Abstract
Patients with nephrotic syndrome require steroids for long time and sometimes repeatedly resulting in various adverse effects. Deflazacort (DFZ) had been described as equally effective and with fewer side effects as compared with other steroids. This review evaluates the literature on efficacy and toxicity of DFZ as compared with other therapies for nephrotic syndrome. A systematic review of Pubmed database and Cochrane Central Register of Controlled Trials with last search date of 20th April 2011. Search terms included “nephrotic AND deflazacort” without any limitations. Randomized control trials comparing DFZ vs placebo or other therapies in subjects with nephrotic syndrome were included. Two authors extracted data independently. Three studies meet inclusion criteria and data were synthesized qualitatively. The limited evidence suggested that DFZ appeared to be equally effective in inducing remission or decreasing proteinuria in patients with nephrotic syndrome. It caused significantly less decrease in bone mineral content (BMC) in spine as compared with prednisolone. The results related to weight change, blood pressure change, Cushingoid symptoms, and urinary calcium excretion were inconsistent between included studies. By reviewing the available limited evidence, DFZ appears to be of similar efficacy for nephrotic patients, but there were inconsistent results regarding side effect profile of DFZ as compared with other steroids except for decrease in BMC where DFZ was better. There is need for larger randomized controlled trials to evaluate effectiveness and adverse effect profile of DFZ as compared with other steroids in nephrotic syndrome.
Keywords: Bone mineral content, deflazacort, nephrotic syndrome, prednisolone
Introduction
Nephrotic syndrome has an incidence of three new cases per 100 000 population each year in adults[1] and about 2/100 000 in children.[2,3] Nephrotic syndrome may be primary, or secondary to various systemic diseases and drugs.[1,4] Corticosteroids (specifically prednisolone [PDN]) form first line of treatment for nephrotic syndrome in children and it is used for prolonged period and sometimes repeatedly for relapses.[4,5] Although there is lack of clinical guidelines for management of nephrotic syndrome in adults, it is managed by controlling edema, using angiotensin-converting enzyme inhibitors with controversial role of steroids.[1,6] The response rates to corticosteroids in adult minimal change disease is variable (remission in 37% to 50% within four weeks, 51% to 76% within eight weeks, and 76% to 97% within 16 weeks with failure in 10% and relapse in about two third patients) as compared with similar disease in children.[7] Cyclophosphamide, cyclosporine, chlorambucil, and other immunosuppressive have been used for patients with either steroid-resistant or frequently relapsing nephrotic syndrome. Immunosuppressive therapy for nephrotic syndrome is not without adverse effects which such as infection, malignancy, peptic ulceration, diabetes mellitus, infertility, kidney failure, bone marrow suppression, hypertrichosis, and alopecia.[1,6] Important side effects of steroids in adults include fall in bone mineral content (BMC), Cushingoid appearance, and increased blood pressure. In children particularly, corticosteroids have known adverse effects such as obesity, impaired growth, hypertension, impaired glucose tolerance, osteoporosis, Cushingoid symptoms, and adrenal suppression and these are more prevalent in those children who relapse frequently requiring multiple courses of corticosteroids.[4]
Deflazacort (DFZ) is an oxazoline derivative of PDN with anti-inflammatory and immunosuppressive activity.[8] The potency ratio of DFZ vs PDN is estimated to be 1.28 (6 mg of DFZ : 5 mg PDN).[9] The use of DFZ in Duchenne Muscular Dystrophy,[10,11] Juvenile Idiopathic arthritis (previously, juvenile chronic or rheumatoid arthritis),[12] chronic inflammatory diseases in adults,[13] renal transplantation,[14–16] various hematological disorders (non-Hodgkin's lymphoma, idiopathic thrombocytopenic purpura, etc.),[17] drug-resistant epilepsies in children,[18] and type 1 autoimmune hepatitis[19] is found to be as efficacious as other steroids with less worrying adverse-effect profile.
Although therapeutic effects are inseparable from adverse metabolic effects of steroids, the goal of corticosteroid therapy should be to achieve maximum clinical benefit with minimum side effects. DFZ appeared to have almost similar efficacy with fewer side effects for various immune-mediated diseases as compared with PDN or other steroids. In management of nephrotic syndrome, steroids are used for long duration resulting in many adverse effects. Thus, it will be prudent to find a drug with similar efficacy but fewer side effects for patients with nephrotic syndrome.
