Abstract
Chronic wounds continue to be a major challenge for the medical profession, and plastic surgeons are frequently called in to help in the management of such wounds. Apart from the obvious morbidity to the patient, these problem wounds can be a major drain on the already scarce hospital resources. Sometimes, these chronic wounds can be more taxing than the underlying disease itself. Although many newer methods are available to handle such situations, the role of stem cells in the management of such wounds is an exciting area that needs to be explored further. A review of literature has been done regarding the role of stem cells in the management of chronic wounds. The abnormal pathology in such wounds is discussed and the possible role of stem cells for optimal healing in such cases would be detailed.
KEY WORDS: Adult stem cells, chronic wound, stem cells
NORMAL WOUND HEALING
Wound healing requires a concerted effort of remodeling of various components of the connective tissue in the presence of appropriate cytokines and growth factors. Wound healing is a complex process involving a cascade of events, orchestrated by interactions between many cell types, soluble factors and matrix components. Various steps like microbial control, subsidence of inflammation, regeneration of connective tissue, angiogenesis and epithelialisation should take place in a time-bound sequence.[1]
Any acute wound initially goes through the phase of “haemostasis and inflammation”. Immediately after the injury, the cell membranes release potent vasoconstrictors like prostaglandin 2 alpha and thromboxane A2. The collagen in the wound sets off the clotting cascade through both the intrinsic and the extrinsic pathways. The resulting fibrin clot contains collagen, thrombin and fibronectin. These in turn lead to the release of inflammatory cytokines and growth factors. Neutrophils undergo chemotaxis into the wound in the presence of interleukin-1, tumour necrosis factor (TNF)-alpha, transforming growth factor (TGF)-beta, platelet factor-4 and bacterial products. These cells get rid of the wound bacteria and devitalized tissue.
The wound then enters into a “proliferative phase” in the vital presence of the activated macrophage. Collagenases secreted by the activated macrophage debride the wound. Interleukins and TNFs stimulate fibroblasts and TGF stimulates keratinocytes. Epithelialisation, angiogenesis, granulation tissue and collagen deposition are the cardinal events that take place.[2] In the proliferative phase, the fibrin clot is replaced by a template of fibrocytes that lay down collagen. Proliferation of endothelial cells results in neovascularisation and capillary formation into the wound bed. This is facilitated by TNF-alpha and is essential for wound healing.
The proliferative phase ends with the formation of granulation tissue. Platelet-derived growth factor (PDGF) and epidermal growth factor secreted, respectively, by platelets and macrophages lead to fibroblast activation. In response to TGF-beta 1 secreted by the macrophages, fibroblasts situated inside the wound transform into myofibroblasts. These myofibroblasts are less capable of proliferation, but are responsible for wound contraction. Stimulated by PDGF, fibroblasts lay down a provisional matrix composed of type III collagen, glycosaminoglycans and fibronectin. Healthy granulation tissue requires an adequate supply of nutrients via capillaries.
An organised deposition of a matrix of collagen is the hallmark of the third phase of “remodeling or maturation”. This phase usually begins 1 week after the injury, and extends up to 1 year [Figure 1]. In the early part of the third phase (4–5 weeks), a lot of collagen deposition takes place and the tissue is indurated. This softens later, as the collagen is resorbed and aligned. Regression of vessels leads to the scar tissue becoming paler with time. If there are problems with matrix deposition, the strength of the wound is considerably impaired. If excess collagen is laid down, hypertrophic scar or keloids result. The collagen in granulation tissue is fundamentally different from the collagen in the normal dermis. It has thinner fibres, probably due to greater hydroxylation and glycosylation of the lysine residues. The strength of the wound reaches a maximum of 80% at around 3 months after injury.[2]
Figure 1.
