
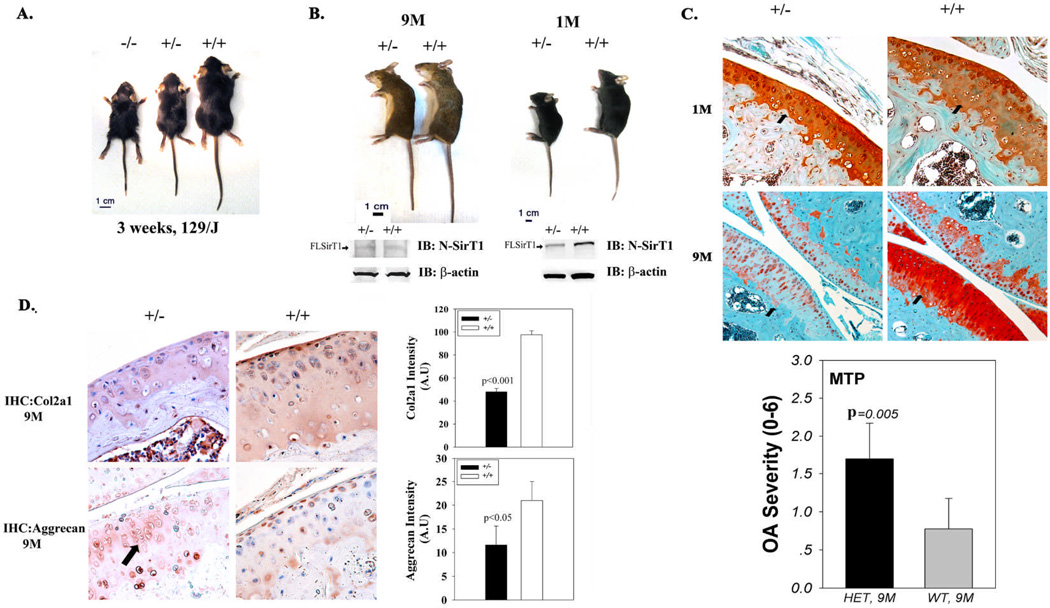
Photographs of 129/J lines at 3 weeks post-natally, reveal that both SirT1−/− and SirT1 +/− are significantly smaller than WT (Figure 1A). Examining SirT1 +/− and WT mice at 1M and 9M, reveals that independent of age SirT1 +/− mice appeared smaller and more arched, possibly indicating the presence of several musculoskeletal defects (Figure 1B). Additional immunoblot analyses in Figure 1B (left lower panel) show that 1M SirT1+/− possess reduced full length SirT1 (FLSirT1, 110kDa) as compared to WT mice, in consistence with their genotype. However, 9M SirT1+/− and WT mice exhibited significantly impaired protein levels of FLSirT1 as compared to their equivalent 1M mice (Figure 1B, right lower panel). In all, it appears that levels of FLSirT1 are significantly reduced in articular chondrocytes with age.
Figure 1. Adult (9M) SirT1 +/− mice possess enhanced OA as compared to WT mice.
A. photo of 3 week littermates of the 129/J strain. B. Photo of young (1M) and adult (9M) SirT1 +/− vs. WT (SirT1 +/+) mice. Lower panel of B present immunoblots of freshly isolated articular chondrocytes from each genotype, probed with the N-SirT1 antibody. C. Left panel depicts SirT1 +/− vs. WT articular cartilage sections from the medial tibial plateau stained with Safranin O (1M: upper and 9M: lower micrographs) at x20 magnification. Adult mice (9M) were blindly ranked for OA (right panel), based on the OARSI semi-quantitative scoring for mice (12). HET denotes haploinsufficient SirT1+/− and WT denotes SirT1+/+ mice. In the graph, MTP indicates the medial tibial plateau. Black arrows in the micrographs indicate MTP of each representative section. D. Immunocytochemistry for collagen 2α1 (Col2a1; upper panels) and aggrecan (lower panels) in adult (9M) mice. Right upper graph exhibits densitometry for Col2a1 level in the extracellular matrix, while the lower right graph exhibit densitometry for aggrecan levels in murine articular chondrocytes. A.U denotes arbitrary units of intensity. Black arrow in SirT1+/− aggrecan staining, point towards irregular enlarged cell clusters. Black bars represent SirT1 +/− and white bars represent WT mice. Bars represent a mean and standard deviation of n=6 (with 2 repetitions per sample). Arrows in the immunoblots indicate the FLSirT1 band (110kD).