Objective of this systematic review is to evaluate the efficacy and toxicity of DFZ for nephrotic syndrome and whether DFZ is effective for inducing and maintaining remission in patients with nephrotic syndrome, similar or more effective than other steroids or therapies? and have fewer side effects as compared to other steroids or therapies. The review included randomized control trials (RCT) comparing DFZ as compared with placebo or other therapies in patients with nephrotic syndrome for efficacy (remission or not, time to remission, number of relapses) and adverse effects.
Materials and Methods
Pubmed was searched with words “nephrotic AND deflazacort” without any limitations up to 20th April 2011. The Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2 was also searched with words “nephrotic AND deflazacort” on 20th April 2011. DARE database and Google scholar were also searched with key words “nephrotic AND deflazacort.” We searched ASN (American Society of Nephrology), WCN (World Congress of Nephrology), and ERA-EDTA (European Renal Association-European Dialysis and Transplantation Association) conference proceedings available online for additional relevant study. References of included studies were reviewed to find further related studies. Two authors individually screened abstract of studies found in search to locate studies eligible to be included in review. The potential eligible studies were assessed for full text to include finally in review. Search results were described in flow diagram as per PRISMA statement.[20] Both authors individually extracted data from included studies. Both the review authors assessed for risk of bias in included studies related to random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. Corresponding authors of included studies were contacted through email for additional information if needed. Meta-analysis was planned if sufficient data became available.
Results
The search results along with selection of studies have been shown in Figure 1. The search of ASN, WCN, and ERA-EDTA conference proceedings did not reveal any additional study. Full texts of three studies were assessed for eligibility and all three were selected for qualitative synthesis as per inclusion criteria of review.[21–23] One crossover RCT was excluded.[24] The corresponding authors of two included studies (Olgaard et al.[22] and Liern et al.[23]) were contacted through email for additional information and we got more unpublished data from Liern et al. but not from Olgaard et al. The characteristic of included studies is shown in Table 1. All three studies finally selected for the review were randomized controlled trials and published in English, except for study by Liern et al.[23] which was published in Spanish but the English version was also available. The included studies involved a total of 91 participants. Only children were included in study by Broyer et al.[21] and Liern et al.,[23] whereas only adults were included in study by Olgaard et al.[22] Olgaard et al. enrolled consecutive newly diagnosed cases of nephrotic syndrome and Broyer et al. enrolled steroid-dependent nephrotic patients and details of nephrotic state was not defined in study by Liern et al. All included studies were conducted at one center each; Broyer et al.[21] in France, Olgaard et al.[22] in Denmark, and Liern et al.[23] in Argentina. The intervention received were DFZ and PDN (on dose ration of 1.2 : 1) in studies by Broyer et al. and Olgaard et al. and DFZ and Methylprednisolone (MPD) (in dose ratio of 1.5 : 1) in study by Liern et al. None of the included study clearly defined the primary and secondary outcome. Broyer et al.[21] recorded time to achieve remission, number of relapse during study period, bone mineral density of lumbar spine, growth velocity, and clinical signs of Cushing syndrome. Olgaard et al.[22] mainly evaluated the effect of DFZ vs PDN on bone metabolism by measuring BMC of spine, arm, forearm, and mandible. Liern et al.[23] mainly evaluated recovery of different immunoglobulin sub-classes in patients with NS treated with MPD vs DFZ.
Figure 1.
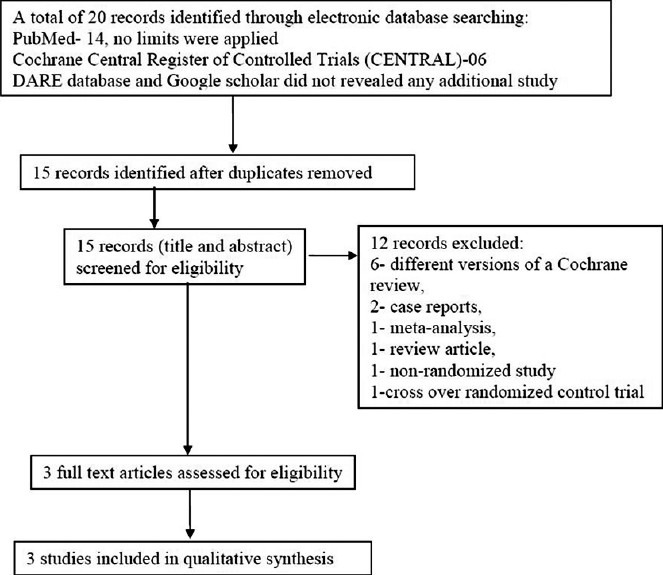
Flow diagram of study selection
Table 1.
