Abstract
Prophylactic ureteric stenting has been shown to reduce ureteric leaks and collecting system obstruction following renal transplantation and is in widespread use. However, the optimal time for removal of ureteric stents after renal transplantation remains unclear. Aim of this study was to compare the result of early versus late removal of ureteric stents after kidney transplantation of the laparoscopically retrieved live related donor grafts. Eligible patients were live donor kidney transplant recipients with normal urinary tracts. All recipients underwent extravesical Lich–Gregoire ureteroneocystostomy over 4F/160 cm polyurethane double J stents by a uniform technique. They were randomized on seventh postoperative day for early removal of stents on postoperative day 7 (Group I), or for late removal on postoperative day 28 (Group II). The incidence of urinary tract infections, asymptomatic bacteriuria, and urological complications were compared. Between 2007 and 2009, 130 kidney transplants were performed at one centre of which 100 were enrolled for the study, and 50 each were randomized into the two groups. Donor and recipient age, sex, native renal disease, immunosupression, number of rejection episodes, and antirejection therapy were similar in the two groups. The occurrence of symptomatic urinary tract infection during the follow-up period of 6 months was significantly less in the early stent removal group [5 out of 50 (10%) in Group I, vs 50 out of 15 (30%) in Group II, P=0.02]. Asymptomatic bacteriuria was documented in 2 out of 50 (4%) in Group I and 4 out of 50 (8%) in Group II (P=0.3). There was no statistically significant difference in the rate of ureteric leak, ureteric obstruction, or hematuria in the two groups (P=1.0). We conclude that, in kidney transplant recipients of laparoscopically retrieved live donor grafts, early stent removal at the end of first week reduces the incidence of urinary tract infection without increasing the rate of urine leak or ureteric obstruction.
Keywords: Renal transplantation, ureteric leak, ureteric stenting, urinary tract infections
Introduction
Major urologic complications such as urine leak and ureteric obstruction continue to cause significant morbidity after renal transplantation, albeit a steady decrease in its incidence to less than 10% in recent years;[1] this decrease may be due to the almost universal use of ureteric stents and adaptation of the technically sound extravesical Lich–Gregoire ureteroneocystostomy technique.[1,2] Two metanalysis have recommended universal prophylactic stent insertion with endoscopic removal at a designated time after transplantation in an effort to reduce urologic complication rates.[1,3] However, the benefits of prophylactic stenting may be outweighed by their complications that include increase in the incidence and severity of urinary tract infections (UTI), hematuria, stent migration, stent encrustation, and forgotten stents.[4–8] As a consequence, many experienced units employ a policy of selective stenting for difficult vesicoureteric anastomosis.[9] In recent years, there has been an increase in the number of kidneys procured through laparoscopic live donor nephrectomy that appeared to incur an increased rate of ureteric complication rates in its learning phase.[10,11] Most units employ prophylactic ureteric stents when transplanting live donor kidneys procured by laparoscopic techniques.
Although it is established that the prolonged presence of stents is likely to increase the incidence of stent related complications, the optimal time of stent removal is debatable. As ureteric stents are more likely to prevent complications that occur in the first week, routine stent insertion with early removal may simultaneously reap the benefit of a prophylactic stent, at the same time obviating its adverse effects due to its sustained presence. We conducted a randomized controlled trial of routine ureteric stenting with early versus late removal in recipients of kidney transplants from laparoscopically retrieved live related donor allografts.
Patients and Methods
Between January 2007 and December 2009, 100 consecutive consenting patients undergoing living donor renal transplantation were included in the study, if they did not have any exclusion criteria. Patients were excluded within 7 days following transplantation if any of the following was detected: a leak from the vescicoureteric anastomosis diagnosed by urine leak from the wound, by the presence of perinephric urine collection (confirmed as urine by biochemical analysis of the fluid), or the presence of a radiologically confirmed leak on a nephrostogram; transplant kidney hydronephrosis on ultrasound scan; impaired graft function defined as creatinine rise more than 25% due to delayed graft function (DGF) or rejection, or was unwilling to participate in the study. DGF was defined as the need for dialysis during the first week after kidney transplantation. Diagnosis of acute rejection was made if it was biopsy proven. Patients were randomized by computer-generated random numbers created by study coordinator and kept in sealed opaque envelopes that were opened on the seventh postoperative day by nurses in the transplant ward. Patients were assigned to the early-stent removal group (Group I) or to a late stent removal group (Group II). In the former, stents were removed on the same day of randomization while in the latter, the ureteric stent was removed on the 28th day, both under local anesthesia after giving injection Ceftazimide 1 gm intravenously 30 min before the procedure. The protocol was reviewed and accepted by the institution's ethics committee.
