Abstract
Hyperbaric oxygen therapy (HBOT) is the use of 100% oxygen at pressures greater than atmospheric pressure. Today several approved applications and indications exist for HBOT. HBOT has been successfully used as adjunctive therapy for wound healing. Non-healing wounds such as diabetic and vascular insufficiency ulcers have been one major area of study for hyperbaric physicians where use of HBOT as an adjunct has been approved for use by way of various studies and trials. HBOT is also indicated for infected wounds like clostridial myonecrosis, necrotising soft tissue infections, Fournier's gangrene, as also for traumatic wounds, crush injury, compartment syndrome, compromised skin grafts and flaps and thermal burns. Another major area of application of HBOT is radiation-induced wounds, specifically osteoradionecrosis of mandible, radiation cystitis and radiation proctitis. With the increase in availability of chambers across the country, and with increasing number of studies proving the benefits of adjunctive use for various kinds of wounds and other indications, HBOT should be considered in these situations as an essential part of the overall management strategy for the treating surgeon.
KEY WORDS: Air embolism, compartment syndrome, crush syndrome, decompression sickness, diabetes mellitus, diabetic foot, gas gangrene, hyperbaric medicine, hyperbaric oxygen therapy, hyperbaric, hyperbaric oxygenation, necrotising fasciitis, osteomyelitis, osteoradionecrosis, radiation injuries, radiation necrosis, reperfusion injury, soft tissue infections, surgical flaps, transcutaneous oximetry
INTRODUCTION
Wound healing is a subject of great interest and involvement for the surgeon. While much of the physiology of wound healing is understood, gaps still exist in our understanding of the phenomenon. The surgeon attempts to modify the wound milieu by various means at his disposal. One such method is hyperbaric oxygen therapy (HBOT).
HBOT is the use of 100% oxygen at pressures greater than atmospheric pressure. The patient breathes 100% oxygen intermittently while the pressure of the treatment chamber is increased to greater than 1 atmosphere absolute (ATA).[1] HBOT has had very exciting and interesting beginnings. In 1620, Drebbel developed a one-atmosphere diving bell.[2] Thereafter, a British clergyman named Henshaw built and ran the first well-known chamber called the domicilium that was used to treat a multitude of diseases. The idea of treating patients under increased pressure was continued by the French surgeon Fontaine, who built a pressurised, mobile operating room in 1879. Dr. Orville Cunningham, a professor of anaesthesia, ran what was known as the “Steel Ball Hospital.” The structure, erected in 1928, was six stories high and 64 feet in diameter and could reach 3 atmospheres pressure. The hospital was closed in 1930 because of the lack of scientific evidence indicating that such treatment alleviated disease and was broken down during World War II for scrap.[3,4] The modern age of hyperbaric medicine began in 1937, when Behnke and Shaw used a hyperbaric chamber to treat decompression sickness (DCS), but it was not until 1955 that HBOT was used for conditions other than DCS. That year, Churchill-Davidson began to use oxygen therapy in a hyperbaric chamber to treat radiotherapy-induced damage in cancer patients. In 1956, Boerema of Holland even performed the first reported heart surgery in a hyperbaric chamber.[2,4]
Since then, the number of indications for which HBOT has been used has been steadily increasing so much so that one article mentioned 132 documented past and present indications for which it has been used.[1] Undersea and Hyperbaric Medicine Society (UHMS) has recognised various indications as “Approved” indications [Table 1].[5] These indications had also been approved by the British Hyperbaric Association.[6] The 2004 European consensus conference in addition recommended HBOT for some additional conditions based on sufficient evidence in the form of expert consensus opinion [Table 2].[7]
Table 1.
UHMS approved indications for HBOT[5]
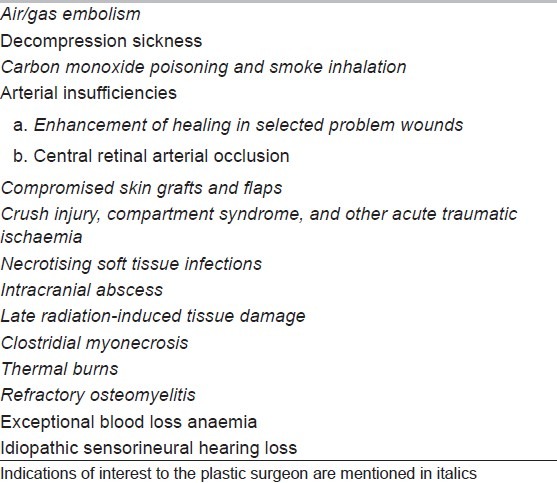
Table 2.
