Abstract
Wound bed preparation has been performed for over two decades, and the concept is well accepted. The ‘TIME’ acronym, consisting of tissue debridement, infection or inflammation, moisture balance and edge effect, has assisted clinicians systematically in wound assessment and management. While the focus has usually been concentrated around the wound, the evolving concept of wound bed preparation promotes the treatment of the patient as a whole. This article discusses wound bed preparation and its clinical management components along with the principles of advanced wound care management at the present time. Management of tissue necrosis can be tailored according to the wound and local expertise. It ranges from simple to modern techniques like wet to dry dressing, enzymatic, biological and surgical debridement. Restoration of the bacterial balance is also an important element in managing chronic wounds that are critically colonized. Achieving a balance moist wound will hasten healing and correct biochemical imbalance by removing the excessive enzymes and growth factors. This can be achieved will multitude of dressing materials. The negative pressure wound therapy being one of the great breakthroughs. The progress and understanding on scientific basis of the wound bed preparation over the last two decades are discussed further in this article in the clinical perspectives.
KEY WORDS: Chronic wound, negative pressure therapy, ulcer, wound, wound bed
INTRODUCTION
The concept of wound bed preparation has been around for more than two decades and has gained international recognition for the management of wounds, especially those that are difficult to treat and are slow to heal. This concept is not static; it is dynamic and rapidly evolving, providing a framework for a structured approach to wound management.
Normal wound healing is a complex series of overlapping events that are interlinked and interdependent. The phases of wound healing are well described and consist of coagulation, inflammation, proliferation and remodelling. Unfortunately, not all wounds will follow this complex model of wound healing. In this article, the authors would like to revisit the concept of wound bed preparation and its definition, aims and importance and to discuss the elements, principles and available interventional options with an emphasis on the clinical perspective.
The definition of wound bed preparation
Wound bed preparation is a concept emphasizing a holistic and systematic approach to evaluate and remove barriers to the healing process to allow the wound healing process to progress normally.[1] It guides the development of appropriate treatment strategies targeting both the patient in general and the underlying disease that caused the wound. To this end, therapeutic agents are optimized to accelerate endogenous healing or increase the effectiveness of advanced therapies.[2]
The goal of wound bed preparation
The overall goal of wound bed preparation is to create an optimal wound healing environment by producing a well-vascularized, stable wound bed with little or no exudates.[3,4] The wound bed preparation is particularly applied to address chronic wounds that fail to progress through the normal healing process. It is performed via removing senescent or abnormal cells, reducing the bacterial load, decreasing the level of wound exudates and increasing the formation of healthy granulation tissue. When these goals are met, the final phase of wound healing will occur.
Why is wound bed preparation important?
Wound bed preparation was initially developed for chronic wounds to maximally benefit from modern but costly wound therapies. It has had positive impacts on the way in which chronic wounds are managed. This concept has helped identify the major components of chronic wound care and organize these components to create a purposeful approach to wound care intervention that utilizes current medical standards and practices. Wound bed preparation also has the potential to ensure that full benefits are derived from advanced wound-care products to overcome the barriers to wound improvement.[5]
The origins of wound bed preparation
The concept of wound bed preparation was introduced by Dr. Vincent Falanga (Professor of Dermatology and Biochemistry at the Boston University School of Medicine) and Dr. Gary Sibbald (Professor of Public Health Sciences and Medicine at the University of Toronto) in 2000 based on their extensive experience in the management of chronic wounds.[3,6] It initially focused on the management of the wound exudates, bacterial balance and devitalized tissue. In 2003, the International Wound Bed Preparation Advisory Board established an algorithmic approach to this process with the development of the ‘T.I.M.E.’ acronym.[7,8] Dr. Sibbald updated the concept in 2006 with an emphasis on treating the cause and patient general factors that impair wound healing and on patient-centred concerns prior to treating the local wound factors.[9] It was further updated and made more comprehensive in 2011 with links to evidence-based literature, expert opinions and clinical practice-based strategies.[10]
The components of wound bed preparation
There are four components of wound bed preparation, which address the different pathophysiological abnormalities underlying chronic wounds:
Tissue management
Inflammation and infection control
Moisture balance
Epithelial (edge) advancement.
The T.I.M.E. framework comprises the comprehensive strategies that can be applied to the management of different types of wounds to maximize the potential for wound healing.
Wound bed preparation in acute, subacute and chronic wounds
There are diverse wound patterns in acute wounds, ranging from post-traumatic abrasions, lacerations and burns to high-energy-explosive wounds. In acute wounds, the normal healing process has not been impaired and healing is ensured if the wound conditions are not compromised. Foreign bodies that may have been embedded in these wounds should be identified and removed to avoid infection. Crushed or devitalized tissue should be debrided early and aggressively for soft tissue closure or reconstruction. Because bacterial contamination or colonization is more common during the early stage of an acute wound (before infection sets in) debridement of any contaminated tissue should be performed to minimize the risk for subsequent infection. In heavily contaminated wounds, delayed closure or secondary healing may be preferable to immediate repair.