Surgical technique
All donor nephrectomies were laparoscopic procedures. Great care was taken to preserve adequate periureteric tissue and to avoid dissection in the “golden triangle” defined by the gonadal vein stump, renal helium, and lower pole of the kidney. The gonadal vein was not routinely preserved with the graft. Renal arteries supplying the lower pole of the kidney were always reconstructed in the recipient by end to side anastomosis to the external iliac artery. The ureter was shortened to facilitate the creation of a nonredundant, tension free anastomosis, and all recipients underwent extravesical Lich–Gregoire ureteroneocystostomy. The ureter-to-mucosa anastomosis was performed using running 5-0 PDS, and the bladder muscle was closed with interrupted sutures of 3-0 PDS. All patients received 4F/16 cm Poluurethane double J stents (Biorad, Bangaluru). An 18 Fr Foley's catheter was routinely inserted intraoperatively and removed on the sixth postoperative day. Surgical drains were used in all patients and were removed when drainage was less than 30 ml/day of serious fluid.
Antibiotics, immunosuppression, and other drugs
All patients received prophylactic antibiotics (Injection Cefotaxme 1 gm every 12 h) for 48 h after surgery and sulfamethaxazole/trimethoprim (400/80 mg) half a tablet daily at night for 6 months for urinary tract infection prophylaxis. A standard triple immunosuppressive regimen using Cyclosporine (5 mg/kg/day), Azathioprine (2 mg/kg/day), and Prednisolone (0.5 mg/kg/day) were given to all patients. IL-2 receptor blocker (Daclizumab) induction was added for patients designated to be at high immunologic risk based on HLA mismatch, followed by maintenance therapy compromising Tacrolimus, Prednisolone, and Azathioprine. Azathioprine was substituted with Mycophenolate Mofetil (35 mg/kg/day) in patients with DGF. Episodes of biopsy proven rejection were treated with three intravenous doses of Methylprednisolone (500 mg/day).
Follow up
Urological complications within the first 6 months of surgery were prospectively identified. During the period, patients were evaluated with urine culture on the seventh day, at 3 weeks, 3 months, and 6 months and at any time if there was fever or symptoms of UTI. A diagnosis of UTI was made if there were any two of the following three criteria: (1) fever (oral temperature >99°F), (2) positive urine culture (>100 000 colony forming unit/ml), and (3) symptoms of UTI (dysuria, pain over the graft, or suprapubic area). Ultrasonogram of graft kidney was done on the fifth postoperative day, 4 weeks, 3 months, and 6 months after transplantation or at any time if a rise of serum creatinine was noted. DTPA Renogram was done on the fifth postoperative day and after 6 months.
Statistical analysis
The two groups were compared by Chi-square test and P value was calculated; P, of <0.05 was considered statistically significant.
Results
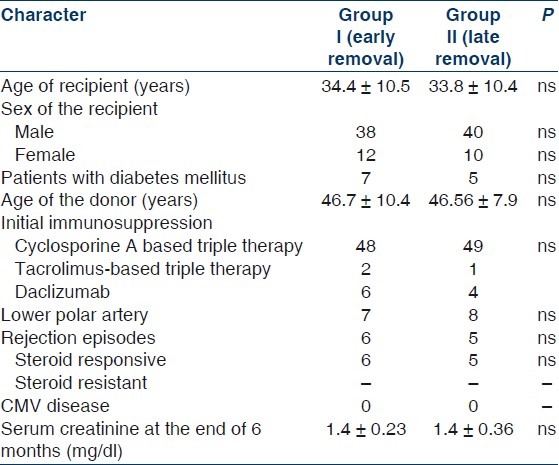
During the study period, 130 transplants were performed; 100 eligible patients were enrolled. Fifty were randomized to Group I and 50 to Group II. Baseline characteristics were similar in the two groups [Table 1].
Table 1.