Characteristics of included studies evaluating deflazacort in patients with nephrotic syndrome
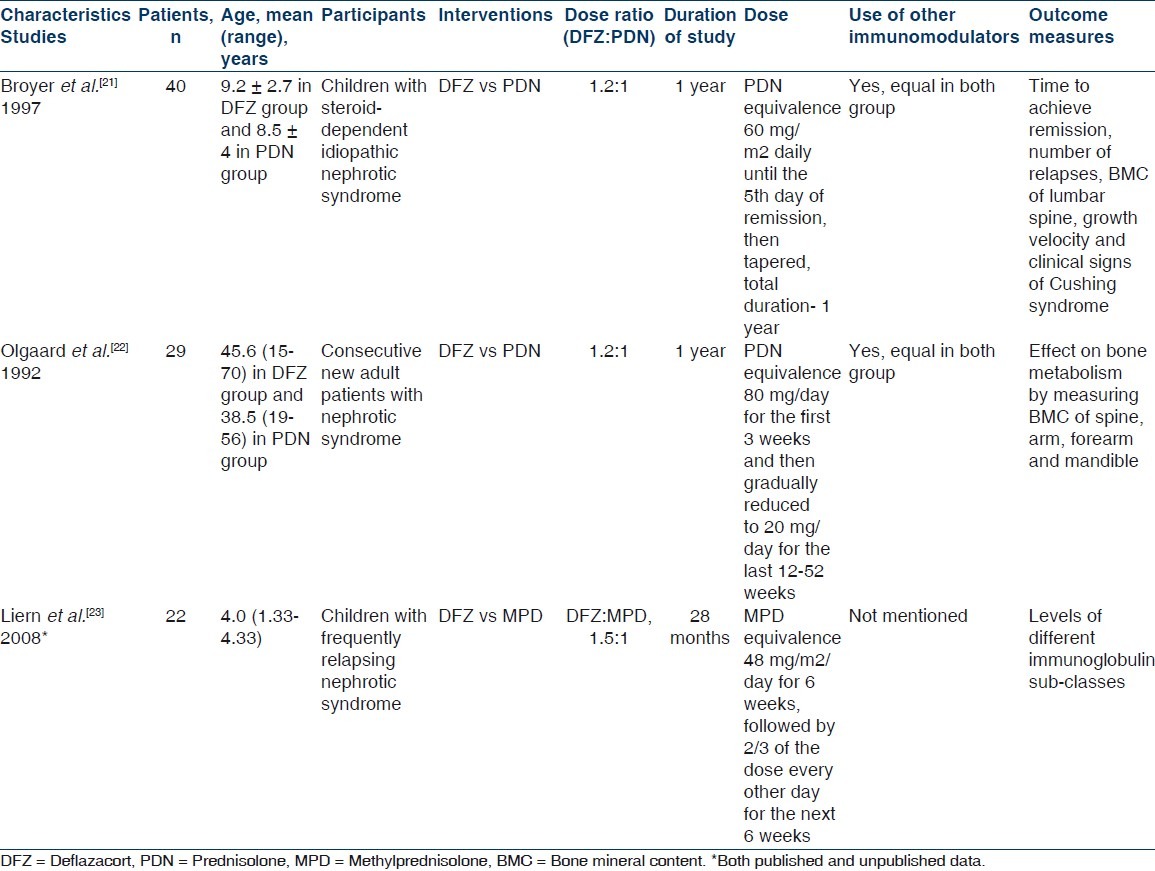
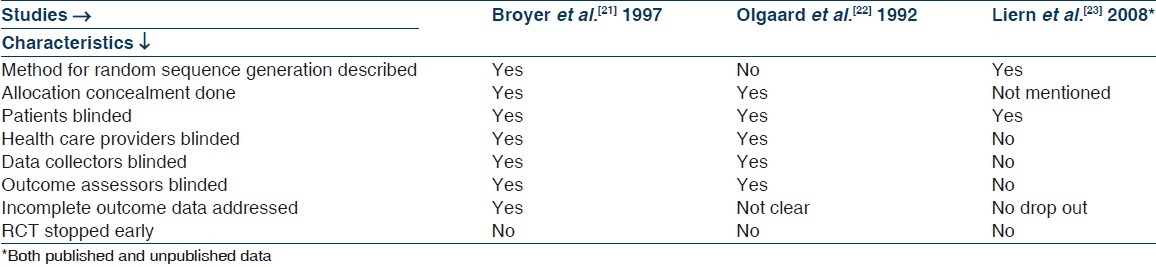
Quality measures of included studies are shown in Table 2. Liern et al.[23] did not describe about allocation concealment, whereas in rest of the two studies, it was done properly. Subjects were blinded in all three included studies and investigators were also blinded in studies by Broyer et al.[21] and Olgaard et al.[22] In study by Liern et al.,[23] there was no drop out; in study by Broyer et al.,[21] drop outs were similar in both groups and reason were given by authors. Details of drop outs were not given clearly in study by Olgaard et al.[22] No study protocol was available for any of the study, so it is difficult to comment on selective reporting in included studies. None of the included studies were stopped early.
Table 2.
Quality measures of included randomized controlled trials
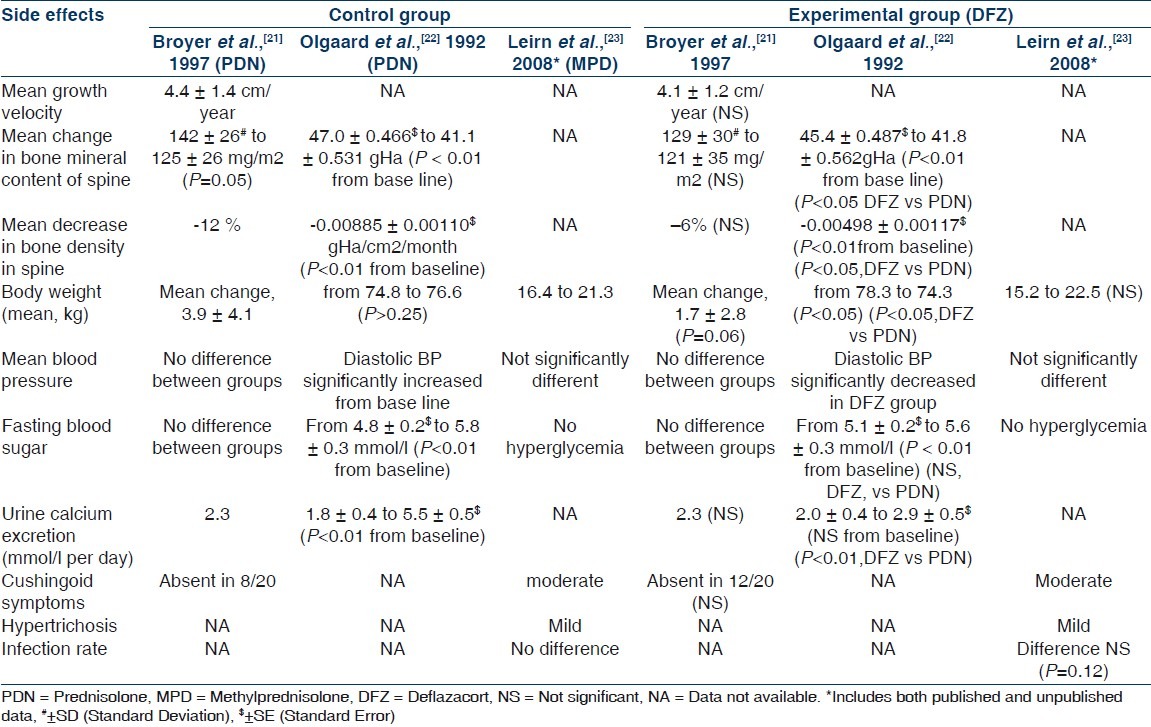
Summaries of results of all three included studies are illustrated in Tables 3 and 4. Because the participants (Broyer et al.[21] included steroid dependent nephrotic children, Olgaard et al.[22] included newly diagnosed adult nephrotics, and Liern et al.[23] included frequently relapsing nephrotic children) reported outcome measures (measurement of effectiveness and metabolic effects of steroids in study by Broyer et al.,[21] evaluation of osteoporosis in study by Olgaard et al.,[22] and it was assessment of recovery of immunoglobulins in nephrotic patients in study by Liern et al.