Abstract
Epidemiological studies provide useful information for clinical practice and investigations. This report aimed to determine glomerular diseases frequencies in a region of Morocco. All native renal biopsies (January 2000 to December 2007) on adults were reviewed, but only glomerular diseases were analyzed. The diagnosis of each case was based on histological, immunopathological and clinical features. We have performed 171 renal biopsies in 161 patients (101 males and 60 females), the mean age was (range) 40.4 ±15 years (16–72). Clinical indications that lead to renal biopsy were: nephrotic syndrome (60.3%), renal failure of unknown aetiology (31.6%), asymptomatic urinary abnormalities (6.2%) and nephritic syndrome(1.9%). Primary glomerular diseases were reported in 84 patients (52%). The most common histological lesion was minimal change disease (26%). Idiopathic membranous glomerulopathy was the second most common lesion (23%) followed by membranoproliferative glomerulonephritis (17%), IgA nephropathy (12%), focal and segmental glomerulosclerosis (9.4%) and crescentic glomerulonephritis (6%). Secondary glomerular diseases were reported in 53 patients (33%). Lupus nephritis was the secondary glomerular disease most frequent (45%) followed by amyloïdosis (19%), diabetic nephropathy (15%), and Good pasture's syndrome (7.6%). The most common complications of the procedure were pain at biopsy site in 4%, gross hematuria in 11.1%, perirenal hematoma in 5% and hematuria requiring nephrectomy in 0.6% patients. Minimal change disease was the most frequent primary glomerulopathy and lupus nephritis was the most frequent secondary glomerulopathy in our group. The reasons for these findings are unclear. This information is an important contribution to the understanding the prevalence of renal diseases in North Africa.
Keywords: Epidemiology, glomerular diseases, Morocco, renal biopsy
Introduction
Glomerular diseases (GD) remain the most common cause of end-stage renal disease worldwide.[1] The glomeruli can be injured by a variety of external factors, systemic diseases[2,3] and hereditary diseases.[4] All GD present with a variable range proteinuria, hematuria, hypertension and impaired renal function.[5–9] Moreover, GD may manifest as insidious development of uremia secondary to chronic GD.[10] The structural changes due to the deposition of immune complexes cause changes in the electrical charge of the glomerular basement membrane and modify the permeability to proteins.[11] There is usually associated inflammatory proliferative response within the injury glomerulus involving the endothelial, mesangial, or epithelial cells.[12,13]
Renal biopsy has a fundamental role in the evaluation of the patterns of GD. In some patients, the renal biopsy appears essential to determine an accurate diagnosis and prognosis and the choice of an appropriate treatment.[14,15] This study aimed to determine the prevalence of primary and secondary GD on adults and is intended to serve as a resource for nephrologists, researches to stimulate new analysis and investigations, to improve treatment of renal disease, and help to develop protocols for preventive medicine.
Patients and Methods
We analysed during a period of 8 years (2000–2007) the results of renal native biopsies on adults (age >16 years). We recorded the following variables for each patient: name, date of birth, gender, clinical and laboratory conditions observed at the time of renal biopsy were reported as follows: (i) nephrotic proteinuria (> 3.5 g/24 h); (ii) nephritic syndrome: combination of hematuria, arterial hypertension and reduced renal function (sCr> 110 μmol/l); (iii) asymptomatic urinary abnormalities (AUA): low-grade proteinuria (< 3.5g/24 h) with or without microhematuria; (iv) renal failure of unknown aetiology (sCr> 1.4 mg/dl). More than one of these four syndromes overlapped in some patients.
Patients were excluded if they had bleeding problems, uncontrolled hypertension, single kidney for percutaneous method, and morbid obesity or were uncooperative. Prior to the biopsy procedure, clotting profile including platelet count, bleeding time, clotting time, and prothrombine time were performed. Only a small numbers of patients needed correction for one or more abnormalities of these parameters before performing the biopsy.
