Abstract
Researchers have identified several of the cellular events associated with wound healing. Platelets, neutrophils, macrophages, and fibroblasts primarily contribute to the process. They release cytokines including interleukins (ILs) and TNF-α, and growth factors, of which platelet-derived growth factor (PDGF) is perhaps the most important. The cytokines and growth factors manipulate the inflammatory phase of healing. Cytokines are chemotactic for white cells and fibroblasts, while the growth factors initiate fibroblast and keratinocyte proliferation. Inflammation is followed by the proliferation of fibroblasts, which lay down the extracellular matrix. Simultaneously, various white cells and other connective tissue cells release both the matrix metalloproteinases (MMPs) and the tissue inhibitors of these metalloproteinases (TIMPs). MMPs remove damaged structural proteins such as collagen, while the fibroblasts lay down fresh extracellular matrix proteins. Fluid collected from acute, healing wounds contains growth factors, and stimulates fibroblast proliferation, but fluid collected from chronic, nonhealing wounds does not. Fibroblasts from chronic wounds do not respond to chronic wound fluid, probably because the fibroblasts of these wounds have lost the receptors that respond to cytokines and growth factors. Nonhealing wounds contain high levels of IL1, IL6, and MMPs, and an abnormally high MMP/TIMP ratio. Clinical examination of wounds inconsistently predicts which wounds will heal when procedures like secondary closure are planned. Surgeons therefore hope that these chemicals can be used as biomarkers of wounds which have impaired ability to heal. There is also evidence that the application of growth factors like PDGF will help the healing of chronic, nonhealing wounds.
KEY WORDS: Cytokines, growth factors, matrix metalloproteinases, platelet-derived growth factor, wound healing
In the last 30 or so years, researchers have identified several of the cellular and biochemical events associated with wound healing. The process is becoming clearer, with the understanding of the cells and chemicals that help wounds to heal, and of those that inhibit healing. Investigators are trying to analyze the chemicals in chronic wounds in order to determine their condition and fitness for closure. A major advance is the clinical application of some of these chemicals to improve outcomes in wound healing.
In this paper we look at the biology of normal and abnormal healing, see if wounds analysis can predict poor healing, and review some literature on the clinical applications of this knowledge.
CELLULAR EVENTS ASSOCIATED WITH NORMAL WOUND HEALING
Wounds heal in four overlapping phases: haemostasis, inflammation, proliferation and remodelling.[1,2]
Phase I: Haemostasis
Haemostasis results from the activation of platelets, which initiate the coagulation cascade. Platelets also release substances that initiate and influence wound healing. One of these is platelet-derived growth factor (PDGF), a protein that can be isolated from platelets using chromatography techniques.[3] Other factors, produced by platelets and other cells, include the transforming growth factors (TGFs), the fibroblast growth factors (FGFs), and vascular endothelial growth factor (VEGF).
Phase II: Inflammation
Inflammation begins within 24 hours, and lasts for 2 weeks or more. Inflammatory cells secrete enzymes and various mediators that result in the classical hallmarks of inflammation: pain, redness, warmth, and swelling. While several other cells are involved in the process, in terms of healing the key players are the neutrophils, macrophages, and the T-lymphocytes.[2]
Neutrophils are the first cells to respond to the platelet products.[2] From the circulation they reach the affected area in response to the chemotatic properties of some of the mediators. Here they marginate, adhere to vascular endothelial cells and subsequently migrate out to the extravascular space, with the help of cell adhesion molecules (CAMs). Fibroblasts too carry CAMs, which also function as receptors for cell-cell interaction.[4] Deficiency of adhesion molecules delays healing.[5] Elastase and collagenase, released by neutrophils, help their migration through capillary walls into the extracellular spaces of the wound for phagocytosis. The enzymes also lyse and remove damaged structural proteins. Elastase can destroy some of the growth factors.[6] In addition, neutrophils produce TNF-α and IL-1 that will recruit fibroblasts and epithelial cells.
Macrophages enter the wound, and participate in the phagocytic process. In addition, macrophages release growth factors and cytokines that help bring in the proliferative phase of healing. These factors include PDGF, TGF-β, β-FGF, TNF-α, interleukin 1 (IL-1), and IL-6.
Lymphocytes are the last cells to infiltrate wounds,[6] but are important in the production of IL-2, which helps recruit fibroblasts.
Phase III: Proliferation
Proliferation itself consists of the following three phases: fibroplasia, granulation, and epithelialization. It effectively begins with fibroblast migration into the wound, a process initiated primarily by the PDGF that has been released by platelets and macrophages.[2] PDGF stimulates fibroblastic proliferation, chemotaxis, and collagenase production.