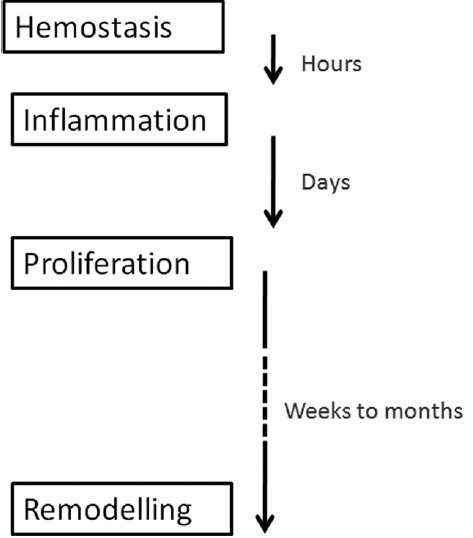
Time line of the stages of normal wound healing
ABNORMALITIES IN A CHRONIC WOUND
The definition of a chronic wound is not clearly found in the literature. These wounds develop whenever there is prolongation of one of the phases of normal healing. These fail to heal when other wounds of similar size would have otherwise healed.[3] According to Leaper and Durani,[4] any wound that that has not shown a 20–40% reduction in area after 2–4 weeks of optimal treatment should be labeled as chronic. A chronicity may be considered when there is no complete healing after 6 weeks or if there is poor response to a treatment change.
Wound healing is said to be impaired when such sequential progression of events for normal healing does not take place and, instead, local injury and chronic inflammation persist.[1,5–7]
Wound healing is a complex process that is susceptible to abnormalities at many levels. These factors can be classified into local and systemic. Low oxygen tension in the wound results in endothelial cell apoptosis, decreased neutrophil and fibroblast activity.[8] Oedema in the wound increases the distance between capillaries at the tissue level and hence local perfusion of oxygen is decreased. Most chronic wounds are hypothesized to have a local environment with reduced oxygen levels.[8] Indeed, hyperoxia recruits bone marrow-derived progenitor cells into diabetic and ischaemic wounds.[9] The resident fibroblasts in chronic wounds may be phenotypically senescent and show decreased responsiveness to TGF and PDGF.[10] The presence of bacteria in the wound increases pro-inflammatory mediators and decreases growth factor levels. All chronic wounds have quantifiable bacterial counts. The local tissue responds to bacterial presence with inflammation. The neutrophils and macrophages release proteases and oxidants, which degrade cytokines and extracellular matrix. But, these also place undue stress on the viable neighbouring cells. Combined, tissue hypoxia and bacterial proliferation are two major local obstacles in the healing of any wound.[7]
TISSUE ENGINEERING
Tissue engineering is a developing field with a lot of promise in wound management.[11] The components of tissue engineering[12] are (1) gene therapy, (2) cytokines and other growth factors, (3) scaffold and (4) cells. Stem cells come under the cellular component.
Stem cell
A stem cell is a cell that can self-replicate and give rise to more than one type of mature daughter cell. Thus, stem cell incorporates a broad range of cells with different capacities for proliferation and differentiation.
Totipotent stem cells are capable of giving rise to an intact organism, including germinal tissues. Pleuripotent stem cells can give rise to cells derived from all three germ layers. Multipotent or organ-specific stem cells are capable of giving rise to the cells that comprise a single organ system or tissue. Therefore, a stem cell is characterized by (1) unlimited self-renewal capacity, (2) long-term viability, (3) multi-lineage potential,[12] (4) participation in tissue repair and (5) preservation of somatic homeostasis.[13,14] Stem cells can be harvested from embryonic or adult tissues. A classical stem cell must be capable of asymmetric cell division, i.e. it must produce as progeny, one exact multipotent replica cell and a transit amplifying (TA) cell[13] that performs a more specialized function.[15,16] Thus, replication of a single cell can potentially result in whole populations of cells. This will lead on to produce tissue regeneration along with retention of a population of identical stem cells. It is these characteristics of clonogenicity and pleuripotency that result in their therapeutic impact.[15,17]
Embryonic stem cells are formed from the inner cell mass of a pre-implantation blastocyst.[18] These have the potential to differentiate into any type of cell (multipotent). With suitable media and in the presence of growth factors, these can give rise to keratinocytes. However, the stability and oncogenicity of embryonic stem cells have to be further defined. On the contrary, stem cells harvested from adult tissues have been well studied.[12] The bone marrow is the richest adult source of these cells. Cells have also been harvested from the peripheral blood, umbilical cord blood, amniotic membrane,[19] adipose tissue, etc. Organ systems also harbour indigenous holoclones of cells, with high-renewal capacity and low levels of differentiation.[13] These are called resident stem cells. In the skin, these are the dermal sheath cells of the hair follicle.[20,21]