All renal tissues were obtained by the percutaneous method using a biopsy gun under X-ray guidance without injection of the contrast agent, or under ultrasound (USD) guidance. The proposed biopsy site, usually the lower pole of the left kidney, was marked on the X-ray film available. Its position in relationship to the spinous processes and the iliac crest was measured and marked on the back of the patient. Under USD, the biopsy site was located directly. The marker served as a guide to the position of the lower pole of the kidney. A very fine needle is initially used to confirm the location and depth of the kidney. If the needle had entered the kidney, the hub of the biopsy needle would be seen to swing with the movement of the diaphragm, and hence the kidney. A special biopsy needle is then used to obtain the kidney biopsy while the patient is holding their breath. A slight resistance could usually be felt when the needle entered into the kidney tissue. Two to three pieces of tissue are usually taken.
Renal biopsies were processed for light and immunofluorescence (IF) microscopy in all specimens. In all cases, sections were stained with Hematoxylin and Eosine (H and E), Masson's trichrome, periodic acid-schiff (PAS) and Silver Jone's stain. The IF microscopy panel included Ig A, Ig M, Ig G, C3, C1q, fibrinogen and, if necessary, kappa and lambda light chains.
Immediately after the biopsy the patient remains lying flat on their back for 24 h. During this time, pain, blood pressure, pulse rate, urine flow and colour of the urine are monitored frequently and the patient is encouraged to drink large volumes of fluid.
Some complications after renal biopsy were recorded and reported as follows: (i) pain at biopsy site; (ii) gross hematuria; (iii) symptomatic hematoma; (iv) hemostasis nephrectomy.
The histological classification of renal diseases was based on the World Health Organization (WHO) recommendations.[16] Primary glomerulonephritis (GN) was classified into eight groups: minimal change disease (MCD), membranous GN (MGN), focal and segmental glomeruloscerosis (FSGS), membranoproliferative GN (MPGN), immunoglobulin A nephropathy (IgAN), crescentic GN (CGN), postinfectious GN and others. Secondary GN was classified into seven groups: lupus nephritis (LN), diabetic nephropathy (DN), amyloidosis (AMYL), Good pasture's disease (GPS), Cryoglobulinemic GN (CryoGN), Henoch–Schönlein purpura (HSP) and others.
Primary or secondary diseases were diagnosed and classified based on clinical features, and laboratory tests were performed on all the material in these patients. The paraclinical evaluation included tests for hepatotropic viruses, human immunodeficiency virus (HIV), serum complement levels, cryoglobulins, antinuclear antibodies (ANA) and other immunological tests. It also included serum protein electrophoresis and others tests according to the clinical features or biopsy diagnosis.
Statistical analysis
Each year's data were stored on a database file. Simple descriptive statistics were used. Data were tested for normal distribution with the Kormogorov-Smirnov test. The quantitative variables were expressed as mean and standard deviation. Numbers and percentages were used for qualitative variables.
Results
General and clinical data
We have performed 171 renal biopsies in 161 patients. The mean age (range) was 40.4±15 years (16–72). There were 101 males and 60 females, with a male/female ratio of 1.68.
The number of renal biopsies during each 2 years increased from 12 cases in 2000–2001 to 71 cases during 2006–2007 [Figure 1].
Figure 1.
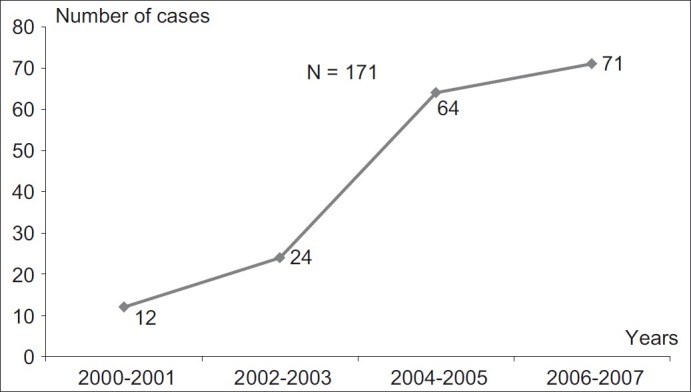
Cases of renal biopsies
The clinical and laboratory indications that led to renal biopsy were: nephrotic proteinuria (60.3%), renal failure of unknown aetiology (31.6%), AUA (6.2%) and nephritic syndrome (1.9%). Secondary renal diseases were rare indications in our practice and accounted for only 11.6% of all biopsies in this study.
