Abstract
Background:
In patients with life-threatening injuries, simple wounds requiring split-thickness skin grafts (SSG) often get neglected. These then need SSG once they are covered with granulation tissue through wound bed preparation. Traditionally, this is done by daily moist dressings. Recombinant human platelet-derived growth factor (rhPDGF) has been shown to improve healing in chronic wounds.
Aim:
The present study was undertaken to compare the efficacy of rhPDGF in wound bed preparation with the current practice of daily saline dressings.
Setting and Design:
A prospective randomised, single-blinded study was carried out for evaluation in traumatic wounds.
Materials and Methods:
The patients were randomised and divided into a control group that was subjected to saline dressings and a test group that was treated with rhPDGF gel. Both the groups were then compared. The statistical analysis was carried out using SPSS 16.0 and the quantitative variables were analysed using unpaired “t” test, while the pre- and post-intervention effects were assessed using paired “t” test. The 95% CI values were also included.
Results:
Of the 155 wounds studied, time taken for appearance of granulation tissue (in days) in the test group had a mean of 13.81 ± 2.68, while that in the control group was 13.36 ± 3.81 (P = 0.401). Complete re-epithelialisation without discharge occurred in the control group with a mean value of 28.9 ± 3.67 days, while that in the test group had a mean of 31.17 ± 4.82 days.
Conclusion:
There was no difference in wound healing between the patients treated with rhPDGF compared to those treated by conventional moist dressings.
KEY WORDS: Platelet-derived growth factor, soft tissue injuries, split-thickness skin grafts, trauma, wound bed preparation, wounds and injuries
INTRODUCTION
Wounds represent a major health burden and drain on our resources through significant morbidity. Two broad categories exist for the classification of wounds: acute and chronic. The ideal treatment for acute wounds, especially for those that are traumatic in aetiology, is early coverage before the contaminated wounds get infected, usually by 3–5 days. However, this is sometimes not possible as the patient may have concomitant major life-threatening injuries that are treated on priority and simple wounds requiring skin grafts get neglected, especially if the patient is treated initially at a primary care centre and then transferred to a larger tertiary care referral centre. The time course between an acute versus chronic wound is a continuum between 4 and 6 weeks. It is during this time that if an acute wound has not healed spontaneously, it is likely to become chronic, a problem wound that requires further intervention.[1] To prevent this from happening, these wounds need coverage as early as possible. The traditional way of wound bed preparation is daily moist dressings till the appearance of granulation tissue. Only then can the wound be covered by an autologous split-thickness skin graft (SSG). However, this is a lengthy process fraught with complications such as pain and bleeding from the wounds due to repeated dressings, and possible sepsis till the wounds are provided with coverage effectively.
Surgical debridement is the mainstay of wound bed preparation for acute wounds. While effective, it has several major disadvantages. Surgical excision is painful and exposes patients to the risks of repeated anaesthesia and significant bleeding. Topical growth factors have been used to good effect in wound bed preparation for chronic wounds. Recombinant human platelet-derived growth factor (rhPDGF) was the first such growth factor to be approved by the Food and Drug Administration (FDA) as a supplement for the treatment of chronic non-healing diabetic ulcers of the lower extremities.[2] It has been found to significantly decrease the time to healing.[3] However, rhPDGF has not been used as an adjunct to wound bed preparation for wounds other than chronic wounds. Many patients with wounds received by tertiary care referral centres are delayed in presentation due to the reasons listed above. Therefore, it is imperative that coverage be done urgently for these wounds to prevent them from converting into chronic or “problem” wounds. Thus, the present study was undertaken to evaluate the efficacy of rhPDGF in wound bed preparation, prior to skin grafting, of traumatic wounds that had not been subjected to coverage in the acute phase of trauma. It only compared the various aspects of wound bed preparation prior to skin grafting between rhPDGF and saline dressings. To the best of the knowledge of the authors, this is the first study of its kind to be published in world literature.
MATERIAL AND METHODS
A prospective randomised, single-blinded study was carried out to evaluate the efficacy of rhPDGF in wound bed preparation in traumatic wounds in patients admitted from May 2009 to May 2011 at a large tertiary referral centre in India. All patients were included in the study after obtaining a signed informed consent from the patients and the study was approved by the hospital ethical committee.
Patients were eligible for inclusion if they had full-thickness post-traumatic wounds requiring autologous SSG for coverage. As an inclusion criterion, the wounds had not been covered in trauma setting either due to delay because of poor general condition of the patient or due to late transfer from a peripheral hospital.