Comparison of the two groups (early vs late removal of ureteric stent)
The incidence of urological complications is shown in Table 2. Five patients (10%) in Group I (early stent removal) and 15 patients (30%) in Group II (late stent removal) developed symptomatic UTI (P value=0.02) within the follow-up period of 6 months [Figure 1]. Bacteriological profiles of UTI are shown in Figure 2. Asymptomatic bacteriuria was documented in 2 (4%) among the 50 patients in Group I and four (8%) in Group II (P value: 0.3). One patient in Group I had ureteric leak after stent removal that was managed by percutaneous nephrostomy; this patient recovered well and had no further complications. There was no ureteric leak in patients with late stent removal. There was no statistically significant difference in incidence of ureteric leak (P value 1.0). There was no case of stent migration breakage, stent related obstruction or hematuria in either group.
Table 2.
Urinary complications in the two groups (early vs late removal of ureteric stent)
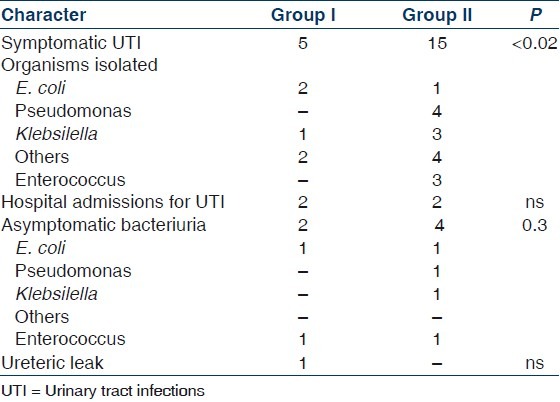
Figure 1.
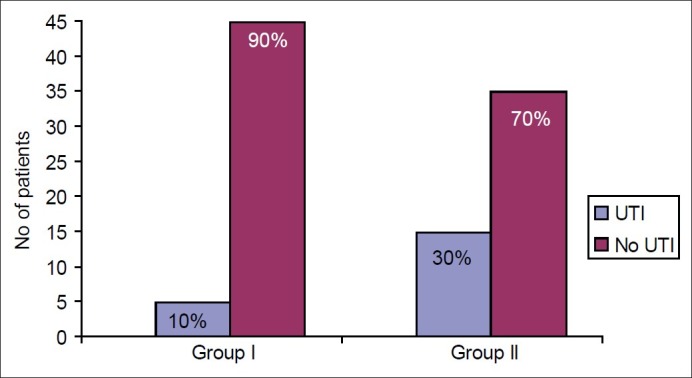
Incidence of urinary tract infections (UTI) (early (Group I) vs late (Group II) removal of ureteric stent). P value=0.02
Figure 2.
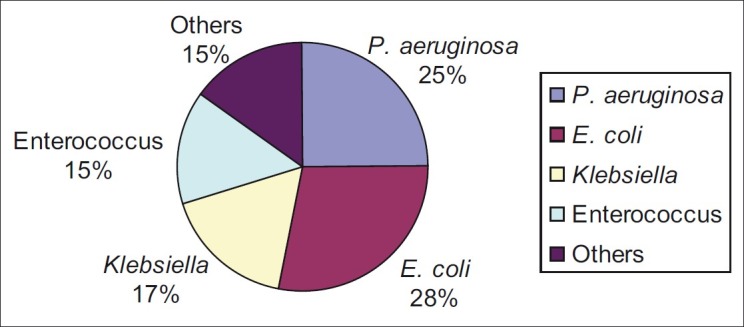
Organisms causing urinary tract infections
Six patients in Group I and five in Group II had acute rejection and required antirejection therapy with parenteral methyl prednisolone. There was no statistical difference in the number of kidneys with lower polar arteries supplying the ureter between the two groups (seven in Group I and eight in Group II). There was no graft or patient loss during the period of the study (6 months). The requirement of hospital admissions for urinary infections was similar in the two groups.
Discussion
In this randomized trial, early removal of ureteric stents resulted in lower incidence of UTI within the first 6-month period. Removal of stents, one week following transplantation did not appear to cause an increase in the ureteric complications such as urine leak or ureteric stenosis. We excluded patients with urine leak that occurred early within the first week from this study because of their likely technical error either at retrieval or implantation. We excluded patients with DGF, as we did not want to confuse the issue by another factor that may produce further worsening of the graft function. We believe, if there is no obvious technical fallibility evident within the first week, it may be wiser to remove the stent at 7 days than to keep it for longer periods.