The renal samples were obtained most commonly by percutaneous method in 170 biopsies (99.4%), using a biopsy gun under X-ray guidance in 159 biopsies (93%) or under USD guidance in 16 biopsies (6.4%); and one biopsy by the surgical method (0.6%) for single kidney. More than one biopsy was performed in some patients, re-biopsy in eight patients and third renal biopsy in one patient, due to inadequate sampling during the first biopsy in five cases or for therapeutic/diagnostic reasons at different time of follow-up for lupus nephritis in four cases. Most of the material obtained is evaluated through light microscopy and IF. However, IF was not performed in the case of the lack of poor quality of a tissue sample. The number of biopsies assessed by light microscopy only was 28.6% of all samples. The electronic microscopy was not used. The mean number of glomerulus (range) by sample was 15.8±10.4 (5–81).
The most common complications of the procedure were pain at biopsy site (4%), gross hematuria (11%), perirenal hematoma (5%) and hematuria requiring nephrectomy (0.6%). Table 1 shows data of the patients with renal biopsy.
Table 1.
Data of the patients with renal biopsy
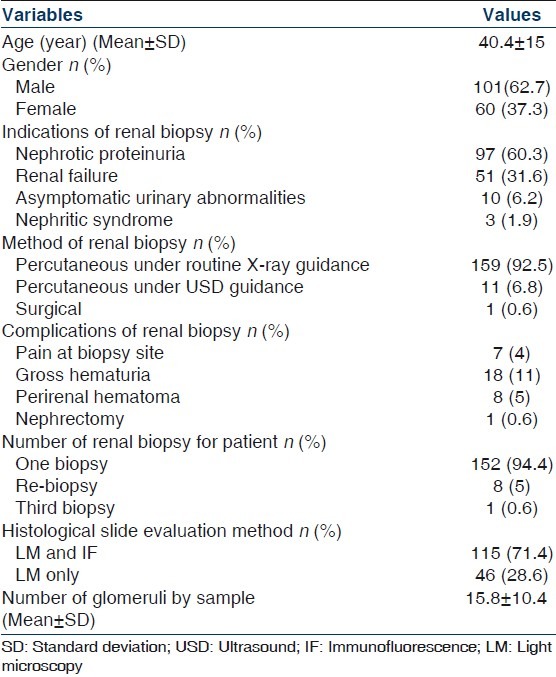
Histopathological data
GD were reported in 137 patients (85%). LN was the most common nephropathy seen in 15% and MCD was the second most common nephropathy recorded in 13% of all renal biopsies. Table 2 shows the distribution of all renal biopsies.
Table 2.
Distribution of diseases
Prevalence of primary GD
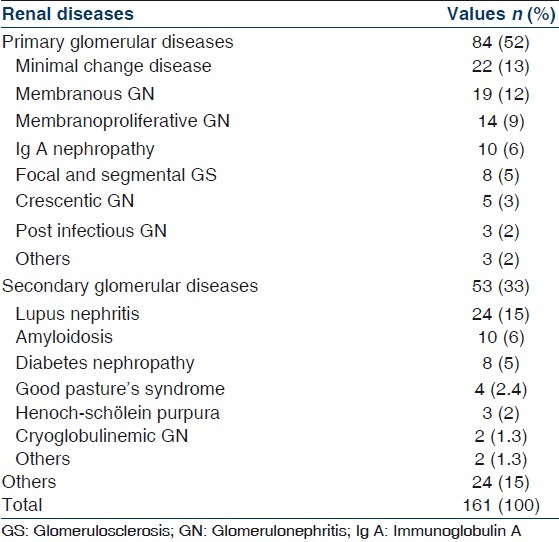
Primary GD were reported in 84 patients (52%). The most common histological lesion was MCD in 22 patients (26%). Idiopathic MGN was the second most common lesion in 19 patients (23%) followed by MPGN in 14 patients (17%), IgAN, FSGS, CGN, postinfectious GN, mesangioproliferative GN and proliferative endocapillary GN. Table 3 indicates the prevalence of different types of primary GD.