Exclusion criteria were the following
Partial-thickness wounds
Acute wounds that were covered before infection set in, i.e. 5–7 days
Burn wounds
Patients with chronic wounds: They were defined as wounds persisting beyond 6 weeks of onset
Patients taken up for flap coverage of the wound
Underlying osteomyelitis
Patients in sepsis
Patients with concomitant malignancy
Diabetes mellitus
Immunocompromised state
Smokers
Patients with history of habitual drug abuse
Ongoing treatment of concomitant major injuries with haemodynamic instability
Patients on immunosuppressants, steroids or any other drugs likely to cause impaired wound healing
Patients with concomitant malignancy
Haemoglobin level below 8 g/dL
Serum albumin level below 3 g/dL
Body mass index less than 18.5 kg/m2
Prior to the start of wound bed preparation, surgical debridement was done to clean the wound bed of gross debris, slough and obvious evidence of infection. Surface area of the wound was recorded after the procedure by obtaining the impression of the ulcer floor on a sheet of cellophane paper and transferring the imprint onto a graph paper. Following this, patients were randomised and divided into control group that was subjected to saline dressings and a test group that was treated with rhPDGF gel, both techniques being compared for wound bed preparation. A sample size of 76 wounds in each arm was judged to be appropriate on the basis of a power analysis. The study was powered to detect a difference of interval for graft bed preparation of 25% between the two groups. A random number generator did the randomisation. Thereafter, the saline or rhPDGF gel was applied to the wounds by the second author. The mode of wound bed preparation was not revealed to the principal investigator, the first author, thus causing blinding and preventing observer bias.
A proprietary preparation of rhPDGF-BB gel (100 μg/g of gel) by the name Plermin (0.01%) (Dr. Reddy's Laboratories Ltd.) was used in the study. The intended dose was around 7μg/cm2 of ulcer per day in an adult with an average weight of 50 kg. For calculating the dose of Plermin, the greatest length of the ulcer, in centimetres, was multiplied by the greatest width and surface area thus obtained was divided by four to give the length of gel, in centimetres, to be used. This appropriate amount of Plermin was applied locally to the wound surface with the help of a cotton swab once daily and the wound covered with gauze dressing. No topical antimicrobial application was used in any of the wounds. The surface area of the wound was assessed at the end of every week by the above-mentioned method. Photographic documentation at change of dressings was performed for comparison of all patients.
The treatment was considered successful if a clean, bleeding surface was seen with no slough. Thereafter, the first author assessed the wound and took the patient up for SSG if it was seen to have healthy granulation tissue. After application of SSG, the first-look dressing was done on the 4th day and subsequently every alternate day till the complete take of SSG. During the study period, the patients were further monitored with twice-weekly hematologic investigations and temperature charting. During the study period, no antibiotics were administered unless fever and features of sepsis supervened. In that case, the patient was treated with appropriate systemic antibiotics and excluded from the study. The primary end point of the study group was the time taken for appearance of healthy granulation tissue, indicating a wound ready to take an SSG, and full re-epithelialisation without drainage. The wounds were monitored at each change of dressing for the appearance of healthy granulation tissue that would hasten the wound bed for SSG cover. Secondary outcomes observed were the percentage of reduction of surface area of the wound, along with presence of complications such as pain and burning, SSG failure or partial take, and mild reactions of peri-wound area. Percentage of SSG take was compared in both the groups at day 14 and 21 after SSG, also as a secondary outcome.
All adverse events that occurred during hospitalisation were recorded and their relationship to the treatment was judged according to their nature and timing in relation to the wound bed preparation by rhPDGF. Requirement of drugs given to relieve pain, if any, was noted during or after application of the dressing.
All investigations including haemogram, total leucocyte count, blood sugar, liver and renal function tests, serum electrolytes, routine urine examination, urine and blood cultures, and serum electrolytes were done twice weekly. Swab cultures were also taken twice weekly. Sepsis was diagnosed when five out of following nine parameters were present in 1 day:
Obvious wound infection
Positive blood culture
Hypothermia (<35.5°C), hyperthermia (>38.5°C)
Low (<3000/mm) or high (>15,000/mm3) TLC
Evidence of pneumonia or any localised abscess
Development of petechial haemorrhages
Confused and disoriented state
Conversion of partial-thickness to full-thickness wounds
Paralytic ileus
All patients with sepsis were discontinued from the study even if it meant exclusion after being recruited in the study.