The uncommitted mesenchymal stem cells (MSCs), residing in the bone marrow stroma, are fibroblast-like cells that optimize the microenvironment of the haematopoietic cells.[5] Approximately 0.0001% of all nucleated cells in the marrow are MSCs. The same kinds of cells are also found in connective tissues across various organs in the body. These MSCs are capable of differentiating into tissues like bone, cartilage, adipose tissue and endothelium, depending on their microenvironment (niche).[22,23] The adult bone marrow aspirate can provide 30% more multipotent stem cells as compared with umbilical cord blood.[17] However, the yield of these cells is still quite low immediately upon harvest.[12] It has been demonstrated that marrow-derived – MSCs – can be expanded (multiplied) due to their property of adhering to tissue culture surfaces.[22,24]
Stem cell actions
Research suggests that stem cells have mainly two actions. Firstly, they help to attenuate the systemic inflammatory response.[15] Infusions of MSCs have been found to upregulate the anti-inflammatory cytokines[25] such as IL-10 and IL-12 while decreasing the concentrations of pro-inflammatory cytokines like interferon-gamma, IL-1, IL-6 and macrophage inflammatory protein-1 alpha. In murine studies, MSCs have been found to have anti-apoptotic effects, upregulation of Bcl-2 and suppression of caspase enzymes in adjacent cells.[25] In mice pre-treated with endotoxin, intrapulmonary infusions of MSCs resulted in reduction in pulmonary vascular congestion, decreased alveolar wall thickening and increased survival.[26] Ex vivo expanded MSCs are known to interact with a broad range of immunocytes, including T lymphocytes, B lymphocytes, NK cells and dendritic cells.
Secondly, MSCs modulate wound healing.[15] Injury results in multiplication of these cells in the marrow. These MSCs then home in to the sites of injury and seed the wound.[27] Inside the microvasculature of the developing granulation tissue, they differentiate into dermal fibroblasts, myofibroblasts,[22] lymphoid tissue and antigen presenting cells.[17,28] Endothelial progenitor cells, again from the marrow, augment new vessel formation (vasculogenesis[18] ). Integrating themselves into the wound, the MSCs are postulated to directly participate in its repair.[18]
Transplanted MSCs are thought to impact locally through five major paths[29] – (1) increased angiogenesis, (2) decreased local inflammation, (3) anti-apoptotic and chemotactic signaling, (4) normalization of extracellular matrix and (5) stimulation of nearby resident stem cells. They have been reported to secrete vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor (TGF beta), hepatocyte growth factor, interleukins 10 and 13 and other cytokines and growth factors (paracrine signaling[18]), which help in remodeling of the extracellular matrix and in neovascularisation. Even in an environment with low oxygen tension, bone marrow MSCs are known to respond positively.[30] Up to 20% of fibroblasts in the wound are postulated to arise from bone marrow precursors.[31] Again, MSCs have the potential to stimulate and differentiate the resident progenitor cells and promote recovery of injured local cells. The immunosuppressive properties of MSCs also help in prolonged wound coverage by delay of allograft rejection.
Only around 5% of the stem cells that are injected into the wound are proved to survive after the initial phase.[29] Engraftment levels of locally delivered stem cells were observed to be too low to significantly regenerate functional tissue, especially in cardiac myocytes and smooth muscle tissue.[32] The injected human MSCs have not been described to transform into keratinocytes and dermal appendage cells.[22] The immuno-attenuative response afforded by the local presence of stem cells can be replicated by cell-free conditioned media derived from stem cells.[29] All these evidence point to the theory that stem cells benefit through paracrine pathways, rather than by replacing functional tissue.