Concerns have been raised in the literature regarding the complications of stents kept for long periods, such as UTI,[12,13] calcification,[14] bleeding,[12] stent migration, ureteric obstruction, and discomfort.[15] In a study conducted by Glazier et al., in the mid-1980s, it was concluded that the use of ureteral stents is safe, but is associated with an increased incidence of urinary tract infection.[16] To reduce infection rates, they recommended stent removal within 14 days, and earlier if possible, particularly in diabetic patients who have received a cadaveric renal transplant.[16] A more recent metanalysis too recommended routine stenting and removal of stents 2 weeks after renal transplantation.[17] In our study, the presence of diabetes did not appear to be a risk factor for increased incidence of UTI, although the number might have been too small to detect this difference. Routine stent removal at one week, prior to the discharge from hospital obviates the risk of forgotten stent, as well as curtails the cost of a second admission for stent removal.
The debate on routine ureteric stenting in renal transplantation continues. A recent metanalysis of seven randomized trials (1154 patients) demonstrated the clinical usefulness of routine ureteric stenting following renal transplantation.[3] Recipients of transplants from living donors have been previously shown to be at higher risk of ureteric complications due to risk of damage to ureteric vascular supply.[8,18] Additionally in the early phase of laparoscopic donor nephrectomy, there were reports of significantly increased ureteric complications in the recipients.[10,11] Thus, the case for routine prophylactic stenting in the era of laparoscopic live donor nephrectomy becomes stronger. All our kidneys were retrieved from live donors laparoscopically. Although preservation of gonadal vein with the specimen has been claimed to be important in the preservation of ureteric blood supply,[19] we did not routinely do so. However, great care was taken to preserve the periureteric fat with its mesentery and to avoid skeletonizing the upper ureter in the “golden triangle” in order to maintain its perfusion. Our overall incidence of ureteric leak was low (1%), probably in part due to the routine use of stents. Its early removal at one week did not increase the risk of delayed ureteric leak or obstruction in this study.
Other factors reported to be associated with ureteric complications include acute rejection, corticosteroid use, and CMV disease.[20,21] There were no differences between the two groups in any of these in our study.
Conclusions
In this prospective randomized trial of kidney transplant recipients of live donor allografts retrieved laparoscopically, early removal of prophylactic ureteric stents 1 week following transplantation significantly decreased the incidence of urinary tract infections occurring in the first 6 months after transplantation. Early removal of stents did not result in increased incidence of ureteric leak or ureteric obstruction. We feel that during the first week following transplantation when healing of urethrovesical anastomosis is expected to occur, the presence of DJ stent may reduce the incidence of ureteric leaks.
We conclude that in kidney transplant recipients of laparoscopically retrieved live donor grafts, early stent removal at the end of first week reduces the incidence of urinary tract infection without increasing the rate of urine leak or ureteric obstruction. Hence, we recommend ureteric stent removal one week after transplant surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mangus RS, Haag BW. Stented versus non stented extravesical ureteroneocystostomy in renal transplantation: A metaanalysis. Am J Transplant. 2004;4:1889–96. doi: 10.1111/j.1600-6143.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 2.Whang M, Geffner S, Baimeedi S, Bonomini L, Mulgaonkar S. Urologic complications in over 1000 kidney transplants performed at the Saint Barnabas healthcare system. Transplant Proc. 2003;35:1375–7. doi: 10.1016/s0041-1345(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 3.Wilson CH, Bhatti AA, Rix DA, Manas DM. Routine intraoperative stenting for renal transplant recipients. Transplantation. 2005;80:877–82. doi: 10.1097/01.tp.0000181197.21706.fa. [DOI] [PubMed] [Google Scholar]