[23]) varied markedly, we focused on describing the studies, their results, their applicability, and their limitations, and on qualitative synthesis rather than meta-analysis. Regarding efficacy of DFZ in nephrotic syndrome as compared with other steroids, the mean time for attaining remission was similar in DFZ group and other steroids in studies by Broyer et al.[21] and Liern et al.[23] [Table 3]. In study by Olgaard et al.,[22] the 24-hour urinary protein decreased significantly among both groups of DFZ and PDN without significant difference between the drugs [Table 3]. Mean number of new relapses during one year of study period were significantly less in DFZ group as compared with PDN group in the study by Broyer et al.[21] [Table 3]. Similarly, the patients free of relapse during study period were significantly more in DFZ group in the same study. The mean numbers of new relapses during study period were not significantly different in study by Liern et al.[23] and such data are not available in study from Olgaard et al.[22] Regarding side effects of steroids in nephrotic syndrome, the mean growth velocity was not significantly different between DFZ and PDN in study by Broyer et al.[21] and such data are not available in other two included studies [Table 4]. In study by Broyer et al.,[21] the mean BMC of spine changed significantly from baseline in PDN group but not in DFZ group. In the same study, mean decrease in bone density was -12% and -6% in PDN and DFZ group, respectively, although it was not statistically significant [Table 4]. Total BMC of lumbar spine and mandible decreased significantly