Thus, in contrast to any specific drug that affects a single pathway for its action, MSCs exhibit their therapeutic power through diverse systemic and local interrelated routes.[18]
Nearly half a century has elapsed since Friedenstein and co-workers[33] first isolated MSCs from rat bone marrow. These cells have been experimented in diseases as diverse as myocardial infarction, segmental bone defect and lung fibrosis.[34] Barrandon et al. cultured different clones of keratinocytes in vitro and introduced the concept of stem cells in wound care.[35] However, stem cells are mostly used in the field of tissue engineering.[12,17] Many dermal analogues have been manufactured to act as a scaffold for keratinocyte regeneration.
Another approach is the local application of stem cells for optimizing the regenerative capability of wounds. Badiavas and Falanga in 2003 introduced the use of bone marrow-derived cells in chronic wounds.[36] Three patients with recalcitrant wounds present for more than 1 year and resistant to standard treatment were chosen for the study. Autologous bone marrow aspirate was topically applied in the first sitting. Part of the aspirate was cultured for subsequent administrations later. The authors reported healing of two of the wounds with three sittings of cell treatment alone.
Ichioka and colleagues used a collagen matrix impregnated with bone marrow aspirate on a chronic leg ulcer that had not healed for more than 1 year.[37] The wound was subsequently skin grafted and healed with full-graft take. The authors postulated that angiogenesis is accelerated soon after application of bone marrow aspirate. Humpert et al. examined the nature of wound healing in a type 2 diabetic patient after the use of topical mononuclear bone marrow cells.[38] They recorded that angiogenesis is restored in the presence of such stem cells. An in vitro study revealed that the levels of bFGF, VEGF and collagen synthesis were much higher in bone marrow stromal cells than in dermal fibroblasts.[39]
The question is whether these stem cells differentiate into elements of connective tissue in the microarchitecture of the wound. Do they produce cytokines and growth factors that promote epithelialisation? The consensus postulated is that the wounds change their character from a chronic non-healing one to an acute regenerating one.[17] Currently, this change of character requires surgical debridement. By removing diseased tissue, debridement recruits fresh fibroblasts, decreases bacterial load and channels the wound into the stages of healing.[10] These are precisely the roles that stem cells have to play.
Burd reports the use of autologous bone marrow on chronic non-healing leg ulcers.[17] The ulcers became more vascular. The wounds as such were definitively closed with skin grafts.
Rasulov et al.[40] document using this procedure in a patient who sustained a 40% Total Burn Surface Area TBSA burn. They applied allogenic fibroblast-like MSCs on the burn wounds prior to skin grafting. They reported that there was a decrease in serous discharge from the wound after the application of stem cells. The patient had less pain after the procedure. On follow-up, they noted neo-angiogenesis, increased epithelialisation and increased graft uptake in the wound.
Ayyappan et al.[41] describe the burn wound as a medium for colonisation of bacteria. They hypothesize that healing would accelerate if inflammatory cells are brought to the wound from the bone marrow. They describe the topical application of autologous bone marrow on two patients with chronic non-healing ulcers. These ulcers epithelialised after such treatment in both the cases. In one patient, a small residual raw area persisted that healed totally with a split-thickness skin graft.
Falanga and colleagues[42] expanded autologous BM-MSCs ex vivo and combined them with fibrin spray for topical application. They studied the efficacy of this system in healing of four acute and six chronic wounds. The acute wounds were defects left to heal secondarily following cancer excision. The authors reported healing of these wounds within 8 weeks with the topical application alone. The chronic wounds that were chosen by the authors were refractory wounds present for more than 1 year. All of those healed within 16–20 weeks, with up to three sittings of the fibrin–MSC delivery system.
Lataillade et al.[43] reported the use of MSCs in a patient who sustained a severe radiation burn injury to his left buttock. The patient underwent multiple sittings of debridement followed by split-skin grafting and local stem cell injection. These cells were cultured from bone marrow aspirate and underwent a two-step expansion process ex vivo, before local injection. Complete healing was noted within 6 months, and without any functional disabilities.