Subacute wounds describe another spectrum of wounds that are not often addressed. The subacute period is generally defined between 72 hours and 3 months of injury. Higher infection rates have been noted in those wounds reconstructed during the subacute period.[11]
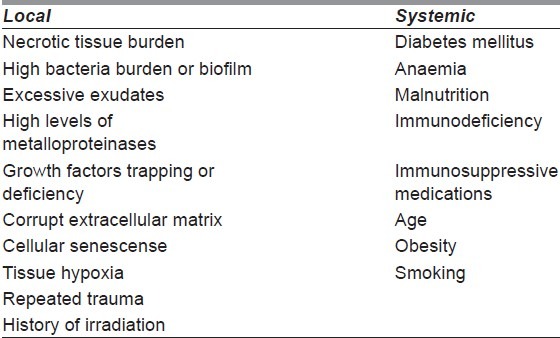
Chronic wounds are wounds that are arrested during certain phases of wound healing and fail to heal in a timely manner, even with standard wound care. Thus, when wounds fail to heal within 3 months, they are considered chronic wounds. There are systemic and local factors that affect the chronic wound healing process[2,12,13] [Table 1].
Table 1.
Local and systemic factors affecting wound healing
Treating the patient before treating the wound
Ligresti et al. has emphasized that the general condition of the patient is a critical part of the treatment scheme for difficult wounds.[14] Because the systemic factors of the patient may delay wound healing, these factors have to be optimized as the initial step in wound bed preparation.[2,15] The patient is assessed with a systematic holistic approach, including physical, psychological and social aspects. The healing ability of the patient has to be optimized.[9] Glycaemic control in diabetes mellitus, the elimination of concurrent medications that impair wound healing, such as steroids or other immunosuppressive medications, the correction of anaemia and the cessation of smoking are among the most important systemic factors to be addressed before focusing on local wound factors. Optimization of nutritional status with adequate protein, antioxidants and trace elements is necessary to ensure healing wound progress in the right direction. Chronic diseases, such as connective tissue diseases, that affect wound healing should be treated and controlled. The vascularity of the extremities is a regional factor to be determined as a wound will heal only if its blood supply is adequate. The pain due to the wound is controlled to ensure that the patient complies with the treatment plan.[9] Other unfavourable features like repeated movements over the joints and pressure point will not allow wound bed preparation for wound healing.
WOUND BED PREPARATION ACCORDING TO AIMS
Management of tissue necrosis
The nonviable necrotic tissue overlying the wound base obscures the assessment of the depth and condition of the wound. In addition, the presence of necrotic tissue is a nidus for bacterial growth and serves as a physical barrier that may obscure the signs of local wound infection. Bacterial colonies in necrotic tissue can produce damaging metalloproteinases that adversely affect extracellular matrix components during the healing process. The bacteria may also compete for the scarce local resources (oxygen, nutrition and building blocks) that are necessary for wound healing.
Tissue management is the process of removing necrotic or devitalized tissue, bacteria and cells that impede the healing process to reduce wound contamination and tissue destruction. The aim is to restore a viable wound base with a functional extracellular matrix. Chronic wounds are converted into acute wounds with the removal of the necrotic burden of senescent cells, the extracellular matrix, inflammatory enzymes and biofilms that contain bacterial colonies[15] [Figure 1a,b].
Figure 1.