from baseline in both DFZ and PDN group in study by Olgaard et al.,[22] but the it was significantly less in DFZ as compared with PDN group for lumbar spine but not for mandible [Table 4]. The mean decrease in bone density/year in lumbar spine was significantly more in PDN group as compared with DFZ group in the same study. Data on BMC were not available from study by Liern et al.[23] Change in body weight was not significantly different between groups in studies by Broyer et al.[21] and Liern et al.[23] but in study by Olgaard et al.,[22] weight decreased significantly in DFZ group as compared with PDN group [Table 4]. Blood sugar levels were not significantly different between the groups in any of included studies. The blood pressure changes were also not significantly different between the groups in any of included studies, except in study by Olgaard et al.[22] where diastolic blood pressure increased significantly in PDN group as compared with DFZ group. Urinary calcium excretion increased significantly in PDN group as compared with DFZ group in study by Olgaard et al.,[22] but there was no difference in urinary calcium excretion between the groups in study by Broyer et al.[21] and such data were not available in study by Liern et al.[23] Cushingoid features were not significantly different among DFZ and other steroids in studies by Broyer et al.[21] and Liern et al.[23] and it was not described in study by Olgaard et al.[22]
Table 3.
Study characteristic and efficacy of deflazacort as compared to other steroids in nephrotic syndrome
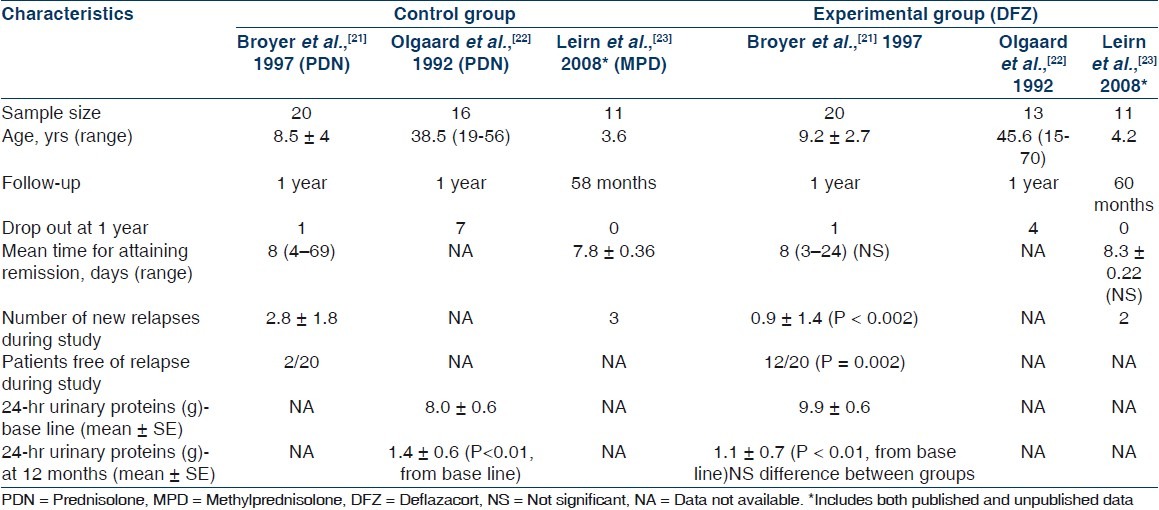
Table 4.