Table 3.
Prevalence of different types of primary glomerular diseases
Prevalence of secondary GD
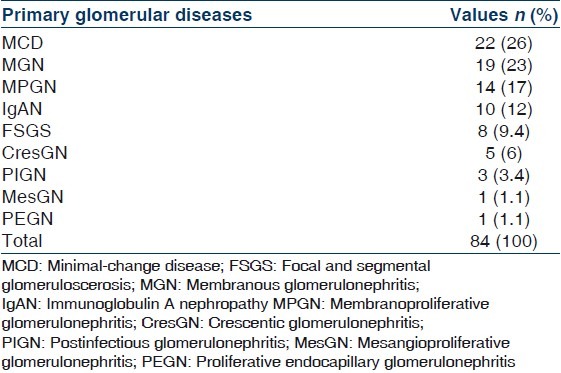
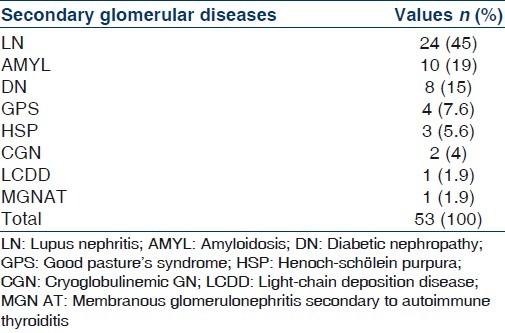
Secondary GD were reported in 53 patients (33%); LN was the secondary GD most frequentin 24 patients (45%) followed by AMYL in 10 patients (19%), DN in 8 patients (15%), GPS, HSP, CryoGN, LCDD and MGN secondary to autoimmune thyroiditis. Table 4 indicates the prevalence of different types of secondary GD.
Table 4.
Prevalence of different types of secondary glomerular diseases
Discussion
Renal biopsy is a fundamental tool in the diagnosis of GD. This report provides information about the occurrence of renal diseases, especially GD, diagnosed by renal biopsy, over a period of 8 years in a single centre on adults. This report is the first systematic review of histological data in our centre.
In this study, which covers the Military Hospital in Rabat, Morocco, the rate of renal-biopsy-proven renal diseases underestimates the true prevalence of disease, as only those above a certain level of severity are likely to be biopsied. Hence, our Figures only reflect the prevalence of moderate to severe renal diseases.
This report showed that renal biopsies were performed more commonly in males that in females (male/female ratio is 1.68). This is similar to other epidemiological studies.[17–20]
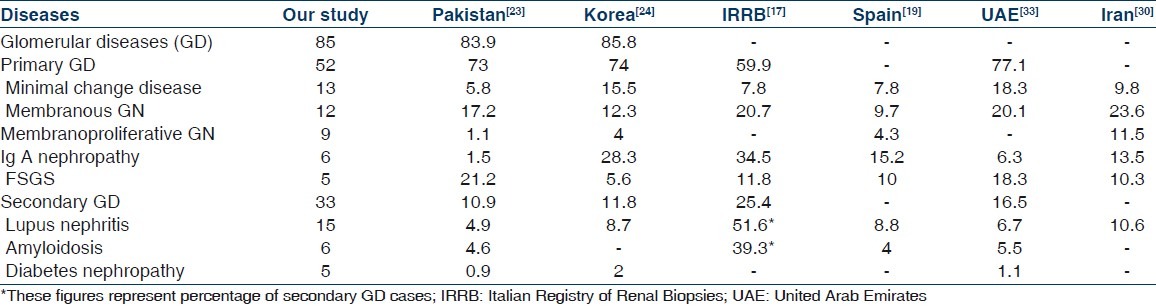
Nephrotic proteinuria was the most frequent indication [of biopsies, accounting for 60.3% of all cases, followed by renal failure for unknown aetiology (31.6%), AUA (6.2%) and nephritic syndrome (1.9%). These data agree with other studies in which nephrotic syndrome is the most frequent indication of renal biopsy.[18,19,21–24] Conversely in the Italian registry, AUA are more common than nephrotic syndrome, perhaps expressing a tendency to biopsy asymptomatic hematuria or proteinuria.[17] Secondary renal diseases indications in our study were only 11.6% of all biopsies. This result is similar to that seen in Pakistan who reported secondary renal diseases for only 1/10 of all biopsies.[23]
In this study, the number of biopsies assessed by light microscopy and IF was 71.4% of all samples. The insufficient tissue did not always allow complete evaluation especially by IF, so that a correct histological diagnosis could not be established. Other reports are similarly incompletes. Only 15.3% out of all samples were evaluated by IF in Nepal,[25] 56.5% in a Japanese study,[21] 78% in Denmark[26] and 89.5% in Italy.[17]
Similarly to other reports,[17,18,23,24,27–31] the GD were the most common histopathological category (85%). LN was the most common nephropathy diagnosed by renal biopsy in our study.