Fibroblasts lay down structural proteins such as collagen [Table 1[7]. They also produce the matrix metalloproteinases (MMPs). These proteolytic enzymes facilitate fibroblast movement within the matrix. Later the fibroblasts decrease their proteolytic activity and start to lay down structural proteins. This step is regulated by two growth factors. One of these is TGF-β, also secreted by both platelets and macrophages. The other is connective tissue growth factor (CTGF), secreted by the fibroblasts themselves.[8] Collagen synthesis involves the hydroxylation of proline, and is affected by vitamin C deficiency.
Table 1.
Main structural proteins in the extracellular matrix

Angiogenesis replaces damaged vasculature with granulation tissue. Epidermal cells, fibroblasts, vascular endothelial cells, and macrophages contribute to angiogenesis by the production of βFGF, TGF-β and VEGF. The proliferative effects of VEGF are regulated by hypoxia, which stimulates VEGF-induced angiogenesis,[2] using adenosine as an intermediary. In fact, adenosine, acting via A2A receptors, is now considered a potent regulator of the early stages of wound healing.[9]
Epithelialization proceeds with the proliferation and migration of the epithelial cells, and is helped by EGF, keratinocyte growth factors (KGFs) and TGF-α. The cells and the extracellular matrix interact closely and continually, stimulating each other.[10]
Phase IV: Remodelling
Remodelling is part of the resolution stage of healing. Inflammatory cells leave, and cells that release the growth factors become fewer. Fibroblasts continue to lay down collagen, even as they too begin to decrease in number.[4] Remodelling takes place by further covalent cross-linking of collagen molecules. In a well-healed wound the final tensile strength may be as high as 80% of that possessed by normal tissue.[2]
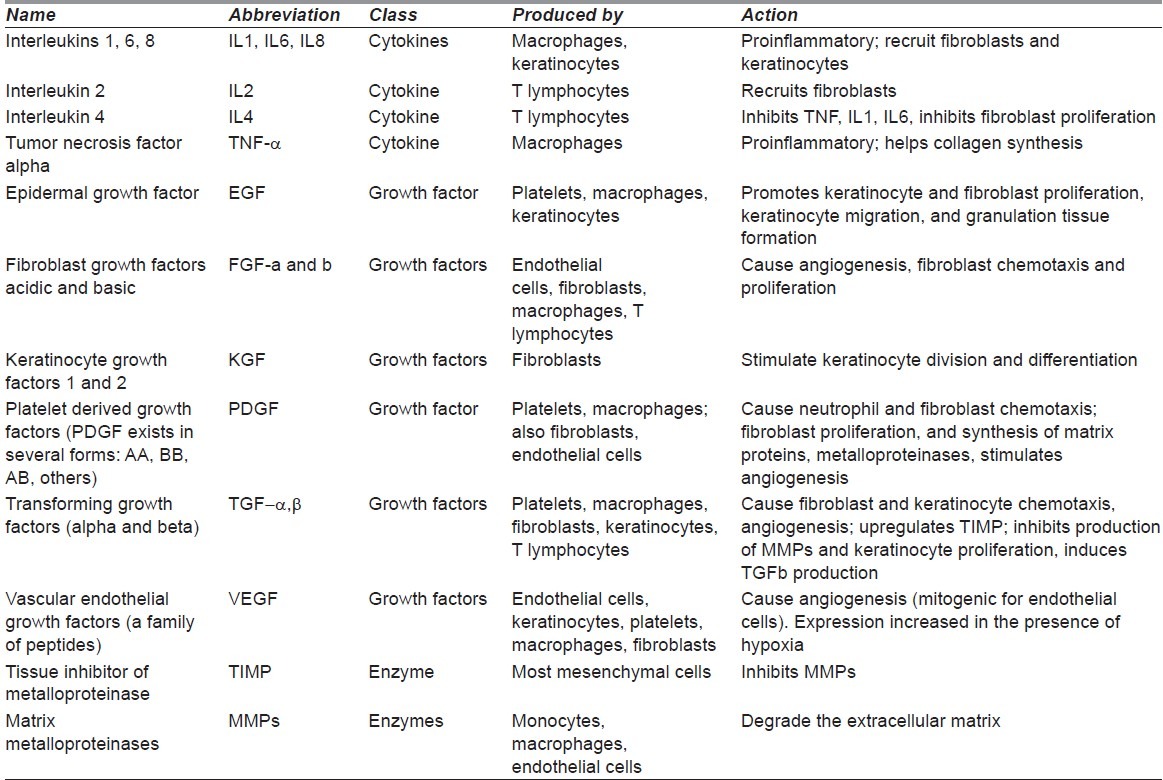
Table 2 lists some of the main cytokines, growth factors, and enzymes involved in the healing process.[1,4,11,12]
Table 2.