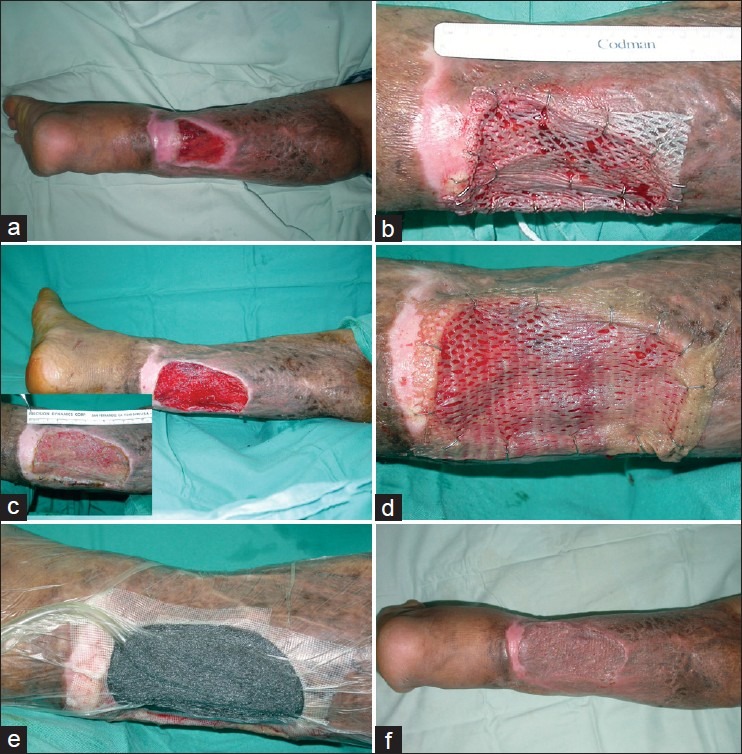
This case demonstrated a multimodality approach for chronic non-healing wound. (a) A 29-year-old man with traumatic injury of his right leg with chronic wound over 3 years. (b) The wound was converted to an acute wound by sharp surgical debridement and covered with skin allograft as a ‘take’ test for skin autograft. (c) The skin allograft prepared the wound bed well and (inset) shows signs of graft rejection at 2 weeks. (d) Split thickness skin graft was applied and (e) secured with negative pressure wound therapy system. (f) Final appearance of the wound after 3 months
The options for debridement include surgical, mechanical, autolytic, enzymatic and biological methods. The surgical method is the fastest mean and allows the accurate assessment of the severity and extent of the wound. It is particularly important in life- or limb-threatening infections with necrotic eschar or gangrene. Surgical debridement is also indicated for wounds with extensive or adhering eschar, in which the rapid clearance of necrotic tissue is required. However, it is non-selective because normal healthy tissue may be removed at the same time.[15] It may also be limited by the bleeding tendency and pain tolerance of the patient and the availability of surgical expertise.
Versajet hydrosurgery (Smith and Nephew, Tampa, FL) represents a technical advance in surgical debridement that uses tangential hydro-dissection. The energy of the saline beam of the Versajet tangentially excises the wound surface with minimal damage to the surrounding healthy tissue. It is as effective in removing bacteria as the high-powered pulse lavage systems.[16] It improves treatment outcomes by decreasing the number of surgeries required for the surgical wound bed preparation of acute and chronic wounds.[17] Versajet has been noted to be a feasible, simple and safe technique that hastens surgical debridement of burns and adds more precision in burn wound excision.[18]
Mechanical debridement involves methods such as wet-to-dry dressing and pressure irrigation. Wet-to-dry dressing is performed by leaving wet gauze in direct contact with wound surfaces and removing it when dry together with any adhering slough tissue. It causes excessive pain as well as bleeding and removes the new healing epithelium when the dressing is changed. Pressure irrigation is the forced irrigation of saline from a syringe through cannulas to remove necrotic tissues that are loose and superficial. However, the irrigation of wounds should not be used when the solution may collect and be trapped in dead spaces.[10]
Edinburg University Solution of Lime (EUSOL) commonly used traditionally for wound debridement is a cheap bleaching and antiseptic agent containing calcium hypochloride and boric acid. The solution was developed around the first world war initially for the treatment of trench foot.[19] EUSOL usually administered as wet-to-dry dressing three to four times a day for chemical debridement of a sloughy wound. Although it has been a subject of controversy over the past two to three decades due to its tissue toxicity and delay healing of clean granulating wound, it still have some role in wound debridement if properly administer by a familiar hand.
Autolytic debridement uses the inherent ability of the body to digest and remove necrotic tissue. A moist-retention dressing is applied to provide a moist wound healing environment that allows the necrotic tissue to be liquefied by endogenous enzymes or phagocytic cells. The application of Hydrogel that is covered by a polyurethane film is an example of the dressing used for this type of debridement. This method is relatively easier to perform, requires limited technical skills and involves minimal pain. However, it is time consuming and carries the risk of invasive infection and wound edge maceration. It is indicated in wounds with a minimal necrotic load or that need more aggressive debridement requiring anaesthesia and in patients who are unable to tolerate pain.[5]
Enzymatic debridement uses manufactured enzyme, such as collagenase and papain-urea, as debriding agents to dissolve necrotic tissue. Papain is a broad-spectrum enzyme that is useful for bulk debridement, whereas collagenase is gentler on viable cells. Enzymatic debridement is suitable for nonsurgical patients and can be effectively combined with moist wound healing. However, it is expensive and has a limited role in the treatment of selected chronic wounds.
In biological debridement, the larvae of green butterfly (Lucila serricata) digest the necrotic tissue and secrete bactericidal enzymes. It is effective in methicillin-resistant staphylococcus aureus (MRSA) and beta haemolytic streptococcus. Biological debridement is considered to be a secondary debridement method after surgical debridement or for patients who are not fit for surgical debridement. The uncomfortable feeling generated by this treatment makes it unpopular.