Side effects of deflazacort as compared to other steroids in nephrotic syndrome
Discussion
There is lack of sufficient evidence for comparing DFZ with other steroids in relation to efficacy and adverse effects in patients with nephrotic syndrome. The identified studies were small in number which seems insufficient to address all objectives of the review. The review included three studies with a total of 91 subjects. Methodologies of included studies varied: All were RCT with proper allocation concealment in two and it was not described in one study; method of random sequence generation described in two studies; participants were blinded in all and in two studies investigators were also blinded. It is difficult to comment on selective reporting in studies as study protocol was not available for any of the study.
The available evidence suggest that DFZ at equipotent dosage appears to be of similar (better in one study) efficacy as compared with PDN or MPD for inducing remission or decreasing proteinuria in patients with nephrotic syndrome. Adverse effects of DFZ as compared with other steroids in patients with nephrotic syndrome were not consistent except for effect on BMC where DFZ had favorable effects as compared with PDN. Effects on blood pressure, weight change, urinary excretion of calcium, and Cushingoid features were not consistent between the studies. The possible explanation for this discrepancy may be difference in participants among studies, e.g., Broyer et al.[21] included steroid-dependent nephrotic children, whereas Olgaard et al.[22] included newly diagnosed adults with nephrotic syndrome. Another reason may be small sample size of included studies.
For this review, the search strategy was broad without any limitations making likelihood that all relevant studies were identified. Two authors were involved individually for study selection and data retrieval and any discrepancy was resolved by discussion. The corresponding authors of two included studies were contacted through email for additional information and we got some additional data from one. Our review had some limitations. A few numbers of studies with small sample size were available for the review. The quality of included studies varied [Table 2] and there were some missing data related to outcome of review. We were unable to perform meta-analysis for reasons described above. There was inconsistency of results regarding adverse effect profile of DFZ as compared with other steroids.
A crossover RCT, excluded from review, compared the treatment sequence of DFZ-prednisone or prednisone-DFZ in ten adult nephrotic patients and reported similar efficacy for both the treatment sequence.[24] Avioli described the equipotent ratio of DFZ to PDN as 1.28:1,[9] although equipotency between DFZ and PDN varies in different conditions, e.g., 1.2:1 for nephrotic syndrome,[24] rheumatoid arthritis,[25] and juvenile chronic arthritis;[12] 1.4 :1 for asthma[25] and polymyalgia rheumatica.[26] Two of the included studies, where DFZ was compared with PDN, used the 1.2:1 ratio. The different effects of DFZ and PDN on T lymphocytes had been reported. Scudeletti et al. showed that a single oral dose of DFZ induced T cell depletion and affected the ratio of helper, inducer/suppressor, cytotoxic T cells for up to 72 hours, while they returned to baseline levels within 24 hours following PDN.[27] This change in ratio of T4/T8 cells had been consistently found in patients treated daily with DFZ, while it was not consistent during PDN therapy.[27,28] These changes in T lymphocyte subsets by DFZ were also noticed in patients after kidney transplantation.[29] This difference in the immune-modulatory effect of DFZ and PDN may explain the different efficacy and side-effect profile in subjects with nephrotic syndrome as dysfunction of T lymphocytes is suspected to be one of the underlying mechanisms in nephrotic patients. A Cochrane review on “Corticosteroid therapy for nephrotic syndrome in children” by Hodson et al. included one study[21] related to DFZ which also is the part of our review.[30] DFZ is costlier and treatment with DFZ 36 mg daily for six months costs £235 (pounds 235) compared with £19 (pounds 19) for PDN 30 mg daily.[31]
Conclusions
Implications for practice
There were insufficient studies comparing DFZ and other steroids for nephrotic syndrome. By reviewing the available evidence, DFZ appeared to be of similar efficacy for nephrotic patients as compared with other steroids and had favorable effect on BMC of spine but there were inconsistent results regarding other side-effect profile of DFZ as compared with other steroids. There was lack of evidence to recommend/not DFZ in place of PDN for treating nephrotic syndrome.