Partial list of cytokines and their actions. Note that most of the molecules exist in several forms (e.g. IL-1 exists as at least two subtypes)
CELLULAR EVENTS ASSOCIATED WITH ABNORMAL WOUND HEALING
Several cellular changes occur in chronic, nonhealing wounds that may differentiate them from acute or healthy wounds. Research that looks into these events is more often experimental than clinical. The evidence from such research indicates that fibroblasts in acute, healthy wounds show more activity, and respond well to inflammatory stimuli. In comparison, fibroblasts in unhealthy wounds respond poorly to inflammatory stimuli. There is also increased proteolytic activity that destroys the extracellular matrix.[2]
FIBROBLAST AND EPITHELIAL CELL ACTIVITY IN HEALTHY AND UNHEALTHY (CHRONIC) WOUNDS
In the chronic, unhealthy wound, fibroblast function is adversely affected. Is this because the environment is unfavourable, or is this because the fibroblasts themselves are damaged? The evidence suggests that both factors play a role.
Secretions from healthy wounds stimulate DNA synthesis in fibroblast cultures.[13] On the other hand, secretions from chronic, nonhealing wounds (e.g., leg ulcers) inhibit the same fibroblasts.[14] Interestingly, Bucalo et al.[15] found that denaturing the chronic wound fluid contents by heating removed the inhibitory effect, and allowed fibroblasts to flourish again.
Again, the fibroblasts from chronic wounds themselves show poorer responses to growth factors than do fibroblasts from acute wounds, indicating that the fibroblasts of chronic wounds are themselves affected.[16,17] Loots and colleagues[18] measured the response of fibroblasts from chronic ulcers to various growth factors, and compared them to fibroblasts from nonlesional controls. Fibroblasts from chronic ulcers responded poorly, and needed higher doses of growth factors for the same response as healthy fibroblasts. The authors wondered if damaged receptors could be a factor in the poor response, but mentioned in their discussion that such receptor changes had been sought by others and not found. It is worth mentioning that epithelial cells from chronic ulcers, like the fibroblasts, also show poor response to growth factors. However, in epithelial cells it has been possible to show a defective expression of receptors for TGF-β.[19]
If fibroblasts become nonresponsive, one would expect that cytokine secretion, representing neutrophil and macrophage activity, would increase in an attempt to cause a fibroblast response. That this indeed does occur was shown by Trengove et al.[20] who reported that levels of the proinflammatory cytokines IL-1 and TNF-α were higher in nonhealing wounds compared to healing wounds. The levels fell significantly when healing began to occur.
Proteolytic activity in unhealthy wounds
Protease (e.g., collagenase) activity, which should fall as the fibroblasts start to lay down collagen, is higher in chronic wounds than acute. Studies show that the levels of MMPs, neutrophil elastase, and cathepsin-G remain high in chronic wounds.[2,21,22] Tissue inhibitors of metalloproteases (TIMPs), which inhibit MMP function, tend to have lower levels in chronic wounds.[23] The overall result of these changes is to increase the enzyme levels, and thus to reduce the proper deposition of matrix proteins. The proteases can also degrade and deplete growth factors like EGF, TGF1-α, and PDGF, molecules that are stable in fresh and clean injuries. Other proteases, not ordinarily involved in wound healing, also increase in fluid secretions of chronic wounds.[2]
BIOMARKERS OF HEALING AND NONHEALING WOUNDS
Surgeons would love to be able to tell if a wound is fit enough for an attempt at closure. The badly infected wounds are obviously unfit, and of course the fresh, clean wounds are obviously fit. The confusion regarding fitness for closure arises in wounds that have enough infection to affect healing, but not enough to produce clinical signs of infection.
Some workers believe that wounds should be classified in terms of colonization by bacteria: sterile, contaminated, colonized, critically colonized, and infected. “Critically colonized” wounds are those without clinical evidence of infection, but with enough bacteria to affect healing.[24] They appear healthy and fit for closure, but will become overtly infected afterwards. These are the wounds which are most likely to be associated with incorrect surgical judgment, and which need the help of the laboratory.