The debridement method should be chosen based on the general patient conditions, wound status, skills of the clinician and availability of resources. Selection of the right method of debridement for a particular type of wound is important to avoid further delays in healing, increases in patient suffering and unnecessary costs of care.[20]
Restoration of the bacterial balance
Chronic wound beds are often colonized by various species of bacteria or fungal organisms due to the prolonged opening of the wound, poor blood flow and underlying disease process. The bacterial balance is achieved by controlling the bacterial burden in terms of its density and pathogenicity.[15] The presence of bacteria in the wound beds ranges from contamination, colonization and critical colonization to invasive infection.
Identifying critical colonization is important because it is the level when wound healing begins to be delayed, even before the occurrence of invasive infection. Critical colonization means the presence of replicating microorganisms that are beginning to cause local tissue damage. It is the point at which the host defences are unable to maintain the balance of organisms at colonization. It is noted clinically by signs such as a change in the colour of the wound bed, friable and unhealthy granulation tissue, abnormal odour, increased serous exudate and pain at the wound site.
Bacterial levels of 106 or more per gram of tissue are generally considered as an infection because wound healing is adversely affected. The presence of replicating microorganisms in the wound causes injury to the host due to the release of toxins, competitive metabolism and inflammation. In acute and subacute wound, infection is recognized clinically as local signs of advancing redness, warmth of the skin surrounding the wound, oedema, increasing pain and tenderness, a foul odour and increased or purulent drainage. Systemic signs include fever, tachycardia and even changes in mental status if sepsis set in. The patient may have an increased white blood cell count. However, chronic wounds more than 3 months old are less likely to have advancing inflammation and constitutional symptoms.[21] Chronic wounds will be devoid of constitutional symptoms. Chronic wound infection is recognized by an increasing ulcer size, increasing exudate production and friable unhealthy granulation tissue.
As chronic wounds are often colonized by bacteria, obtaining and interpreting laboratory data should be performed in correlation with the clinical findings. Although a tissue biopsy may be more ideal, a properly performed deep wound swab is also useful. Apart from a quantitative bacterial count, the presence of four or more organisms in the wound bed can be predictive of delayed wound healing as certain organisms exhibit synergism.[22]
The critically colonized wound should be treated with topical antimicrobial dressings. Sustained-release silver dressings have gained in popularity due to their efficacy, low resistance and broad-spectrum antimicrobial actions, especially when pseudomonas or MRSA infection is a concern. The wound should be cleansed with low toxicity topical antiseptic solutions, e.g. normal saline or chlorhexidine solution, instead of cytotoxic solutions such as povidone-iodine. Topical antiseptics have the advantages of a broad spectrum of bacterial coverage and delivery in high concentrations directly to the wound bed. Wound debridement is an important adjunct as it directly reduces the bacterial burden, including the biofilms. Biofilms are bacterial colonies surrounded by a protective coat of polysaccharides, more easily resistant to the action of antimicrobials. The periodic release of motile bacteria from these colonies may result in infection. Systemic antibiotics are only indicated in the presence of invasive wound infection or sepsis. The host systemic factors that cause immunosuppression should also be addressed, e.g. in controlling diabetes mellitus. Other factors that have clinical implication on wound bed preparation include bone exposure complicated with underlying osteomyelitis. This may require extended systemic antibiotic and surgical management such as debridement, and adequate soft tissue coverage.
Achieving moisture balance
One beneficial effect of a moist wound environment is accelerating wound re-epithelization. In addition, keeping a wound moist does not increase the infection rate. These principles are fundamental parts of moisture balance in wound bed preparation.[1] Achieving moisture balance involves the creation and maintenance of a warm, moist wound bed and the stimulation of components in the moisture that have a positive impact on wound healing, such as growth factors. An appropriate amount of moisture is needed for the optimal effects of growth factors and cytokines and for the growth of proliferating cells, such as keratinocytes, endothelial cells and fibroblasts. On the one hand, excessive wound fluids contain matrix metalloproteinases and serine proteases that can break down or damage essential extracellular matrix materials. Excessive moisture also may lead to the maceration of the wound edges. On the other hand, inadequate moisture may inhibit cellular activities and promote eschar formation.[10] Thus, moisture balance is a delicate process of maintaining a moist healing environment that is optimal for healing. This requirement has led to the development of a vast array of moisture-retentive dressings that promote moist wound healing, ranging from occlusive, semiocclusive, absorptive and hydrating dressings.
Exudates can be managed directly via the use of a number of dressing materials, depending on the moisture status of the wound bed. For example, in a highly exudative wound, an absorptive dressing such as foam will be appropriate, whereas in a dry wound eschar, an occlusive or semi-occlusive dressing such as a hydrocolloid will be suitable to achieve the appropriate moisture balance. The use of compression and limb elevation to eliminate fluid from the wound site should be applied in venous ulcers or in wounds with surrounding oedema. The moisture balance can be achieved indirectly via systemic therapy that reduces oedema, such as in heart failure, or the use of medication to reduce the inflammatory response in certain diseases.[23]
Biological dressing, such as a skin allograft, can help manage chronic wounds. It forms a mechanical barrier against fluid, protein and electrolyte losses, thus preventing tissue desiccation and also microbial invasion. A skin allograft can also be used as a ‘take’ test prior to autologous skin grafting[24,25] [Figure 1b,c].