Further inspection of cartilage morphology via Safranin O staining, did not reveal any significant differences in intensity between the genotypes at 1M (Figure 1C upper panel). However, at the age of 9M, SirT1+/− mice exhibited a decreased Safranin-O staining intensity, reflecting the negatively charged GAGs on aggrecan and other proteoglycans, compared equivalent WT littermates (Figure 1C lower panel). While both strains did not show any signs of OA at 1M, 9M old mice were overall scored higher for OA severity, defined by the OARSI semi-quantitative scoring for mice (11) (Figure 1C lower panel). More specifically, SirT1+/− (9M) displayed a 2-fold increase in OA severity vs. WT of the same age, while OA severity of the medial femoral plateau, displayed no difference amongst the genotypes. Additional immunohistochemical analyses of tibial articular cartilage derived from 9M WT mice (Figure 1D), displayed reduced collagen 2α1 level in adult SirT1+/− compared to WT mice (Figure 1D upper panel). Examining aggrecan levels, show decreased staining in 9 month-old SirT1+/− mice compared to WT (Figure 1D lower panel). Increased aggrecan levels were mostly apparent in the superficial articular zone of 9 month-old WT mice. Interestingly, articular chondrocytes of the midzone in SirT1+/− mice exhibited a larger size and irregular cell clusters as compared to aged-matched WT mice.
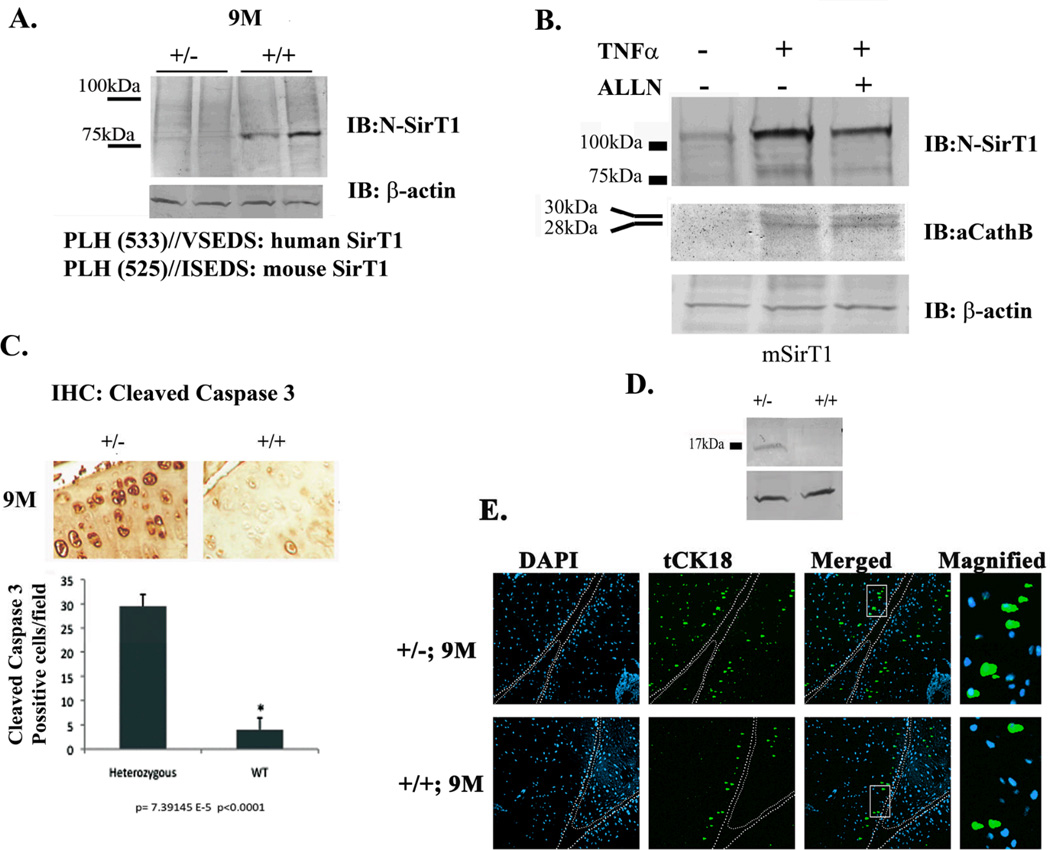
Since FLSirT1 was not detected both in WT and SirT1+/− 9M mice strains, we further attempted to detect the presence of 75SirT1 in those mice (Figure 2A). To do so, we imunoblotted articular cartilage-derived extracts with an N-terminally reactive antibody for SirT1, which detects both FLSirT1 and cleaved 75SirT1 (7). Interestingly, 9M WT mice showed enhanced levels of 75SirT1 compared to SirT1+/− mice. The 75SirT1 fragment is known to be generated in the presence of TNFα and IL1β (7). The lower panel of Figure 2A, presents the predicted cleavage site of mSirT1 based on the site previously characterized for human SirT1 (7). These observations may indicate that overall inflammatory cytokines are systemically upregulated with age, as previously described by Chung et al., (2009)(10).
Figure 2. 9M SirT1+/− mice show enhanced cleaved caspase 3 as compared to WT.