Can biomarkers identify the “critically colonized” wounds: the ones in which healing has been affected? The answer is “probably”. The biomarkers that hold most potential are cytokines and proteases.[25]
Cytokine levels
Cytokine levels are higher in nonhealing wounds than in healing wounds, especially the levels of IL-1, IL-6, and TNF-α.[2] Trengove et al.[20] found that median levels of IL-1 were 9200 U/ml (range 1300-48000) in nonhealing wounds, compared to 2700 (400-14000) U/ml in healing wounds (P = 0.003). Similarly, the levels of IL-6 and TNF-α were also significantly higher in nonhealing wounds. Beidler et al.[26] reported very high values for IL-8 in chronic wounds. Unfortunately, the wound and serum cytokine levels are so variable that it is difficult to use their levels as reliable markers of poor healing.[27] Harris et al.,[28] for example, did not find cytokine levels in the two types of wounds to be significantly different. Forsberg et al.[29] measured cytokine levels in severe military wounds, and tried to predict which wounds would fail to heal: there was almost no correlation.
Protease levels
Protease levels are more likely to prove reliable as biomarkers of poor healing. Utz et al.[30] studied 38 wounds in 25 patients, and collected samples during successive wound debridements. They stated that serum MMP-2 and MMP-7 were statistically predictive of wound healing outcome (p < 0.001): the higher the level, the lower the chance of successful healing. Snyder et al.[22] reported the findings of a consensus panel, which concluded that the wound levels of elastase, metalloproteinases, and, in particular, MMP-9/TIMP ratios could prove good prognosticators of wound behaviour.[23]
Gene expression
Gene expression analysis may be an option in the future. Asada and coworkers[31] reported a fascinating experimental study in which they showed changes on gene expression analysis. Using reverse transcription polymerase chain reaction on wound fluid in rats, they showed that virulence factor bacterial genes were more likely to be expressed in the presence of invasive infection rather than mere colonization of wounds. Several host housekeeping genes (genes constitutively expressed to perform the cell's basic functions[32]), in contrast, were more likely to be expressed in healthier wounds, but not in wounds that were frankly infected. They suggested that gene expression analysis could help establish the status of the wound.
Other substances
Moor et al.[33] reported that wound fluid myeloperoxidase levels were associated with nonhealing and infected wounds. Studies are also on to determine if wound analysis for volatile organic compounds (e.g., various esters, alcohols, and organic compounds) may help in prognostication.[34] Chronic wounds also contain higher quantities of reactive oxygen species.[6] Forsberg et al.[29] found that healing was invariably successful in wounds with procalcitonin levels lower than 220 pg/ml. This suggests that procalcitonin may have potential as a marker of healing.
Tissue bacterial levels
Another biological marker that may be predictive of healing in both chronic and acute wounds is the tissue bacterial level.[35] High tissue levels of bacteria inhibit or impair all the processes of wound healing and prevent satisfactory repair of wound[36,37]
Table 3 lists biomarkers that may have potential in predicting nonhealing wounds.[20,22,23,29–31,33,34]
Table 3.
Biomarkers of nonhealing. The following substances have potential as possible biomarkers of wound healing

APPLICATION: THE CLINICAL USE OF GROWTH FACTORS
Over the last 20 years, researchers have investigated the results of topical application of growth factors on wounds. They have conducted animal and human studies, on both acute and chronic wounds. In an attempt to recreate an experimental “nonhealing” environment, some workers have used the setting of diabetes, ischaemia, and irradiation.
Animal studies
In the laboratory animal PDGF healed wounds in significantly less time and with less scarring compared to controls.[38] Other compounds that have been used with good results include EGF,[38] adenosine A2A receptor agonists,[39–41] fibrin sealant,[42] and even fibronectin.[43]
PDGF was superior to controls when applied topically to acute wounds in diabetic rats,[44] and in rats with impaired healing due to steroid administration.[45] Acute but ischaemic wounds also show accelerated epithelialization following application of PDGF.[46] The factor also shows benefit in irradiated wounds.[47]
Why do chronic ulcers respond worse to growth factors than even ischaemic and irradiated ulcers? One possible reason is that in chronic wounds, the cells (e.g., fibroblasts) fail to respond, perhaps because of a reduction of appropriate receptors. If this is true, then this poor response should be limited to chronically infected wounds, but not to acute wounds with ischaemia or irradiation. The acute irradiated wounds and acute ischaemic wounds showed improved healing when growth factors were applied, perhaps because they were not chronic, and the serum had not yet acquired the presence of inhibitors of growth factors.
If cellular response to growth factors is weaker in chronic wounds than in acute, the resultant impairment in healing should remain only until the environment remained unfavourable. After good wound care, the tissues should once again become responsive to growth factors. This in fact was shown in a recent study. In the first week of therapy chronically infected wounds behaved poorly, whether or not growth factors were administered. However, after a week of good wound care, the lesions in the growth factor group showed improved healing as compared to controls.[48]
PDGF genes, delivered in plasmids,[39] viruses,[49] or in fibroblasts[50] have also resulted in improvement in markers of healing.