Negative pressure wound therapy is an important device that can be used to manage a heavily exudating wound. Foam dressing as a wound filler can maintain adequate moisture in the wound bed while draining the excess wound exudates. Negative pressure wound therapy can also reduce oedema, contributing to improved tissue perfusion. Negative pressure wound therapy also plays an important role in wound bed preparation by reducing the size and complexity of the wounds. The wound immediately contracts (macro-deformation) once the negative pressure is applied. It has micro-deformation effects due to the stretching of small tissue blebs into the foam dressing. These mechanical effects change the cytoskeleton, resulting in cascades of biologic effects, including the stimulation of angiogenesis, reduction of bacterial loads and formation of granulation tissue.[26] This therapy also can be used as a tool for mechanical and autolytic wound debridement as the necrotic tissue adhering to the foam dressing is removed during the dressing changes and the moist wound bed enables the autolytic action of endogenous proteinases present within the moist environment, respectively [Figure 2].
Figure 2.
This is a case of 62-year-old lady with diabetic foot ulcer over the antero-medial aspect of her left ankle. She was referred to our service for wound management. (a) Initial appearance of the wound during first consultation: the wound bed contains mild to moderate amount of slough, pale granulation tissue and exposed tendon. (b) After four cycles of negative pressure wound therapy, the wound size has contracted significantly with healthy red and stable granulation tissue on the wound bed. (c) Six weeks after initiation of treatment the wound has contracted and epithelialized significantly.
Epithelial advancement
The progression of the wound edge in terms of epidermal cell/keratinocyte migration and wound contraction is one of the key indicators of a healing wound. If there is an arrest of these processes, the clinicians should consider the earlier elements discussed (T.I.M.E.), including cellular dysfunction and biochemical imbalances-discussed below, as possible reasons for the failure of wound healing. For instant, keratinocytes will not be able to proliferate and migrate over necrotic tissue, biofilm, hypergranulation tissue, fibrinous slough, presence of callosity or hyperkeratotic edges. These adverse environments should be removed by proper debridement. Control of infection as well as excessive inflammation must be achieved to reduce the level of proteases to a normal level therefore maintaining delicate biochemical balance required for epithelial cells replication. An optimal moisture level of the wound as mentioned earlier will promote epithelialization as evidence by advancing wound edge. Microscopically, cellular senescent may present at the edge of chronic wound that need intervention to achieve healing. With the advance concept of wound bed preparation and its scientific basis, many clinicians acknowledge the problem and take appropriate action needed.
Correction of cellular dysfunction and restoration of the biochemical balance
Other components of wound bed preparation, especially when dealing with chronic, non-healing wounds, are the correction of cellular dysfunction and the restoration of the biochemical imbalance. For multiple reasons, chronic wounds are believed to be stuck in a prolonged inflammatory phase, which results from a variety of factors, including cellular dysfunction and dysregulation caused by the abnormal expression of extracellular matrix molecules and growth factors.
In chronic wounds, the healing process has failed to proceed in an orderly and timely manner to produce anatomic and functional integrity,[12] and these chronic wounds are characterized by a defective remodelling of the extracellular matrix, prolonged inflammation and failure to re-epithelialize.[27–29] In contrast, the normal wound healing process involves complex interactions between cellular components, extracellular matrix molecules and biochemical mediators/enzymes, such as growth factors, cytokines and proteases. These orderly cellular and biochemical processes are lost in the chronic non-healing wound.