The statistical analysis was carried out using SPSS 16.0 and the quantitative variables were analysed using unpaired “t” test while the pre- and post-intervention effect was assessed using paired “t” test. The 95% CI values were also included.
RESULTS
Of all the patients who were recruited initially, four patients from each group developed features of sepsis and were discontinued from the study midway. Twenty-three of the 48 patients in the control group and 24 of the 47 patients in the test group had more than one wound, and therefore the number of wounds was studied in the analysis [Figure 1]. Of the 155 wounds studied, the control group comprised 78 wounds and the test group had 77 wounds. Age ranged from 20 to 48 years with a mean of 31.54 ± 6.92 years. All the patients were males. There were no co-morbidities in the patients studied. At presentation, the wounds were on an average 26.30 days old.
Figure 1.
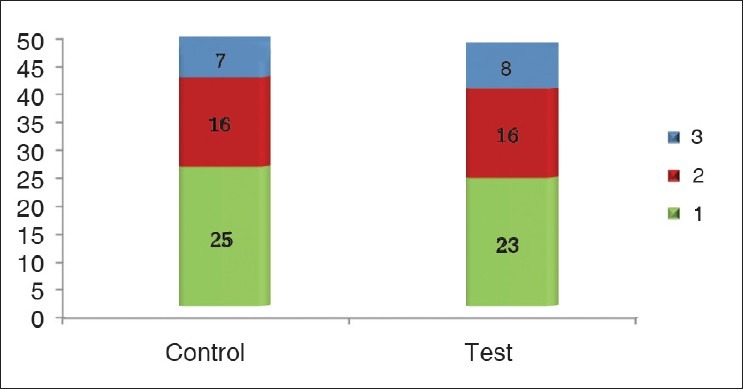
Number of soft tissue defects in each patient
The aetiology of the wounds is shown in Figure 2. The distribution of wounds in both the groups is depicted in Table 1. The largest proportion of wounds studied was in the lower limb (58.97% in control and 58.44% in test group). Many of the subjects had had associated major life-threatening injuries that had been treated earlier [Table 2]. Time taken for appearance of granulation tissue (in days) in the test group had a mean of 13.81 ± 2.68, while that in the control group was 13.36 ± 3.81. On analysis, there was no statistical significance between the two groups (P = 0.401). The actual reduction in wound surface area per week in shown in Table 3. The least wound contraction in both the groups occurred in scalp (8.08% in control vs. 7.68% in test group), while the maximum occurred in the abdomen (20.32% in control vs. 18.68% in test group). The wound surface areas were compared before start of wound bed preparation and after appearance of healthy granulation tissue in both the control and test groups. In the former, the mean reduction of area was 13.55 ± 7.55 cm2, while for the latter, the mean reduction of area was 13.04 ± 5.89 cm2. On analysis, no statistical significance between the two groups was seen in this aspect of wound healing (P = 0.64). Description of SSG take in both the control and test groups is depicted in Table 4. 74.35% of the wounds had a 100% take at 2 weeks after application of SSG in the control group, while the percentage was 67.53% in the test group.
Figure 2.

Etiology of wounds
Table 1.
Location of the wounds
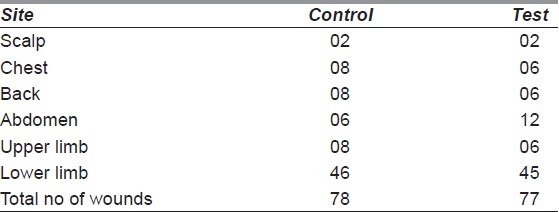
Table 2.
Concomitant major injury
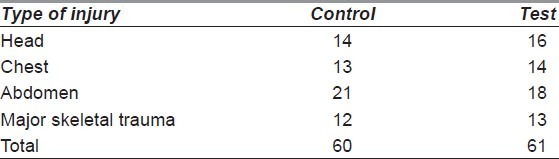
Table 3.
Actual reduction in wound surface area per week (till appearance of granulation tissue) (in percentage)
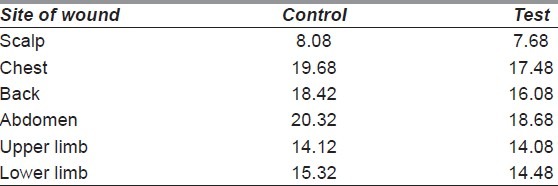
Table 4.
SSG take

Comparison of complete take of SSG at 14 days between the control and test groups further revealed a relative risk (RR) value of 0.89 with CI value 0.72-1.09. The RR was not statistically significant (P = 0.35).