Although not all studies confirm that growth factors improve healing,[51,52] there is nevertheless considerable evidence that small but significant changes take place in angiogenesis, wound contraction, and epithelialization in experimental animals.
Clinical studies on acute wounds
PDGF exists as dimers of A and B chains.[53] As compared to PDGF-AA and PDGF-AB, PDGF-BB is more active[38] and is approved by the US Food and Drug Administration for application to wounds to promote healing.[54] In the last 20 years, several studies have shown that PDGF applied to acute wounds results in earlier angiogenesis and complete healing. Ehrlich and Freedman[55] applied topical PDGF to acute wounds behind the ear in patients undergoing elective surgery. Treated wounds healed significantly faster (16 days vs 20 days) and by epithelialization rather than by contraction in a small study of four patients.
Clinical studies on chronic wounds
In contrast, the use of growth factors in chronic wounds is limited. Fernández-Montequín et al.[56] used an intralesional epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcers. They showed that treatment was well tolerated and safe, but, lacking a control group, could not show improvement in healing.
Platelet-derived growth factor
Kurtz et al.[57] applied PDGF to the wounds of ten patients who had nonhealing wounds three months after abdominoperineal resection for inflammatory bowel disease. Six of the 10 wounds healed after an average of average 80 days. This report shows promise, but in the absence of controls does not confirm the value of PDGF in chronic wounds.
Unfortunately, controlled studies are rare. Shackelford et al.[58] conducted a double-blind randomized placebo-controlled trial in patients with surgical wound separation. They randomized ten patients to treatment with 0.01% recombinant human PDGF gel, and eleven to control groups. Wounds of patients receiving placebo closed 54 days after operation; treated wounds closed in 35 days (P = 0.05). In their discussion they commented that wound closure rates were inferior to operative secondary closure. Nevertheless, this report provides strong evidence of the positive influence of PDGF in this setting of separated (but not chronically infected) wounds.
Recombinant PDGF (becaplermin, marketed as Regranex©), approved in the United States for specific wounds, significantly improved the incidence of complete healing in chronic pressure ulcers.[59] In this study the ulcers were of over 4-week duration, and underwent debridement before drug application. On the other hand, Chan and coworkers were unable to show any benefits with PDGF on burn wounds in diabetic mice.[51]
Other growth factors
Experience with factors other than PDGF is limited, since few are approved for clinical use. bFGF, approved for clinical use in Japan, showed reduced scarring in a prospective study of 230 cases.[60] In contrast, Richard et al.,[61] in a prospective randomized trial, found no benefit with bFGF in chronic ulcers. Tsang et al., in a randomized trial,[62] showed significantly improved healing in chronic diabetic foot ulcers using recombinant EGF, as did a phase III trial from India.[63]
Bao and colleagues[12] reviewed the literature and stated that VEGF gene transfer (e.g., by VEGF-expressing plasmids) may be used for chronic wounds, though at present the evidence is limited.
Personalized wound care
Personalized treatment is now evolving. Tests that detect individual biologic differences may allow individualized, targeted patient management. With the identification of biomarkers, biologic differences can be identified to aid in individualizing treatment. These concepts have recently been applied to predict individualized wound care in combat wounds.[64–67]
Table 4 lists growth factors that may have uses in clinical practice.[12,56–60]
Table 4.
Potentially useful growth factors

Verdict on clinical usage
Costs
Growth factor therapy is somewhat expensive. For the management of a foot ulcer, a patient needs one to two tubes of recombinant human PDGF at about $25 per tube.[68] Langer and Rogowski[69] conducted a systematic survey of the costs involved; admitting that there were weaknesses in the studies they reviewed, they concluded that the overall costs of therapy with PDGF resulted in some small but measurable saving.
Should growth factors be used clinically?
As of today there is very little level 1 evidence (i.e., by randomized controlled trials, Shackelford et al.[58] being an exception here) to prove the value of growth factor therapy for acute or chronic wounds. Acute wounds respond well to PDGF, but heal well without help. Chronic wounds need help, but respond poorly to the factor. Nevertheless, bearing in mind the scanty evidence, there is still enough to warrant the use of PDGF in selected cases. It would be well to warn the patient that the results may be less than spectacular.