The most frequently diagnosed lesion in our patients with primary GD was MCD (26%) followed by MGN (23%) and MPGN (17%). These three entities comprised 66% of primary GD. MCD was a rare disease in others studies in the world,[17–19,21,23,28,29,32–35] while IgAN is the most common glomerular disease worldwide but its detection rate varies widely, mostly depending on biopsy indications and mass urinary screening for AUA.[21] The low prevalence of IgAN in our series (12% of primary GD and 6% of all renal biopsies) is most probably due to very low rate of renal biopsies in patients with AUA and incomplete evaluation by IF.
The MGN is the next most frequent primary GD in this study. MGN used to be the most common cause of idiopathic nephrotic syndrome (INS) in adults and is still cited as the most common cause of INS in adults in widely used renal pathology textbooks.[23,36] In European studies, it is still common and, notably, more common than FSGS.[18,35,36]
FSGS was not rare among our group and represents 9.4% of primary GD and 5% of all renal biopsies. Other authors have reported high frequencies of FSGS among Hispanic population,[22,37] in Ghana[38] and Hong Kong.[39]
In view of that fact of poor socioeconomical conditions, the prevalence of MPGN was 9% of all renal biopsies (17% of primary GD) and similar to that in others countries such as Italy[17] and Serbia.[18] This disease also shows a very divergent prevalence rate.[17–19,21,23,28,29,32–35] The highest rates are reported in studies from Lithuania,[40] Saudi Arabia,[27] Romania[29] and Nepal,[25] while low rates are reported from Central and Western European countries.[17,19,35]
Immunoglobulin M nephropathy (IgMN) has not been observed in our study. The prevalence of this entity shows widely divergent results.[18,23,41] A very high prevalence of IgMN has been reported in Thailand, where it constituted the most common renal pathology in adults, seen in 45% of overall cases.[41] In contrast to this, IgMN as a distinct entity has not been described in reports from European renal biopsy registries except for the Italian registry where an annual frequency varying from 1.2–2.8% of cases was observed.[17,19,28,29,35]
Among secondary GD, LN was the most frequent, constituting 45% of secondary GD (15% of all renal biopsies). This disease has been reported as the most common secondary GD, with a female preponderance, in studies from Thailand,[41] north-western Colombia,[22] Korea,[24] Serbia[18] and Arab countries.[5,20,27,33,42,43]
The prevalence of AMYL in our study was 6% of all renal biopsies (19% of secondary GD) in spite of high prevalence of tuberculosis and other chronic conditions that may be associated with AMYL. By contrast, AMYL was a fairly frequent finding in reports from other Arab and Mediterranean countries, where the prevalence was 40.7% (of patients with secondary nephrotic syndrome) in Jordan,[44] 13% in Lebanon,[45] 14.4% in Tunisia[46] and 32.4% in Turkey.[47] This is mainly related to the high prevalence of Familial Mediterranean Fever in these countries.
DN was found in 5% (15% of secondary GD). We usually do not biopsy diabetic patients unless there are doubts about the role of diabetes in the causation of renal disease. This restrictive biopsy approach accounts for the low prevalence of this disease in our study.