Complete re-epithelialisation without discharge occurred in the control group with a mean value of 28.9 ± 3.67 days, while that in the test group had a mean of 31.17 ± 4.82 days. There was a statistically significant difference in the time taken for complete healing between the two groups with the test group showing delayed healing (P = 0.001).
There were no major complications due to rhPDGF gel application. Only 4 (5.19%) patients had transient burning ranging from 3 to 5 days (average of 4 days) that could be tackled effectively with oral nonsteroidal anti-inflammatory drugs.
DISCUSSION
Wounds have become one of the most dramatic challenges for the health care system the world over. The management of wounds places an enormous drain on healthcare resources; studies have calculated the cost of wounds to the National Health Service (NHS) to be about ≤1 billion a year.[4] In the United Kingdom, around 24,000 admissions a year are for patients with diabetic foot ulceration, thereby costing the NHS some ≤17 million.[5] Foot ulceration is the commonest complication of diabetes that requires hospitalisation, and in the United States, management of this problem is estimated to cost $150 million a year.[6]
The ideal treatment for acute wounds, especially for those that are traumatic in aetiology, is early coverage before the contaminated wounds get infected, usually by 72 h.[7] The International Committee of the Red Cross recommends coverage within 5 days of trauma. However, there may be wounds that cannot be covered in the acute phase of trauma due to poor general condition of the patient owing to associated major life-threatening injuries, or due to delayed referral to a tertiary level care centre because of a step-wise mode of evacuation. These may tend to behave like the problematic chronic wounds that are indolent to heal. There is therefore urgency to cover these wounds before the subversion.
Surgical debridement is often the mainstay of treatment in such wounds. Though effective, it is non-selective and one tends to excise more tissue than is required. There is also the added concern of pain, blood loss and repeated anaesthesia associated with multiple debridements. Topical rhPDGF has been shown to be effective in healing small chronic wounds, especially those with diabetic foot infections.[8–10] Platelet-derived growth factor receptor β-subunit expression has been detected in endothelial cells of the vessels, in the granulation tissue and the wound edge, whereas platelet-derived growth factor receptor α-subunit has not been shown to be expressed in endothelial cells of uninjured skin.[11] This finding suggests that the platelet-derived growth factor β-subunit may be involved in vessel formation during tissue repair. In a quest to identify a suitable adjunct to surgical debridement in wound bed preparation of traumatic wounds, the present study was undertaken to compare topical rhPDGF with the conventional mode of moist dressings for wound bed preparation prior to SSG in traumatic wounds. To the best of the knowledge of the authors, such a study has never been undertaken before.
Topical rhPDGF recommended by the FDA for use is Becaplermin (Regranex). A proprietary preparation of rhPDGF-BB gel (100 μg/g of gel) by the name Plermin (0.01%) (Dr. Reddy's Laboratories Ltd.) was used in the present study as it is an indigenous preparation that is freely available in India. Similar to Becaplermin, it has a molecular weight of 24.5 kDa and is a homodimer composed of two identical polypeptide chains that are bound together by disulphide bonds. It is a low bio-burden (less than 3%), preserved, sodium carboxymethylcellulose-based topical gel, containing the active ingredient rhPDGF-BB and inactive ingredients like carboxymethylcellulose sodium, glacial acetic acid, I-lysine hydrochloride, m-cresol, methylparaben, propylparaben, sodium acetate trihydrate, sodium chloride, and water as a vehicle.
Time taken for appearance of granulation tissue with topical rhPDGF has also not been studied thus far. In this study, the time taken for appearance of healthy granulation tissue between the two groups was comparable.
In a study of 922 patients involving five centres, with full-thickness ulcers with a baseline area of <10 cm2 treated for up to 20 weeks, Steed, in 2006, reported that patients treated with rhPDGF had a significant increase in complete healing compared to patients given placebo. It also decreased the time to complete healing by 30%.[12] An earlier meta-analysis by Schaffer in 2001, of the clinical studies with topical application of rhPDGF for neuropathic diabetic foot ulcers, showed an increase in healing by 10–15% within 20 weeks of treatment.[13] All the studies from the west had used Becaplermin, while the present study used Plermin. In this study, wounds in the group treated by Plermin took slightly longer to heal, while the SSG take at 14 and 21 days was comparable in both the groups. The reason for this variance from the western studies seems obscure, but there may be a few reasons. It may be a measure of different standardisation technique and preparation of the compound Plermin, causing it not to accelerate wound healing. The wounds in our study also appeared to be larger in surface area. This may have had a negative impact on wound healing promoted by rhPDGF. It may also have been that Plermin is not capable of wound bed preparation in traumatic wounds or the wounds such as in our study, viz. bordering on but not quite the problematic chronic wounds. In addition, the method of using cotton swab once daily to apply the gel and the wound being subsequently covered with absorbent gauze may have contributed towards the negative result. The gel may have dissipated into the absorbent gauze rather than staying on the wound bed. Another delivery method may have given a different result. This could be considered a limitation of the study. All these aspects may be a trigger for further research on the subject.