The surgeon should be aware of the caveats. First, and foremost, growth factors are a small adjunct to good wound care, not a replacement.[54] Second, in patients whose wounds are suitable for secondary closure or cover, these options result in quicker healing. Third, there are several biological therapies: cultured skin, allografts, xenografts, and skin substitutes. They are not part of this review, but should be considered in the appropriate setting.[70] Finally, texts of plastic surgery have begun recommending that PDGF should be “considered” in wounds not responsive to initial comprehensive therapy,[70] therefore today there is certainly a small but definite place for this form of pharmacotherapy in chronic wounds.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Buchanan EP, Lorenz HP. Wound healing, including fetal skin healing. In: Bahman G, Eriksson E, Persing JA, editors. Plastic Surgery: Indications and Practice. Elsevier; 2009. pp. 9–26. [Google Scholar]
- 2.Chin GC, Diegelmann RF, Schultz GS. Cellular and molecular regulation of wound healing. In: Falabella AF, Kirsner RS, editors. Wound Healing. Boca Raton: Taylor & Francis Group; 2005. pp. 17–37. [Google Scholar]
- 3.Antoniades HN, Scher CD, Stiles CD. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979;76:1809–13. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar V, Abbas AA, Fausto N, editors. Robbins and Cotran's Pathologic Basis of Disease. 7th edition. Philadelphia: Elsevier; 2005. Tissue renewal and repair: regeneration, healing, and fibrosis; pp. 87–118. [Google Scholar]
- 5.LeBlanc S, Arabzadeh A, Benlolo S, Breton V, Turbide C, Beauchemin N, et al. CEACAM1 deficiency delays important wound healing processes. Wound Repair Regen. 2011;19:745–52. doi: 10.1111/j.1524-475X.2011.00742.x. [DOI] [PubMed] [Google Scholar]
- 6.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Kirsner RS. Extracellular matrix and wound healing. In: Falabella AF, Kirsner RS, editors. Wound Healing. Boca Raton: Taylor & Francis Group; 2005. pp. 39–48. [Google Scholar]
- 8.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: Down-regulation by cAMP. FASEB J. 1999;13:1774–86. [PubMed] [Google Scholar]
- 9.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–18. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 11.Rumalla VK, Borah GL. Cytokines, growth factors, and plastic surgery. Plast Reconstr Surg. 2001;108:719–33. doi: 10.1097/00006534-200109010-00019. [DOI] [PubMed] [Google Scholar]
- 12.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol. 1991;25:1054–8. doi: 10.1016/0190-9622(91)70306-m. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: A potential mechanism for senescence in venous ulcers. J Vasc Surg. 1999;30:734–43. doi: 10.1016/s0741-5214(99)70113-8. [DOI] [PubMed] [Google Scholar]
- 15.Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen. 1993;1:181–6. doi: 10.1046/j.1524-475X.1993.10308.x. [DOI] [PubMed] [Google Scholar]
- 16.Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho-Kere UK, et al. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl (Stockh) 2000;210:3–17. [PubMed] [Google Scholar]
- 17.Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg. 1998;28:876–83. doi: 10.1016/s0741-5214(98)70064-3. [DOI] [PubMed] [Google Scholar]
- 18.Loot MA, Kenter SB, Au FL, van Galen WJ, Middelkoop E, Bos JD, et al. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol. 2002;81:153–60. doi: 10.1078/0171-9335-00228. [DOI] [PubMed] [Google Scholar]
- 19.Cowin AJ, Hatzirodos N, Holding CA, Dunaiski V, Harries RH, Rayner TE, et al. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J Invest Dermatol. 2001;117:1282–9. doi: 10.1046/j.0022-202x.2001.01501.x. [DOI] [PubMed] [Google Scholar]
- 20.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 21.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 22.Snyder RJ, Driver V, Fife CE, Lantis J, Peirce B, Serena T, et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: A consensus panel discussion. Ostomy Wound Manage. 2011;57:36–46. [PubMed] [Google Scholar]
- 23.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 24.White RJ, Cutting KF. Critical colonization--the concept under scrutiny. Ostomy Wound Manage. 2006;52:50–6. [PubMed] [Google Scholar]
- 25.Hahm G, Glaser JJ, Elster EA. Biomarkers to predict wound healing: The future of complex war wound management. Plast Reconstr Surg. 2011;127(Suppl 1):21S–6S. doi: 10.1097/PRS.0b013e3181fbe291. [DOI] [PubMed] [Google Scholar]