- 4.Khanna P, Abraham G, Mohamed Ali AA, Miriam PE, Mathew M, Lalitha MK, et al. Urinary tract infections in the era of newer immunosuppressant agents: A tertiary care center study. Saudi J Kidney Dis Transpl. 2010;21:876–80. [PubMed] [Google Scholar]
- 5.Ranganathan M, Akbar M, Ilham MA, Chavez R, Kumar N, Asderakis A. Infective complications associated with ureteral stents in renal transplant recipients. Transplant Proc. 2009;41:162–4. doi: 10.1016/j.transproceed.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Osman Y, Ali-El-Dein B, Shokeir AA, Kamal M, El-Din AB. Routine insertion of ureteral stent in live-donor renal transplantation: Is it worthwhile? Urology. 2005;65:867–71. doi: 10.1016/j.urology.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Verma BS, Bhandari M, Srivastava A, Kapoor R, Kumar A. Optimum duration of JJ stenting in live related renal transplantation. Indian J Urol. 2002;19:54–7. [Google Scholar]
- 8.Kumar A, Kumar R, Bhandari M. Significance of routine JJ stenting in live related renal transplantation: A prospective randomized study. Transplant Proc. 1998;30:2995–7. doi: 10.1016/s0041-1345(98)00902-6. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez J, Clase CM, Mahalati K, MacDonald AS, McAlister VC, Belitsky P, et al. Is routine ureteric stenting needed in kidney transplantation? A randomized trial. Transplantation. 2000;70:597–601. doi: 10.1097/00007890-200008270-00011. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira JM, Cangro CB, Fink JC, Schweitzer E, Wiland A, Klassen DK, et al. A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation. 1999;67:722–8. doi: 10.1097/00007890-199903150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Philosophe B, Kuo PC, Schweitzer EJ, Farney AC, Lim JW, Johnson LB, et al. Laparoscopic versus open donor nephrectomy: Comparing ureteric complications in the recipient and improving the laparoscopic technique. Transplantation. 1999;68:497–502. doi: 10.1097/00007890-199908270-00009. [DOI] [PubMed] [Google Scholar]
- 12.Bassiri A, Amiransari B, Yazdani M, Sesavar Y, Gol S. Renal transplantation using ureteral stents. Transplant Proc. 1995;27:2593–4. [PubMed] [Google Scholar]
- 13.Pleass HC, Clark KR, Rigg KM, Reddy KS, Forsythe JL, Proud G, et al. Urologic complications after renal transplantation: A prospective randomized trial comparing different techniques of ureteric anastomosis and the use of prophylactic ureteric stents. Transplant Proc. 1995;1:1091–2. [PubMed] [Google Scholar]
- 14.Haab F, Pedron P, Gattegno B, Thibault P. Re: Insertion of a double pigtail ureteral stent for the prevention of urologic complications in renal transplantation: A prospective randomized study. J Urol. 1997;158:888. [PubMed] [Google Scholar]
- 15.Benoit G, Blanchet P, Eschwege P, Alexandre L, Bensadoun H, Charpentier B. Insertion of a double pigtail ureteral stent for the prevention of urological complications in renal transplantation: A prospective randomised study. J Urol. 1996;156:881–4. [PubMed] [Google Scholar]
- 16.Glazier DB, Jacobs MG, Lyman NW, Whang MI, Manor E, Mulgaonkar SP. Urinary tract infection associated with ureteral stents in renal transplantation. Can J Urol. 1998;5:462–6. [PubMed] [Google Scholar]
- 17.Mongha R, Kumar A. Transplant ureters should be stented routinely. Indian J Urol. 2010;26:450–3. doi: 10.4103/0970-1591.70594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insall RL, Bell R, Hutchison BG, Haywood EF, House AK. A method for the treatment of ureteric complications following renal transplantation. Aust N Z J Surg. 1995;65:654–7. doi: 10.1111/j.1445-2197.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 19.Kocak B, Baker TB, Koffron AJ, Leventhal JR. Ureteral complications in the era of laparoscopic living donor nephrectomy: Do we need to preserve the gonadal vein with the specimen? J Endourol. 2010;24:247–51. doi: 10.1089/end.2009.0414. [DOI] [PubMed] [Google Scholar]
- 20.Shoskes DA, Hanbury D, Cranston D, Morris PJ. Urological complications in 1,000 consecutive renal transplant recipient. J Urol. 1995;153:18–21. doi: 10.1097/00005392-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Karam G, Maillet F, Parant S, Soulillou JP, Giral-Classe M. Ureteral necrosis after kidney transplantation: Risk factors and impact on graft and patient survival. Transplantation. 2004;78:725–9. doi: 10.1097/01.tp.0000131953.13414.99. [DOI] [PubMed] [Google Scholar]