These processes are closely regulated, and disorder in one will affect another. The expression of extracellular matrix molecules, such as fibronectin and thrombospondin, follows a defined temporal course. However, they appear to be over-expressed in the chronic wound, which is believed to result from cellular dysfunction and dysregulation.[30]
The biochemical content of chronic-wound exudates is different from those of an acute wound. The balance of inflammatory cytokines, growth factors, enzymes and their inhibitors is altered, slowing down or even completely blocking the proliferation of cells, such as endothelial cells, fibroblasts and keratinocytes. These effects can prevent wound healing from progressing.[8]
Under normal circumstances, the levels of two pro-inflammatory cytokines, TNF-α and IL-1, peak after a few days and return to very low levels in the absence of infection. However, they are persistently elevated in non-healing wounds. When the chronic wound shows signs of healing, Schultz et al. has shown that the concentrations of inflammatory cytokines decrease to values approaching those in acute healing wounds, indicating a close correlation between low levels of inflammatory cytokines and the progression of wound healing.[8]
Fibroblasts are believed to play a pivotal role in orchestrating cellular and biochemical interactions in normal wound healing. Fibroblasts synthesize collagen fibres to provide strength to the healing wound and more importantly produce fibronectin, thrombospondin, integrins and vitronectin to form a basal lamina. These molecules are also components of the extracellular matrix, which provides a scaffold and template for endothelial cell and keratinocyte migration, for angiogenesis and for epithelialization. Fibroblasts also play a role in modulating the levels of the extracellular matrix molecules by mediating the expression of cytokines and proteases, such as matrix metalloproteinases and elastase. In the chronic wound, the fibronectin level is low due to its rapid degradation as the levels of proteases are higher than in acute wounds and due to the absence or low level of tissue inhibitor of metalloproteinases (TIMPs). Falanga showed that fibroblasts in diabetic wounds respond poorly to mitogenic stimulation with the production of low levels of various growth factors and collagen[31]
Fibroblasts isolated from a chronic wound also display cellular dysfunctions, such as the inability to respond to growth factors such as platelet-derived growth factor (PDGF)-β and transforming growth factor (TGF)-β. Several authors and studies have demonstrated that fibroblasts derived from a variety of wound beds in chronic wounds represent a premature, senescent or undifferentiated phenotype that responds inefficiently to normal stimulatory messages.[12,32–34]
Chronic diabetic wounds increase the activity of fibroblasts, leukocytes and macrophages by creating a local deficiency of glucose[35] and increase the amount of unwanted lactic acid byproducts, which are detrimental to the wound environment.[36] Macrophages isolated from the wounds of diabetic mice showed significant impairment in efferocytosis. Impaired efferocytosis was associated with a significantly higher burden of apoptotic cells in the wound tissue as well as a higher expression of pro-inflammatory cytokines and a lower expression of anti-inflammatory cytokines.[37]
Liu et al. have shown that there is impaired local angiogenesis in chronic diabetic foot ulcers due to the inadequate presence of endothelial progenitor cells. This deficiency is, however, correctable by the use of hyperbaric oxygen therapy, which enhances the mobilization of circulating endothelial progenitor cells to the wound and synergistically works with the administration of exogenous stromal cell-derived factor (SDF)-1α.[38] The failure or insufficient angiogenesis will affect cell migration and the support of the collagen synthesis necessary for the formation of mature granulation tissue and subsequent re-epithelialization.[39]
Similar to other parameters, to correct the cellular and biochemical abnormalities, the causative factor(s) should be removed or treated first. Such issues, for example, involve glycaemic control in diabetes, pressure redistribution in a pressure ulcer, revascularization in arterial insufficiency and compressive treatment in venous ulceration.[10]
One of the classical ways to manage the chronic wound is by converting the wound to an acute wound by removing the wound edges using surgery. Multiple studies using biopsies and biochemical analyses have found that the cellular dysfunction and chemical abnormalities are found in this region.[12,32,33,34,40,41] The biochemical imbalance usually can be managed by controlling the exudates by means of different dressing materials, such as negative pressure wound therapy and the multitude of modern dressings discussed above.
However, even with adequate wound bed preparation using the abovementioned techniques, there are wounds that remain recalcitrant to treatment or slow to progress. This issue may be the consequence of persistent cellular and biochemical derangement from the inappropriate activities of proteolytic enzymes, cytokines and growth factors produced within the wound, leading to prolonged inflammation, poor angiogenesis, extracellular matrix degradation and failure of cellular proliferation and migration.[42] Treatments aiming to reverse these abnormalities using advanced therapies may prove beneficial in the initiation of the healing process, as shown in several studies.[43–49]
The use of advanced treatment may be expensive and unavailable to many clinicians and practitioners, and such treatments need to be further evaluated regarding their cost-effectiveness before their use is universally accepted. In addition, they should be used in a well-prepared wound bed to achieve the maximum benefit.[8] The advanced treatment modalities being used include engineered skin constructs,[43] platelet-derived growth factor,[48] keratinocyte growth factor,[45] granulocyte-macrophage colony stimulating factor,[44] esterified hyaluronic acid,[46,48] protease modulating matrix,[50] immunoglobulin,[51] and many more. These treatments in general produce/replace growth factors, stimulate angiogenesis, modulate inflammatory cells, promote cell proliferation and control excessive protease activity.