With regard to postbiopsy complications, we recorded a high rates of clinically serious complications compared with the experience of other authors[28,48] who reported gross hematuria in 1.12% and 5–7% or severe perirenal hemorrhage in 1.12% and 0.2–1.4%, respectively. In our study, one patient needed open surgical intervention for nephrectomy and there is no death during the procedure of renal biopsy. A large number of renal biopsies were performed under X-ray guidance in our cases (93%), but still a USD guided biopsy was lower (6.8%) despite the wide availability of USD. In Europe,[28,49] USD guidance was reported as predominant.
In conclusion, MCD was the most frequent primary glomerulopathy and LN was the most frequent secondary glomerulopathy in our report. The reasons for these findings are unclear. This information provides a contribution toward understanding the epidemiology of GD in North Africa, with possible implications for the planning of future research Table 5.
Table 5.
Comparison of some common glomerular diseases in our series with others study (all figures are in percentages)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Couser WG. Pathogenesis of glomerular damage in glomerulonephritis. Nephrol Dial Transplant. 1998;13(Suppl 1):10–5. doi: 10.1093/ndt/13.suppl_1.10. [DOI] [PubMed] [Google Scholar]
- 2.Myers BD, Guash A. Mechanisms of proteinuria in nephrotic humans. Pediatr Nephrol. 1994;8:107–12. doi: 10.1007/BF00868285. [DOI] [PubMed] [Google Scholar]
- 3.Cameron JS. Systemic lupus erythematosus. In: Nielson EG, Couser WG, editors. Immunologic Renal Disease. Philadelphia: Lippincott-Raven; 1997. pp. 1055–94. [Google Scholar]
- 4.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 5.Mitwalli AH, Al Wakeel JS, Al Mohaya SS, Malik HG, Abu-Aisha H, Hassan OS, et al. Pattern of glomerular disease in Saudi Arabia. Am J Kidney Dis. 1996;27:797–802. doi: 10.1016/s0272-6386(96)90516-8. [DOI] [PubMed] [Google Scholar]
- 6.Madaio MP. Renal biopsy. Kidney Int. 1990;38:529–43. doi: 10.1038/ki.1990.236. [DOI] [PubMed] [Google Scholar]
- 7.Azerus JM, Brenner BM. Acute renal failure. 3rd ed. New York: Churchill Livingstone; 1993. [Google Scholar]
- 8.Pontier PJ, Patel TG. Racial differences in the prevalence and presentation of glomerular disease in adults. Clin Nephrol. 1994;42:79–84. [PubMed] [Google Scholar]
- 9.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 10.Albers FJ. Clinical characteristics of atherosclerotic renovascular disease. Am J Kidney Dis. 1994;24:636–41. doi: 10.1016/s0272-6386(12)80225-3. [DOI] [PubMed] [Google Scholar]
- 11.Hudson BG, Kalluri R, Gunwar S, Noelken ME, Mariyama M, Reeders ST. Molecular characteristics of the Good pasture autoantigene. Kidney Int. 1993;43:135–9. doi: 10.1038/ki.1993.22. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ. The glomerular response to injury: progression or resolution? Kidney Int. 1994;45:1769–82. doi: 10.1038/ki.1994.230. [DOI] [PubMed] [Google Scholar]
- 13.Churg J, Habib R, White RH. Pathology of the nephritic syndrome in children: a report for the International Study of Kidney Diseases in Children. Lancet. 1970;760:1299–302. doi: 10.1016/s0140-6736(70)91905-7. [DOI] [PubMed] [Google Scholar]
- 14.Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C, et al. Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis. 2000;35:448–57. doi: 10.1016/s0272-6386(00)70197-1. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM. Renal pathology with clinical and functional correlations. 2nd ed. Philadelphia, PA: Lippincott; 1994. pp. 85–115. [Google Scholar]