Yoshikawa and colleagues[44] in 2008 treated 20 patients with chronic wounds with MSCs. The wounds were of diverse origin and included post-burn, diabetic and decubitus ulcers. The MSCs were cultured and expanded and impregnated into a collagen sponge that served as a dermal replacement. Some of the wounds had to subsequently undergo an autologous split-skin grafting. The authors reported that 18 of the 20 wounds healed satisfactorily.
Adipose-derived stem cells (ASCs) are similar to MSCs in morphology, differentiation and paracrine secretion under hypoxic conditions.[45] Plastic surgeons, lately adipose-tissue engineers, take advantage of their abundance in liposuction specimens for cell-assisted lipotransfer.[46,47] ASCs were used along with suitable scaffolds to regenerate bone in human calvarial[48] and maxillectomy[49] defects. Healing of radiation ulcers[50] and gastrointestinal fistulae[51] has also been reported.
PROBLEMS AND POSSIBILITIES
A pilot study was conducted in our hospital on burn patients using bone marrow MSCs expanded in vitro (publication pending). Two wounds of similar size were compared. Both wounds underwent closure by split-skin grafting. The test wound was injected with up to 2–3 million MSCs at the time of grafting. While a majority of the test wounds healed faster than their counterparts, we did encounter some areas of concern for clinical application of stem cells.
The ideal mechanism of delivery of stem cells has not been elucidated. Falanga refined the local delivery of stem cells by incorporating them in fibrin as a spray.[42] He postulated that incorporating into fibrin might be ideal for transporting cells and soluble mediators into sites of injury.[16] Bartosh et al. envisaged that 3D constructs of MSCs, with enhanced anti-inflammatory properties, could be mass produced as off-the-shelf medication.[52] Future technology could involve the use of biogels,[53] biodegradable matrices[14] and microparticles that integrate into the wound bed without the risk of foreign body reaction or infection.[18] Dermal matrix carrier has been found to improve the therapeutic effects of bone marrow and ASCs in murine models.[54,55] Composite skin with xenogenic dermis seeded with epidermal stem cells and dermal papilla cells has also favoured wound healing.[56] Impregnating the carrier with growth factors that induce the commitment of resident stem cells into keratinocytes is also another possibility. Genetic modulation and transfection of cutaneous stem cells for induction into keratinocyte lineage has also been postulated.[19,57,58] MSC mobilization strategies with factors such as Granulocyte Macrophage Colony Stimulating Factor GM-CSF are envisaged.[59] Development of smart biomaterials that would augment the transit of circulating stem cells is also sought after. This can potentially avoid the application of exogenous cells, using the patient's own cells for wound healing.[7]
The amount of stem cells required for optimisation of the wound is also not standardised. The number and location of stem cells within skin may contribute to rapid healing seen in the foetus.[13] However, cells migrate to distant organs like spleen,[60] when therapeutically administered numbers exceed 1 ×106. Adequate culture, expansion and characterisation of stem cells take 3 weeks to 1 month in the laboratory. This timeframe can give rise to significant delay in coverage of large wounds.