Implications for research
This review highlights the need for larger randomized controlled trials with sufficient follow-up period to evaluate effectiveness and adverse effect profile of DFZ as compared with other steroids in subjects with nephrotic syndrome, especially children with first episode of nephrotic syndrome. Further research is also needed for defining accurate equipotency ratio of DFZ as compared with PDN.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008;336:1185–9. doi: 10.1136/bmj.39576.709711.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arneil GC. 164 children with nephrosis. Lancet. 1961;2:1103–11. doi: 10.1016/s0140-6736(61)91026-1. [DOI] [PubMed] [Google Scholar]
- 3.Schlesinger ER, Sultz HA, Mosher WE, Feldman JG. The nephrotic syndrome: Its incidence and implications for the community. Am J Dis Child. 1968;116:623–32. [PubMed] [Google Scholar]
- 4.Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, et al. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–57. doi: 10.1542/peds.2008-1559. [DOI] [PubMed] [Google Scholar]
- 5.Manrique-Rodríguez S, Fernandez-Llamazares CM, Sanjurjo-Saez M. Pharmacotherapeutic review and update of idiopathic nephrotic syndrome in children. Pharm World Sci. 2010;32:314–21. doi: 10.1007/s11096-010-9380-2. [DOI] [PubMed] [Google Scholar]
- 6.Kodner C. Nephrotic syndrome in adults: Diagnosis and management. Am Fam Physician. 2009;80:1129–34. [PubMed] [Google Scholar]
- 7.Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fuijimi S. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis. 2002;39:503–12. doi: 10.1053/ajkd.2002.31400. [DOI] [PubMed] [Google Scholar]
- 8.Joshi N, Rajeshwari K. Deflazacort. J Postgrad Med. 2009;55:296–300. doi: 10.4103/0022-3859.58942. [DOI] [PubMed] [Google Scholar]
- 9.Avioli LV. Potency ratio: A brief synopsis. Br J Rheumatol. 1993;32(Suppl 2):24–6. doi: 10.1093/rheumatology/32.suppl_2.24. [DOI] [PubMed] [Google Scholar]
- 10.Campbell C, Jacob P. Deflazacort for the treatment of Duchenne Dystrophy: A systematic review. BMC Neurol. 2003;3:7. doi: 10.1186/1471-2377-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houde S, Filiatrault M, Fournier A, Dubé J, D’Arcy S, Bérubé D, et al. Deflazacort use in Duchenne muscular dystrophy: An 8-year follow-up. Pediatr Neurol. 2008;38:200–6. doi: 10.1016/j.pediatrneurol.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Loftus J, Allen R, Hesp R, David J, Reid DM, Wright DJ, et al. Randomized, double-blind trial of deflazacort versus prednisone in juvenile chronic (or rheumatoid) arthritis: A relatively bone-sparing effect of deflazacort. Pediatrics. 1991;88:428–36. [PubMed] [Google Scholar]
- 13.Gray RE, Doherty SM, Galloway J, Coulton L, de Broe M, Kanis JA. A double-blind study of deflazacort and prednisone in patients with chronic inflammatory disorders. Arthritis Rheum. 1991;34:287–95. doi: 10.1002/art.1780340306. [DOI] [PubMed] [Google Scholar]
- 14.Ferraris JR, Pasqualini T. Therapy with a new glucocorticoid: Effect of deflazacort on linear growth and growth hormone secretion in renal transplantation. J Rheumatol suppl. 1993;37:43–6. [PubMed] [Google Scholar]
- 15.Schärer K, Feneberg R, Klaus G, Paschen C, Wüster C, Mehls O, et al. Experience with deflazacort in children and adolescents after renal transplantation. Pediatr Nephrol. 2000;14:457–63. doi: 10.1007/s004670050792. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris JR, Krmar R, Flores D, Giogieri S, Díaz L, Tessler J. Pharmacokinetics of deflazacort in renal transplanted and hemodialyzed children. Clin Nephrol. 1998;50:172–7. [PubMed] [Google Scholar]