- 16.Chug J, Sobin LH. Renal disease. Classification and atlas of glomerular diseases. Tokyo: Igaku Shoin; 1982. [Google Scholar]
- 17.Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol Dial Transplant. 1997;12:418–26. doi: 10.1093/ndt/12.3.418. [DOI] [PubMed] [Google Scholar]
- 18.Naumovic R, Pavlovic S, Stojkovic D, Basta-Jovanovic G, Nesic V. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant. 2009;24:877–85. doi: 10.1093/ndt/gfn564. [DOI] [PubMed] [Google Scholar]
- 19.Rivera F, López-Gómez JM, Pérez-García R. Spanish Registry of Glomerulonephritis. Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant. 2002;17:1594–602. doi: 10.1093/ndt/17.9.1594. [DOI] [PubMed] [Google Scholar]
- 20.Al Arrayed A, George SM, Malik AK, Al Arrayed S, Rajagopalan S, Al Arrayed A, et al. The spectrum of glomerular diseases in the Kingdom of Bahrain: An epidemiological study based on renal biopsy interpretation. Transplant Proc. 2004;36:1792–5. doi: 10.1016/j.transproceed.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Research Group on Progressive Chronic Renal Disease. Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1850 biopsied cases. Nephron. 1999;82:205–13. doi: 10.1159/000045404. [DOI] [PubMed] [Google Scholar]
- 22.Arias LF, Henao J, Giraldo RD, Carvajal N, Rodelo J, Arbeláez M. Glomerulardiseases in a Hispanic population: review of a regional renal biopsy database. Sao Paulo Med J. 2009;127:140–4. doi: 10.1590/S1516-31802009000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mubarak M, Kazi JI, Naqvi R, Ahmed E, Akhter F, Naqvi SA, et al. Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology (Carlton) 2011;16:87–92. doi: 10.1111/j.1440-1797.2010.01410.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al. Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406–10. doi: 10.1093/ndt/gfp091. [DOI] [PubMed] [Google Scholar]
- 25.Garyal, Kafle RK. Histopathological spectrum of glomerular disease in Nepal: a seven-year retrospective study. Nepal Med Coll J. 2008;10:126–8. [PubMed] [Google Scholar]
- 26.Heaf J, Lokkegaard H, Larsen S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant. 1999;14:1889–97. doi: 10.1093/ndt/14.8.1889. [DOI] [PubMed] [Google Scholar]
- 27.Huraib S, Al Khader A, Shaheen FA, Abu Aisha H, Souqiyyeh MZ, Al Mohana F, et al. The spectrum of glomerulonephritis in Saudi Arabia: the results of the Saudi registry. Saudi J Kidney Dis Transpl. 2000;11:434–41. [PubMed] [Google Scholar]
- 28.Rychlík I, Jancová E, Tesar V, Kolsky A, Lácha J, Stejskal J, et al. The Czech Registry of Renal Biopsies. The Czech regestry of renal biopsies. Occurrence of renal diseases in the 1994-2000. Nephrol Dial Transplant. 2004;19:3040–9. doi: 10.1093/ndt/gfh521. [DOI] [PubMed] [Google Scholar]
- 29.Covic A, Schiller A, Volovat C, Gluhovschi G, Gusbeth-Tatomir P, Petrica L, et al. Epidemiology of renal disease in Romania: a 10 year review of two regional biopsy databases. Nephrol Dial Transplant. 2006;21:419–24. doi: 10.1093/ndt/gfi207. [DOI] [PubMed] [Google Scholar]
- 30.Naini AE, Harandi AA, Ossareh S, Ghods A, Bastani B. Prevalence and clinical findings of biopsy-proven glomerulonephritidis in Iran. Saudi J Kidney Dis Transpl. 2007;18:556–64. [PubMed] [Google Scholar]
- 31.Abdou N, Boucar D, El Hadj Fary KA, Mouhamadou M, Abdoulaye L, Mamadou Mourtala KA, et al. Histopathological profiles of nephropathies in Senegal. Saudi J Kidney Dis Transpl. 2003;14:212–4. [PubMed] [Google Scholar]