The current status of stem cells in chronic wounds would be as an additional tool to the existing therapeutic armamentarium. The first worldwide clinical trial involving cadaveric MSCs will be underway this year.[61] Future goals of stem cell therapy for chronic wounds should focus on rapid expansion of cells in vitro. Another option would be to maximise the number of precursor cells resident in the wound.[32] Effective delivery and optimal engraftment of these cells are also of paramount importance. A bioengineered dermal–epidermal composite enriched with expanded autologous stem cells that can immune-modulate the wound it covers remains a challenge for reconstructive surgery.[11]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489–93. [PubMed] [Google Scholar]
- 2.Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: An overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 3.Izadi K, Ganchi P. Chronic wounds. Clin Plast Surg. 2005;32:209–22. doi: 10.1016/j.cps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Leaper DJ, Durani P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int Wound J. 2008;5:361–8. doi: 10.1111/j.1742-481X.2007.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125:510–6. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117(7 Suppl):72S–109S. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- 7.Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63:866–81. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr Surg. 2006;117(7 Suppl):35S–41S. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez OC. Angiogenesis and vasculogenesis: Inducing the growth of new blood vessels and wound healing by stimulation of bone marrow–derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45:39A–47A. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 11.Bottcher-Haberzeth S, Biedermann T, Reichmann E. Tissue engineering of skin. Burns. 2010;36:450–60. doi: 10.1016/j.burns.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Hedrick MH, Daniels EJ. The use of adult stem cells in regenerative medicine. Clin Plastic Surg. 2003;30:499–505. doi: 10.1016/s0094-1298(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 13.Roh C, Lyle S. Cutaneous stem cells and wound healing. Pediatr Res. 2006;59:100R–3R. doi: 10.1203/01.pdr.0000203572.51876.ba. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Dai Y, Hu Q, Li J, Yuan J, Li J, et al. An experimental study on the repair of full skin loss of nude mice with composite graft of epidermal stem cells. Burns. 2006;32:416–22. doi: 10.1016/j.burns.2005.10.014. PMID: 16621316. [DOI] [PubMed] [Google Scholar]
- 15.Butler KL, Goverman J, Ma H, Fischman A, Yu YM, Bilodeau M, et al. Stem Cells and Burns: Review and Therapeutic Implications. J Burn Care Res. 2010;31:874–81. doi: 10.1097/BCR.0b013e3181f9353a. [DOI] [PubMed] [Google Scholar]
- 16.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73–8. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007;33:282–91. doi: 10.1016/j.burns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Ko SH, Nauta A, Wong V, Glotzbach J, Gurtner GC, Longaker MT. The Role of Stem Cells in Cutaneous Wound Healing: What Do We Really Know? Plast Reconstr Surg. 2011;127(Suppl):10S–20S. doi: 10.1097/PRS.0b013e3181fbe2d8. [DOI] [PubMed] [Google Scholar]
- 19.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahoda CAB, Reynolds AJ. Hair follicle dermal sheath cells: Unsung participants in wound healing. Lancet. 2001;358:1445–8. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, Bartfeld S, Clevers H. Tissue-Resident Adult Stem Cell Populations of Rapidly Self-Renewing Organs. Cell Stem Cell. 2010;7:656–70. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Schneider RKM, Neuss S, Stainforth R, Laddach N, Bovi M, Knuechel R, et al. Three-dimensional epidermis-like growth of human mesenchymal stem cells on dermal equivalents: Contribution to tissue organization by adaptation of myofibroblastic phenotype and function. Differentiation. 2008;76:156–67. doi: 10.1111/j.1432-0436.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 23.Sellheyer K, Krahl D. Skin mesenchymal stem cells: Prospects for clinical dermatology. J Am Acad Dermatol. 2010;63:859–65. doi: 10.1016/j.jaad.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 25.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Kelly ML, Meldrum DR. Stem cells in sepsis. Ann Surg. 2009;250:19–27. doi: 10.1097/SLA.0b013e3181a77b9c. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 27.Rea S, Giles NL, Webb S, Adcroft KF, Evill LM, Strickland DH, et al. Bone marrow-derived cells in the healing burn wound—More than just inflammation. Burns. 2009;35:356–64. doi: 10.1016/j.burns.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Korbling M, Estrov Z. Adult stem cells for tissue repair- a new therapeutic concept? N Engl J Med. 2003;349:570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 29.Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–81. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–7. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 32.Hirschi KK, Goodell MA. Hematopoietic, vascular and cardiac fates of bone marrow-derived stem cells. Gene Ther. 2002;9:648–52. doi: 10.1038/sj.gt.3301722. [DOI] [PubMed] [Google Scholar]