- 26.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49:1013–20. doi: 10.1016/j.jvs.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg. 2008;48:1272–7. doi: 10.1016/j.jvs.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Harris IR, Yee KC, Walters CE, Cunliffe WJ, Kearney JN, Wood EJ, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol. 1995;4:342–9. doi: 10.1111/j.1600-0625.1995.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg JA, Elster EA, Andersen RC, Nylen E, Brown TS, Rose MW, et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–8. doi: 10.2106/JBJS.G.00265. [DOI] [PubMed] [Google Scholar]
- 30.Utz ER, Elster EA, Tadaki DK, Gage F, Perdue PW, Forsberg JA, et al. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010;159:633–9. doi: 10.1016/j.jss.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Asada M, Nakagami G, Minematsu T, Nagase T, Akase T, Huang L, et al. Novel biomarkers for the detection of wound infection by wound fluid RT-PCR in rats. Exp Dermatol. 2012;21:118–22. doi: 10.1111/j.1600-0625.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 32.Butte AJ, Dzau VJ, Glueck SB. Further defining housekeeping, or “maintenance,” genes Focus on “A compendium of gene expression in normal human tissues”. Physiol Genomics. 2001;7:95–6. doi: 10.1152/physiolgenomics.2001.7.2.95. [DOI] [PubMed] [Google Scholar]
- 33.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17:832–9. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas AN, Riazanskaia S, Cheung W, Xu Y, Goodacre R, Thomas CL, et al. Novel noninvasive identification of biomarkers by analytical profiling of chronic wounds using volatile organic compounds. Wound Repair Regen. 2010;18:391–400. doi: 10.1111/j.1524-475X.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 35.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77:637–50. doi: 10.1016/s0039-6109(05)70572-7. [DOI] [PubMed] [Google Scholar]
- 36.Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg. 1990;17:485–92. [PubMed] [Google Scholar]
- 37.Robson MC. Wound healing and wound closure. In: Heggers JP, Robson MC, editors. Quantitative bacteriology: Its role in the armamentarium of the surgeon. Boca Raton FL: CRC Press; 1991. –43.pp. 54 [Google Scholar]
- 38.Gope R. The effect of epidermal growth factor & platelet-derived growth factors on wound healing process. Indian J Med Res. 2002;116:201–6. [PubMed] [Google Scholar]
- 39.Victor-Vega C, Desai A, Montesinos MC, Cronstein BN. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel) Inflammation. 2002;26:19–24. doi: 10.1023/a:1014417728325. [DOI] [PubMed] [Google Scholar]
- 40.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–92. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: A role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774–88. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogford JE, Tawil B, Jia S, Mustoe TA. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen. 2009;17:405–10. doi: 10.1111/j.1524-475X.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 43.Lariviere B, Rouleau M, Picard S, Beaulieu AD. Human plasma fibronectin potentiates the mitogenic activity of platelet-derived growth factor and complements its wound healing effects. Wound Repair Regen. 2003;11:79–89. doi: 10.1046/j.1524-475x.2003.11112.x. [DOI] [PubMed] [Google Scholar]
- 44.Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY. Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci. 2007;45:193–201. doi: 10.1016/j.jdermsci.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Engrav LH, Richey KJ, Kao CC, Murray MJ. Topical growth factors and wound contraction in the rat: Part II.Platelet-derived growth factor and wound contraction in normal and steroid-impaired rats. Ann Plast Surg. 1989;23:245–8. doi: 10.1097/00000637-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Uhl E, Rosken F, Sirsjo A, Messmer K. Influence of platelet-derived growth factor on microcirculation during normal and impaired wound healing. Wound Repair Regen. 2003;11:361–7. doi: 10.1046/j.1524-475x.2003.11508.x. [DOI] [PubMed] [Google Scholar]
- 47.Mustoe TA, Purdy J, Gramates P, Deuel TF, Thomason A, Pierce GF. Reversal of impaired wound healing in irradiated rats by platelet-derived growth factor-BB. Am J Surg. 1989;158:345–50. doi: 10.1016/0002-9610(89)90131-1. [DOI] [PubMed] [Google Scholar]
- 48.Blume P, Driver VR, Tallis AJ, Kirsner RS, Kroeker R, Payne WG, et al. Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011;19:302–8. doi: 10.1111/j.1524-475X.2011.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liechty KW, Nesbit M, Herlyn M, Radu A, Adzick NS, Crombleholme TM. Adenoviral-mediated overexpression of platelet-derived growth factor-B corrects ischemic impaired wound healing. J Invest Dermatol. 1999;113:375–83. doi: 10.1046/j.1523-1747.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 50.Breitbart AS, Laser J, Parrett B, Porti D, Grant RT, Grande DA, et al. Accelerated diabetic wound healing using cultured dermal fibroblasts retrovirally transduced with the platelet-derived growth factor B gene. Ann Plast Surg. 2003;51:409–14. doi: 10.1097/01.SAP.0000084461.83554.71. [DOI] [PubMed] [Google Scholar]