A. Protein extracts from duplicate SirT1 +/− vs. duplicate WT mice were obtained and immunoblotted with an N-SirT1 antibody. Lower panel shows the predicted cleavage site for human and mouse SirT1, which generate a similar molecular weight cleaved SirT1 product. B. Stably transfected human chondrocytes expressing mSirT1 were treated as indicated above the blot and expressed enhanced 75SirT1 and active Cathepsin B (aCathB) in the presence of TNFα. C. Immunohistochemistry of the MTP at x20 magnification (upper panel) and equivalent densitometry for chondrocytes positive for cleaved caspase 3 in adult SirT1 +/− and WT mice. D. Immunoblot analyses for cleaved caspase 3 in adult SirT1 +/− vs. WT mice. A.U denotes arbitrary units of intensity. E. shows increased truncated cytokeratin 18 (tCK18) pronounced in SirT1+/− (2-fold) vs. WT. mice. DAPI was used to stain the nuclei and white bars differentiate between the medial tibial plateau (left) and the medial femoral plateau (left). Bars represent a mean and standard error of n=6 (with 2 repetitions per sample). Protein extracts were obtained from 3 different mice or 3 different stable cell lines expressing mSirT1.
Using mSirT1 stably expressing chondrocyte lines, confirmed in-vitro that mSirT1 is cleaved in the presence of TNFα, a process which is deterred upon the addition of the Cathepsin B enzymatic inhibitor ALLN (Figure 2B). The protein levels of active Cathepsin B (aCathB), responsible for cleavage of FLSirT1, are also enhanced in TNFα-treated mSirT1 chondrocyte lines.
Enhanced chondrocyte death in OA articular cartilage (13), may stem from increased exposure to proinflammatory cues (5,6). Since FLSirT1 is associated with enhanced viability in human chondrocytes (2,3), we initially postulated that lack of FLSirT1 may elicit enhanced chondrocyte death in both strains. Contrarily, chondrocytes positive for cleaved caspase 3 were 6-fold higher in adult SirT1+/− as compared to WT. mice, which also corresponds with immunoblot analyses (Figure 2C and 2D). The presence of cleaved caspase 3 is associated with end-stage events of apoptotic cell death. Additionally, early apoptotic events characterized by enhanced cytokeratin 18 cleavage were more pronounced in SirT1+/− (2-fold) vs. WT. mice (Figure 2E). Variable results obtained from these in-situ assays, could stem from their semi-quantative character. Regardless the overall findings display a marked increase in apoptosis in 9M heterozygote mice vs. WT. Based on the fact that the majority of SirT1 in adult WT mice consists of the cleaved 75SirT1 fragment, we postulate it too may contribute to sustaining chondrocyte viability in adult mice, which is consistent with our in-vitro findings in human samples (4).
The involvement of Cathepsin B in OA pathology has been previously illustrated by Baici et al., (1995) (14). Baici and colleagues found enhanced secreted Cathepsin B in articular cartilage of OA patient as compared to normal samples (14). Despite the destructive effects of Cathepsin B on cartilage extracellular matrix (ECM), its intracellular activity may also impact cell biology and viability under proinflammatory stress (15). Our previous report shows that Cathepsin B targets FLSirT1 for site-specific cleavage generating a stable undegraded 75SirT1 form (7), which is transferred to the mitochondria and participates in prolonging chondrocyte survival (4). Here we show that 75SirT1 is elevated in adult mice, possibly due to systemic autoimmunity apparent in synovium (4,5), reported to be enhanced with age (13). Expectedly, cleavage and inactivation of FLSirT1 may deter the expression of various ECM genes as seen for collagen 2α1 and aggrecan (1), however it appears from our preliminary data that this variant possesses a protective effect against apoptosis, similar to that of FLSirT1 (2,3).
In closing,75SirT1 is generated with age, possibly as a response to systemic inflammatory cues. The presence of 75SirT1 correlates with enhanced cell survival in-vivo, which establishes an alternative role for SirT1 as a longevity factor. Regardless, generating 75SirT1 may come at the cost of FLSirT1-mediated ECM expression, ultimately leading ECM instability and cartilage breakdown..
ACKNOWLEDGMENTS
This work was supported by the Marie Curie European IRG reintegration grant and Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health. The authors thanks Prof. Michael W. McBurney (Ottawa Hospital Research Institute) for his contribution of 129/J mice strains.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by Sirt1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62(5):1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R. SirT1 regulation of apoptosis of human chondrocytes. . Arth. & Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, Gagarina V, Lee EJ, Dvir-Ginzberg M. 75kDa SirT1 blocks TNFα-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. doi: 10.1002/art.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatsugi N, Tsukazaki T, Osaki M, Koji T, Tamashita S, Shindo H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J Orthop Sci. 2000;5:150–156. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. TNFα-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis and Rheumatism. 2011;63(8):2363–2373. doi: 10.1002/art.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6(4):376–378. [PubMed] [Google Scholar]
- 9.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The Mammalian SIR2 Protein Has a Role in Embryogenesis and Gametogenesis. Molecular and Cellular Biology. 2003;23(1):38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nature Protocols. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 13.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondroyctes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Baici A, Lang A, Hörler D, Kissling R, Merlin C. Cathepsin B in osteoarthritis: cytochemical and histochemical analysis of human femoral head cartilage. Annal. Rheum. Dis. 1995;54:289–297. doi: 10.1136/ard.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000 Nov;106(9):1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]