Wounds that reached more than 15% of wound area reduction at 1 week had a 68% probability of healing versus 32% for those that did not reach the figure.[14] As a means to track wound healing progress, Attinger et al.[15] recommended that wound area should reduce by 10–15% per week. If this is not achieved, it is recommended that other types of intervention be sought to heal the problematic wound. In the present study, the wound contraction in both the groups was similar, with the minimum contraction occurring in the scalp while the maximum occurred in the abdominal region.
rhPDGF appears to be a reasonably safe drug for use. Most adverse events have been mild to moderate and transient. The incidence of wound-related infections, including cellulitis, wound infection and osteomyelitis, was 26.3% for the placebo-treated group and 11.4% for rhPDGF-BB–treated group.[12] In our study, only 5.19% patients had burning after application of Plermin that was self-limiting.
There was no difference in wound healing between the patients treated with the agent (Plermin) compared to those treated by conventional moist dressings. Thus, in the present form and application method used, this agent cannot be recommended for wound bed preparation in traumatic wounds prior to split-thickness skin grafting. Even though this study is suggestive, it is recommended that more research, especially multi-centre randomised controlled trials, may be directed towards re-evaluating the efficacy of rhPDGF in bed preparation of various types of wounds.
ACKNOWLEDGMENTS
The authors wish to thank Mrs. S. R. Patrikar, Lecturer, Department of Community Medicine, Armed Forces Medical College, Pune, India, for her invaluable support and thorough analysis of the results of the study. They also wish to thank Dr. A. Chauhan, Surgical Oncologist at Command Hospital, Lucknow, India, for assistance in the initial part of the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hansen SL, Mathes SJ. Plastic surgery. New York: Elsevier; 2006. Problem wounds and principles of closure; pp. 901–50. [Google Scholar]
- 2.Nagai MK, Embil JM. Becaplermin: Recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin Biol Ther. 2002;2:211–8. doi: 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- 3.Wiemen TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet derived growth factor BB (Becaplermin) in patients with chronic neuropathic diabetic ulcers. Diabetes Care. 1998;21:822–7. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 4.Harding KG. The future of wound healing. In: Leaper DJ, Harding KJ, editors. Wounds: biology and management. Oxford: Oxford University Press; 1998. pp. 191–9. [Google Scholar]
- 5.Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy and ulceration in diabetes. Diabetes Care. 1998;21:42–8. doi: 10.2337/diacare.21.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Reiber GE. Diabetic foot care: Financial implications and practical guidelines. Diabetes Care. 1992;15:29–31. doi: 10.2337/diacare.15.1.s29. [DOI] [PubMed] [Google Scholar]
- 7.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–92. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, et al. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: An open-label clinical evaluation of efficacy. Wound Repair Regen. 2000;8:162–8. doi: 10.1046/j.1524-475x.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 9.Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: A phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen. 1999;7:141–7. doi: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 10.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: A combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335–46. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 11.Peus D, Jungtäubl H, Knaub S, Leuker A, Gerecht K, Ostendorf R, et al. Localization of platelet-derived growth factor receptor subunit expression in chronic venous leg ulcers. Wound Repair Regen. 1995;3:265–72. doi: 10.1046/j.1524-475X.1995.30306.x. [DOI] [PubMed] [Google Scholar]
- 12.Steed DL. Clinical evaluation of recombinant human platelet derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S–9S. doi: 10.1097/01.prs.0000222526.21512.4c. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer M, Coerper S, Becker HD. Gene therapy in diabetic foot. Kongressbd Dtsch Ges Chir Kongr. 2001;118:825–8. [PubMed] [Google Scholar]
- 14.Lavery LA, Barnes SA, Keith MS, Seaman JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care. 2008;31:26–9. doi: 10.2337/dc07-1300. [DOI] [PubMed] [Google Scholar]
- 15.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117:72S–109S. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]