- 51.Chan RK, Liu PH, Pietramaggiori G, Ibrahim SI, Hechtman HB, Orgill DP. Effect of recombinant platelet-derived growth factor (Regranex) on wound closure in genetically diabetic mice. J Burn Care Res. 2006;27:202–5. doi: 10.1097/01.BCR.0000202898.11277.58. [DOI] [PubMed] [Google Scholar]
- 52.Karr BP, Bubak PJ, Sprugel KH, Pavlin EG, Engrav LH. Platelet-derived growth factor and wound contraction in the rat. J Surg Res. 1995;59:739–42. doi: 10.1006/jsre.1995.1232. [DOI] [PubMed] [Google Scholar]
- 53.Hammacher A, Mellstrom K, Heldin CH, Westermark B. Isoform-specific induction of actin reorganization by platelet-derived growth factor suggests that the functionally active receptor is a dimer. EMBO J. 1989;8:2489–95. doi: 10.1002/j.1460-2075.1989.tb08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts SA. Platelet-derived growth factor for foot ulcerations. An effective adjunct to good wound care. Adv Nurse Pract. 2001;9:60–3. [PubMed] [Google Scholar]
- 55.Ehrlich HP, Freedman BM. Topical platelet-derived growth factor in patients enhances wound closure in the absence of wound contraction. Cytokines Cell Mol Ther. 2002;7:85–90. doi: 10.1080/13684730310001643. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Montequin JI, Betancourt BY, Leyva-Gonzalez G, Mola EL, Galan-Naranjo K, Ramirez-Navas M, et al. Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: Treatment up to complete wound closure. Int Wound J. 2009;6:67–72. doi: 10.1111/j.1742-481X.2008.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurtz MP, Svensson E, Heimann TM. Use of platelet-derived growth factor for delayed perineal wound healing in patients with inflammatory bowel disease: A case series. Ostomy Wound Manage. 2011;57:24–31. [PubMed] [Google Scholar]
- 58.Shackelford DP, Fackler E, Hoffman MK, Atkinson S. Use of topical recombinant human platelet-derived growth factor BB in abdominal wound separation. Am J Obstet Gynecol. 2002;186:701–4. doi: 10.1067/mob.2002.121867. [DOI] [PubMed] [Google Scholar]
- 59.Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: A phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen. 1999;7:141–7. doi: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 60.Ono I, Akasaka Y, Kikuchi R, Sakemoto A, Kamiya T, Yamashita T, et al. Basic fibroblast growth factor reduces scar formation in acute incisional wounds. Wound Repair Regen. 2007;15:617–23. doi: 10.1111/j.1524-475X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 61.Richard JL, Parer-Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, et al. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double-blind, placebo-controlled study. Diabetes Care. 1995;18:64–9. doi: 10.2337/diacare.18.1.64. [DOI] [PubMed] [Google Scholar]
- 62.Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26:1856–61. doi: 10.2337/diacare.26.6.1856. [DOI] [PubMed] [Google Scholar]
- 63.Mohan VK. Recombinant human epidermal growth factor (REGEN-D 150): Effect on healing of diabetic foot ulcers. Diabetes Res Clin Pract. 2007;78:405–11. doi: 10.1016/j.diabres.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Ginsburg GS, Willard HF. Genomic and personalized medicine: Foundations and applications. Transl Res. 2009;154:277–87. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Jones J, Libermann TA. Genomics of renal cell cancer: The biology behind and the therapy ahead. Clin Cancer Res. 2007;13:685s–92s. doi: 10.1158/1078-0432.CCR-06-1867. [DOI] [PubMed] [Google Scholar]
- 66.Auffray C, Chen Z, Hood L. Systems medicine: The future of medical genomics and healthcare. Genome Med. 2009;1:2. doi: 10.1186/gm2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawksworth JS, Stojadinovic A, Gage FA, Tadaki DK, Perdue PW, Forsberg J, et al. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250:1002–7. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 68.Lantis JC, 2nd, Boone D, Gendics C, Todd G. Analysis of patient cost for recombinant human platelet-derived growth factor therapy as the first-line treatment of the insured patient with a diabetic foot ulcer. Adv Skin Wound Care. 2009;22:167–71. doi: 10.1097/01.ASW.0000305466.25177.a8. [DOI] [PubMed] [Google Scholar]
- 69.Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9:115. doi: 10.1186/1472-6963-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pomahac B, Hirsch T, Eriksson E. Wound management. In: Bahman G, Eriksson E, Persing JA, editors. Plastic Surgery: Indications and Practice. Elsevier; 2009. pp. 27–36. [Google Scholar]