CONCLUSION
Over the last two decades, the approach to wound management has progressed and evolved, especially with a better understanding of the scientific basis of wound healing. Wound bed preparation has been introduced to guide the clinician systematically in wound assessment and management, particularly in those wounds that are difficult to heal. Every wound is unique and therefore should be assessed and treated individually, principally correcting the underlying cause(s) and systematically going through each of the T.I.M.E. components of wound bed preparation. An advanced knowledge of the scientific basis of wound bed preparation has broadened our armamentariums in handling chronic wounds, including simple mechanical tissue management, enzymatic agents, a variety of modern dressings, advances in mechanical and bioengineering innovations, biochemical agents such as growth factors and stem-cell therapies. However, these advanced therapies may not be available to all clinicians, and they should be evaluated from time to time until they are fully established with hard clinical evidence and proven cost-effectiveness. This area is constantly being updated and ever evolving science. Therefore it is difficult to include in this review comprehensively whatever available in practice and in the literature.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Falanga V. Wound Bed Preparation in Practice. EWMA Position Document. London: Medical Education Partnership Ltd; 2004. Wound bed preparation: Science applied to practice; pp. 2–5. [Google Scholar]
- 2.Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611–26. doi: 10.1016/j.suc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347–52. [PubMed] [Google Scholar]
- 4.Dowsett C. The role of the nurse in wound bed preparation. Nurs Stand. 2002;16:69–72. doi: 10.7748/ns2002.07.16.44.69.c3234. [DOI] [PubMed] [Google Scholar]
- 5.Knox KR, Datiashvili RO, Granick MS. Surgical wound bed preparation of chronic and acute wounds. Clin Plast Surg. 2007;34:633–41. doi: 10.1016/j.cps.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Sibbald RG, Williamson D, Orsted HL, Campbell K, Keast D, Krasner D, et al. Preparing the wound bed--debridement, bacterial balance, and moisture balance. Ostomy Wound Manage. 2000;46:14–22. [PubMed] [Google Scholar]
- 7.Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: Focus on infection and inflammation. Ostomy Wound Manage. 2003;49:23–51. [PubMed] [Google Scholar]
- 8.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, et al. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–28. doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 9.Sibbald RG, Orsted HL, Coutts PM, Keast DH. Best practice recommendations for preparing the wound bed: Update 2006. Adv Skin Wound Care. 2007;20:390–405. doi: 10.1097/01.ASW.0000280200.65883.fd. [DOI] [PubMed] [Google Scholar]
- 10.Sibbald RG, Goodman L, Woo KY, Krasner DL, Smart H, Tariq G, et al. Special considerations in wound bed preparation 2011: An update(c) Adv Skin Wound Care. 2011;24:415–36. doi: 10.1097/01.ASW.0000405216.27050.97. [DOI] [PubMed] [Google Scholar]
- 11.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–92. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. J Plast Reconstr Surg. 2006;117:35–41. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher J. Differences between acute and chronic wounds and the role of wound bed preparation. Nurs Stand. 2008;22:62–8. doi: 10.7748/ns2008.02.22.24.62.c6412. [DOI] [PubMed] [Google Scholar]
- 14.Ligresti C, Bo F. Wound bed preparation of difficult wounds: An evolution of the principles of TIME. Int Wound J. 2007;4:21–9. doi: 10.1111/j.1742-481X.2006.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panuncialman J, Falanga V. The science of wound bed preparation. Clin Plast Surg. 2007;34:621–32. doi: 10.1016/j.cps.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Granick MS, Tenenhaus M, Knox KR, Ulm JP. Comparison of wound irrigation and tangential hydrodissection in bacterial clearance of contaminated wounds: Results of a randomized, controlled clinical study. Ostomy Wound Manage. 2007;53:64–6. [PubMed] [Google Scholar]
- 17.Granick M, Boykin J, Gamelli R, Schultz G, Tenenhaus M. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen. 2006;14()(Suppl 1):S1–10. doi: 10.1111/j.1743-6109.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 18.Gravante G, Delogu D, Esposito G, Montone A. Versajet hydrosurgery versus classic escharectomy for burn debridment: A prospective randomized trial. J Burn Care Res. 2007;28:720–4. doi: 10.1097/BCR.0B013E318148C9BD. [DOI] [PubMed] [Google Scholar]
- 19.Patton MA. Eusol: The continuing controversy. BMJ. 1992;304:1636. doi: 10.1136/bmj.304.6842.1636-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falabella AF. Debridement and wound bed preparation. Dermatol Ther. 2006;19:317–25. doi: 10.1111/j.1529-8019.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 21.Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001;9:178–86. doi: 10.1046/j.1524-475x.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 22.Trengove NJ, Stacey MC, McGechie DF, Mata S. Qualitative bacteriology and leg ulcer healing. J Wound Care. 1996;5:277–80. doi: 10.12968/jowc.1996.5.6.277. [DOI] [PubMed] [Google Scholar]