- 17.Gobbi M, Scudeletti M. Deflazacort in the treatment of haematologic disorders. Eur J Clin Pharmacol. 1993;45(Suppl 1):S25–8. doi: 10.1007/BF01844200. [DOI] [PubMed] [Google Scholar]
- 18.Grosso S, Farnetani M, Mostardini R, Cordelli D, Berardi R, Balestri P. A comparative study of hydrocortisone versus deflazacort in drug-resistant epilepsy of childhood. Epilepsy Res. 2008;81:80–5. doi: 10.1016/j.eplepsyres.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Bae SH, Kim JS, Kim DH. Deflazacort for Type-1 Autoimmune Hepatitis in a Korean Girl. J Korean Med Sci. 2006;21:758–60. doi: 10.3346/jkms.2006.21.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 21.Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P. A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatr Nephrol. 1997;11:418–22. doi: 10.1007/s004670050308. [DOI] [PubMed] [Google Scholar]
- 22.Olgaard K, Storm T, van Wowern N, Daugaard H, Egfjord M, Lewin E, et al. Glucocorticoid-induced osteoporosis in the lumbar spine, forearm, and mandible of nephrotic patients: A double-blind study on the high-dose, long-term effects of prednisone versus deflazacort. Calcif Tissue Int. 1992;50:490–7. doi: 10.1007/BF00582160. [DOI] [PubMed] [Google Scholar]
- 23.Liern M, Diéguez S, Canepa C. Recovery of total immunoglobulin and immunoglobulin subclasses in nephrotic syndrome: Deflazacort vs methylprednisone. Nefrologia. 2008;28:563. [PubMed] [Google Scholar]
- 24.Piccoli A, Gastaldon F, Pillon L, Mussap M, Faggian D, Plebani M, et al. Bioequivalence of deflazacort and prednisone in the treatment of idiopathic nephrotic syndrome. A pilot study. Curr Ther Res Clin Exp. 1993;54:588–97. [Google Scholar]
- 25.Markham A, Bryson HM. Deflazacort: A review of its pharmacological properties and therapeutic efficacy. Drugs. 1995;50:317–33. doi: 10.2165/00003495-199550020-00008. [DOI] [PubMed] [Google Scholar]
- 26.Krogsgaard MR, Lund B, Johnsson B. A longterm prospective study of the equipotency between deflazacort and prednisolone in the treatment of patients with polymyalgia rheumatica. J Rheumatol. 1995;22:1660–2. [PubMed] [Google Scholar]
- 27.Scudeletti M, Pende D, Barabino A, Imbimbo B, Grifoni V, Indiveri F. Effect of single oral doses of prednisone and deflazacort on human lymphocyte distribution and functions. Analysis with monoclonal antibodies. Adv Exp Med Biol. 1984;171:335–44. [PubMed] [Google Scholar]
- 28.Scudeletti M, Piccardo C, Piovano P, Imbimbo B, Indiveri F. Effects of a new heterocyclic glococorticoid, deflazacort(DFC), on the functions of lymphocytes from patients with rheumatoid arthritis(RA) Int J Immunopharmacol. 1987;9:133–9. doi: 10.1016/0192-0561(87)90087-7. [DOI] [PubMed] [Google Scholar]
- 29.Elli A, Rivolta R, Di Palo FQ, Parenti M, Vergallo G, Palazzi P, et al. A randomized trial of deflazacort versus 6-methylprednisolone in renal transplantation, immunosuppressive activity and side effects. Transplantation. 1993;55:209–12. [PubMed] [Google Scholar]
- 30.Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev. 2007;4:CD001533. doi: 10.1002/14651858.CD001533.pub4. [DOI] [PubMed] [Google Scholar]
- 31.Deflazacort - an alternative to prednisolone? Drug Ther Bull. 1999;37:57–8. doi: 10.1136/dtb.1999.37857. [DOI] [PubMed] [Google Scholar]