- 33.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 34.Barry FP, Murphy JM, O’Brien T, Mahon B. Mesenchymal stem cell transplantation for tissue repair. Semin Plast Surg. 2005;19:229–39. [Google Scholar]
- 35.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci. 1987;84:2302–6. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–6. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 37.Ichioka S, Kouraba S, Sekiya N, Ohura N, Nakatsuka T. Bone marrow-impregnated collagen matrix for wound healing: Experimental evaluation in a microcirculatory model of angiogenesis, and clinical experience. Br J Plast Surg. 2005;58:1124–30. doi: 10.1016/j.bjps.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 38.Humpert PM, Bartsch U, Konrade I, Hammes HP, Morcos M, Kasper M, et al. Locally applied mononuclear bone marrow cells restore angiogenesis and promote wound healing in a type 2 diabetic patient. Exp Clin Endocrinol Diabetes. 2005;113:538–40. doi: 10.1055/s-2005-872886. [DOI] [PubMed] [Google Scholar]
- 39.Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55:414–9. doi: 10.1097/01.sap.0000178809.01289.10. [DOI] [PubMed] [Google Scholar]
- 40.Rasulov MF, Vasilchenkov AV, Onishchenko NA, Krasheninnikov ME, Kravchenko VI, Gorshenin TL, et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139:141–4. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 41.Ayyappan T, Chadha A, Shaikh MF, Naik N, Desai I, Kadam, Shah C, et al. Topically applied autologous bone marrow in healing of chronic non-healing raw areas—a pilot study. Indian J Burns. 2004;12:42–7. [Google Scholar]
- 42.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 43.Lataillade JJ, Doucet C, Bey E, Huet C, Clairand I, Bottollier-Depois JF, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–94. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–77. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 45.Tholpady SS, Llull R, Ogle RC, Rubin JP, Futrell JW, Katz AJ. Adipose tissue: Stem cells and beyond. Clin Plast Surg. 2006;33:55–62. doi: 10.1016/j.cps.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterodimas A, de Faria J, Nicaretta B, Papadopoulos O, Papalambros E, Illouz YG. Cell-assisted lipotransfer. Aesthet Surg J. 2010;30:78–81. doi: 10.1177/1090820X10362730. [DOI] [PubMed] [Google Scholar]
- 48.Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J Craniomaxillofac Surg. 2004;32:370–3. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De-La-Quintana P, Trebol J, et al. Treatment of enterocutaneous fistula in Crohn's disease with adipose-derived stem cells: A comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24:27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
- 52.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–9. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altman AM, Matthias N, Yan Y, Song YH, Bai X, Chiu ES, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29:1431–42. doi: 10.1016/j.biomaterials.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 55.Leonardi DF, Nardi N, Meuer R. The use of the association of stem cells and tissue engineering in promoting regeneration and neovascularization of skin wounds. Burns. 2009;35S:S1–47. [Google Scholar]
- 56.Shao-Hai Q, Po L, Ju-Lin X, Bin S, Ying-Bin X, Chang-Neng K, et al. Experimental study on repairing of nude mice skin defects with composite skin consisting of xenogeneic dermis and epidermal stem cells and hair follicle dermal papilla cells. Burns. 2008;34:385–92. doi: 10.1016/j.burns.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Heng BC, Cao T, Liu H, Phan TT. Directing stem cells into the keratinocyte lineage in vitro. Exp Dermatol. 2005;14:1–16. doi: 10.1111/j.0906-6705.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 58.Pereira CT, Herndon DN, Perez-Polo JR, Burke AS, Jeschke MG. Scar trek: Follicular frontiers in skin replacement therapy. Genet Mol Res. 2007;6:243–9. [Google Scholar]
- 59.Mansilla E, Marýn GH, Drago H, Sturla F, Salas E, Gardiner C, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage. New evidence for their use in regenerative medicine. Transplant Proc. 2006;38:967–9. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 60.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–7. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansilla E, Aquino VD, Roque G, Tau JM, Maceira A. Time and regeneration in burns treatment: Heading into the first worldwide clinical trial with cadaveric mesenchymal stem cells. Burns. 2012;38:450–2. doi: 10.1016/j.burns.2011.09.007. [DOI] [PubMed] [Google Scholar]