- 32.Narasimhan B, Chacko B, John GT, Korula A, Kirubakaran MG, Jacob CK. Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol. 2006;19:205–10. [PubMed] [Google Scholar]
- 33.Yahya TM, Pingle A, Boobes Y, Pingle S. Analysis of 490 kidney biopsies: data from the United Arab Emirates Renal Diseases Registry. J Nephrol. 1998;11:148–50. [PubMed] [Google Scholar]
- 34.Huraib SO, Abu-Aisha H, Mitwalli A, Mhmoud K, Memon NA, Sulimani F. The spectrum of renal disease found by kidney biopsies at King Khalid University Hospital. Saudi Kidney Dis Transpl Bull. 1990;1:15–9. [Google Scholar]
- 35.Carvalho E, do Sameiro Faria M, Nunes JP, Sampaio S, Valbuena C. Renal diseases: A 27-year renal biopsy study. J Nephrol. 2006;19:500–7. [PubMed] [Google Scholar]
- 36.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis. 1997;30:621–31. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 37.Dragovic D, Rosenstock JL, Wahl SJ, Panagopoulos G, DeVita MV, Michelis MF. Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic pat-terns. Clin Nephrol. 2005;63:1–7. doi: 10.5414/cnp63001. [DOI] [PubMed] [Google Scholar]
- 38.Adu D, Anim-Addo Y, Foli AK, Blankson JM, Annobil SH, Reindorf CA, et al. The nephrotic syndrom in Ghana: clinical and pathological aspects. Q J Med. 1981;50:297–306. [PubMed] [Google Scholar]
- 39.Lai FM, Lai KN, Chan KW, Au TC, Tong KL, Vallance-Owen J. Pattern of glomerulonephritis in Hong Kong. Pathology. 1987;19:247–52. doi: 10.3109/00313028709066558. [DOI] [PubMed] [Google Scholar]
- 40.Razukeviciene L, Bumblyte IA, Kuzminskis V, Laurinavicius A. Membranoproliferative glomerulonephritis is still the most frequent glomerulonephritis in Lithuania. Clin Nephrol. 2006;65:87–90. doi: 10.5414/cnp65087. [DOI] [PubMed] [Google Scholar]
- 41.Parichatikanond P, Chawanasuntorapoj R, Shayakul C, Choensuchon B, Vasuvattakul S, Vareesangthip K, et al. An analysis of 3555 cases of renal biopsy in Thailand. J Med Assoc Thai. 2006;89(Suppl 2):S106–11. [PubMed] [Google Scholar]
- 42.Shaker IK, Al-Saedi AJ, Al-Salam S, Saleem MS, Al-Shamma IA. Spectrum of glomerular disease in Iraqi patients from a single center. Saudi J Kidney Dis Transpl. 2002;13:515–9. [PubMed] [Google Scholar]
- 43.Khalifa EH, Kaballo BG, Suleiman SM, Khalil EA, El-Hassan AM. Pattern of glomerulonephritis in Sudan: histopathological and immunofluorescence study. Saudi J Kidney Dis Transpl. 2004;15:176–9. [PubMed] [Google Scholar]
- 44.Said R, Hamzeh Y, Tarawneh M. The spectrum of glomerulopathy in Jordan. Saudi J Kidney Dis Transpl. 2000;11:430–3. [PubMed] [Google Scholar]
- 45.Moukheibir N. Computer analysis of renal disease in Lebanon. J Med Liban. 1970;23:19–29. [PubMed] [Google Scholar]
- 46.Verroust P, Ben-Maiz H, Morel-Maroger L, Mahfoud A, Geniteau M, Benayed H, et al. A clinical and immunopathological study of 304 cases of glomerulonephritis in Tunisia. Eur J Clin Invest. 1979;9:75–9. doi: 10.1111/j.1365-2362.1979.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 47.Sokmen C, Ozdemir AI. The spectrum of renal diseases found by kidney biopsy in Turkey. Ann Intern Med. 1967;67:603–5. doi: 10.7326/0003-4819-67-3-603. [DOI] [PubMed] [Google Scholar]
- 48.Ponticelli C, Mihatsch MJ, Imbasciati E. Renal biopsy: performance and interpretation. In: Davison AM, et al., editors. Oxford Textbook of Clinical Nephrology. 2nd ed. Oxford: Oxford University Press; 1999. pp. 158–9. [Google Scholar]
- 49.Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C, et al. Current indications for renalbiopsy: a questionnaire-based survey. Am J Kidney Dis. 2000;35:448–57. doi: 10.1016/s0272-6386(00)70197-1. [DOI] [PubMed] [Google Scholar]