- 23.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117:72 S–109. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- 24.Mat Saad AZ, Khoo TL, Dorai AA, Halim AS. The versatility of a glycerol-preserved skin allograft as an adjunctive treatment to free flap reconstruction. Indian J Plast Surg. 2009;42:94–9. doi: 10.4103/0970-0358.53017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mat Saad AZ, Halim AS, Khoo TL. The use of glycerol-preserved skin allograft in conjunction with reconstructive and flap surgery: Seven years of experience. J Reconstr Microsurg. 2011;27:103–8. doi: 10.1055/s-0030-1268208. [DOI] [PubMed] [Google Scholar]
- 26.Borgquist O, Gustafsson L, Ingemansson R, Malmsjo M. Micro- and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg. 2010;64:789–93. doi: 10.1097/SAP.0b013e3181ba578a. [DOI] [PubMed] [Google Scholar]
- 27.Hasan A, Murata H, Falabella A, Ochoa S, Zhou L, Badiava E, et al. Dermal fibroblasts from venous ulcers are unresponsive to action of transforming growth factor-beta I. J Dermatol Sci. 1997;16:59–66. doi: 10.1016/s0923-1811(97)00622-1. [DOI] [PubMed] [Google Scholar]
- 28.Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic leg ulcers is ulcer-dependent. J Invest Dermatol. 1999;112:463–9. doi: 10.1046/j.1523-1747.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 29.Cook H, Davies KJ, Harding KG, Thomas DW. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2 and MMP-2 activity. J Invest Dermatol. 2000;115:225–33. doi: 10.1046/j.1523-1747.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 30.Falanga V, Grinnell F, Gilchrist B, Maddox YT, Moshell A. Workshop on the pathogenesis of chronic wounds. J Invest Dermatol. 1994;102:125–7. doi: 10.1111/1523-1747.ep12371745. [DOI] [PubMed] [Google Scholar]
- 31.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 32.Stanley AC, Park HY, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be stimulated with growth factors. J Vasc Surg. 1997;26:994–9. doi: 10.1016/s0741-5214(97)70012-0. [DOI] [PubMed] [Google Scholar]
- 33.Henderson EA. The potential effect of fibroblast senescence on wound healing and the chronic wound environment. J Wound Care. 2006;15:315–8. doi: 10.12968/jowc.2006.15.7.26932. [DOI] [PubMed] [Google Scholar]
- 34.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen L, Holstein P, Larsen K, Bullow J. Glucose metabolism in chronic diabetic foot ulcers measured in vivo usingmicrodialysis. Clin Physiol. 1998;18:355–9. doi: 10.1046/j.1365-2281.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- 36.Stolle LB, Riegels-Nielsen P. The metabolism if the diabetic foot. In vivo investigation with microdialysis. Acta Orthop Scand. 2004;75:106–8. doi: 10.1080/00016470410001708210. [DOI] [PubMed] [Google Scholar]
- 37.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS ONE. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu ZJ, Velazquez OC. Hyperoxia, Endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–82. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30–9. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jude EB, Blakytny R, Bulmer J, Boulton AJ, Ferguson MW. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet Med. 2002;19:440–7. doi: 10.1046/j.1464-5491.2002.00692.x. [DOI] [PubMed] [Google Scholar]
- 41.Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor-1 (IGF-1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190:589–94. doi: 10.1002/(SICI)1096-9896(200004)190:5<589::AID-PATH553>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol. 1992;141:1085–95. [PMC free article] [PubMed] [Google Scholar]
- 43.Fivenson D, Scherschun L. Clinical and economic impact of Apligraf for the treatment of non-healing venous leg ulcers. Int J Dermatol. 2003;42:960–5. doi: 10.1111/j.1365-4632.2003.02039.x. [DOI] [PubMed] [Google Scholar]
- 44.Da Costa RM, Ribeiro Jesus FM, Aniceto C, Mendes M. Randomised, double-blind, placebo-controlled, dose-ranging study of granulocytemacrophage colony stimulating factor in patients with chronic venous leg ulcers. Wound Repair Regen. 1999;7:17–25. doi: 10.1046/j.1524-475x.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- 45.Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, et al. Randomised trial of topically applied Repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001;9:347–52. doi: 10.1046/j.1524-475x.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 46.Colletta V, Dioguardi D, Di Lonardo A, Maggio G, Torasso F. A trial to assess the efficacy and tolerability of Hyalofill-F in non-healing venous leg ulcers. J Wound Care. 2003;12:357–60. doi: 10.12968/jowc.2003.12.9.26530. [DOI] [PubMed] [Google Scholar]
- 47.Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care. 2002;11:335–41. doi: 10.12968/jowc.2002.11.9.26438. [DOI] [PubMed] [Google Scholar]
- 48.Sibbald RG, Torrance G, Hux M, Attard C, Milkovich N. Cost-effectiveness of becaplermin for non-healing neuropathic diabetic foot ulcers. Ostomy Wound Manage. 2003;49:76–84. [PubMed] [Google Scholar]
- 49.Edmonds M, Foster A. Hyalofill: A new product for chronic wound management. Diabetic Foot. 2000;3:9–30. [Google Scholar]
- 50.Vevas A, Sheehan P, Pham HT. A randomised controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcer. Arch Surg. 2002;137:822–7. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 51.Felts AG, Grainger DW, Slunt JB. Locally delivered antibodies combined with systemic antibiotics confer synergistic protection against antibiotic-resistant burn wound infection. J Trauma. 2000;49:873–8. doi: 10.1097/00005373-200011000-00014. [DOI] [PubMed] [Google Scholar]