Additional indications recommended by 2004 European Consensus Conference[7]

Apart from the approved indications, a number of areas are being explored to determine if HBOT might be of some clinical benefit.[8] These areas include senility, stroke, multiple sclerosis, sports injuries, high altitude illness, myocardial infarction, brain injuries, migraine, glaucoma, head injuries, management of chronic fatigue in HIV-positive patients[9] and enhancement of survival in free flaps.[10]
ADMINISTRATION OF HBOT
HBOT is administered inside chambers that are pressurised using air or oxygen to pressures more than atmospheric. Broadly, there are two types of chambers, multiplace which can hold more than one patient and monoplace chambers designed to cater for a single patient. Multiplace chambers use masks or hoods to administer oxygen to the patient and are more suitable for management of critical patients. Monoplace chambers were once made of metal, but are now made of transparent acrylic and are pressurised directly using oxygen, and therefore the patient does not need to wear a mask or hood. For hyperbaric chambers, monitoring and intensive care equipment are available where equipments are placed outside the chamber and probes fixed to the patient via biomedical connectors fixed to the bulkhead of the chamber.[3] Only equipment certified for use inside a hyperbaric chamber may be used. In case of lack of clarity on certification, the clinician must refer the equipment manuals or contact the manufacturer for certification of use.
Most therapy is given at 2 or 3 ATA and the average duration of therapy is 60–90 min. Number of therapies may vary from 3 to 5 for acute conditions to 50–60 for radiation illnesses.[1]
MECHANISM OF ACTION
HBOT has two primary mechanisms of action, hyperoxygenation and a decrease in bubble size. Hyperoxygenation is an application of Henry's law and results from an increase in dissolved oxygen in plasma as a result of increased partial pressure of arterial oxygen. A pressure of 3 ATA results in 6 ml of O2 being dissolved per 100 ml of plasma, thus rendering as much O2 delivery as by haemoglobin bound O2. Hyperoxygenation is valuable in management of crush injury, compartment syndrome, flap salvage and acute blood loss anaemia. Decrease in bubble size is an application of Boyle's law according to which the volume of a bubble decreases directly in proportion to increasing pressure and is the primary mechanism at work in management of decompression sickness and arterial gas embolism.[3,4,11–13]
Secondary mechanisms of action include vasoconstriction, angiogenesis, fibroblast proliferation, leukocyte oxidative killing, toxin inhibition and antibiotic synergy. Hyperoxia in normal tissues causes vasoconstriction which reduces post-traumatic tissue oedema, contributing to the treatment of crush injuries, compartment syndromes and burns. This vasoconstriction, however, does not cause hypoxia as this is more than compensated by increased plasma oxygen content and microvascular blood flow.
Oxygen is vital for hydroxylation of lysine and proline residues during collagen synthesis and for cross linking and maturation of collagen which is required for strong wound healing. Lack of oxygen is corrected during HBOT, leading to adequate amounts of mature collagen formation.
Hypoxia is a vital stimulant for angiogenesis, but development of adequate capillary network requires adequate amounts of tissue oxygen concentration. HBOT increases the oxygen gradient between the centre and periphery of the wound, thus creating a strong angiogenic stimulus. This along with fibroblastic proliferation leads to increased neovascularisation.
HBOT increases the generation of oxygen free radicals, which oxidise proteins and membrane lipids, damage DNA and inhibit bacterial metabolic functions. Superoxide dismutase, catalase, glutathione and glutathione reductase keep the formation of these radicals in check until the oxygen load overwhelms the enzymes, leading to the detrimental effects on cell membranes, proteins and enzymes. HBOT is particularly effective against anaerobes which lack superoxide dismutase and facilitates the oxygen-dependent peroxidase system by which leukocytes kill bacteria.
Hyperoxia during HBOT further inhibits clostridial toxin production and improves potency of antibiotics like Fluoroquinolones, Amphotericin B and Aminoglycosides, all of which use oxygen for transport across cell membranes.
APPLICATIONS IN PLASTIC SURGERY
The indications of HBOT of interest to the plastic surgeon are discussed below.
Non-healing wounds: Diabetic, vascular insufficiency ulcers
Non-healing wounds are those which fail to heal within a reasonable time frame despite adequate management. Although multifactorial in aetiology, these wounds are typically hypoxic which is where HBOT becomes very effective. The rationale for treatment of chronic non-healing wounds with HBOT uses the known secondary mechanisms of action. HBOT leads to improved angiogenesis through a multifactorial mechanism. First, fibroblast proliferation and collagen synthesis are oxygen dependent, and collagen is the foundational matrix for angiogenesis. In addition, HBOT likely stimulates growth factors, particularly vascular endothelial growth factor (VEGF), involving angiogenesis and other mediators of the wound healing process. Hyperbaric oxygen also has been shown to have direct and indirect antimicrobial activity; in particular, it increases intracellular leukocyte killing. Decreased oedema due to systemic vasoconstriction allows better diffusion of oxygen and nutrients through tissues while also relieving pressure on the surrounding vessels and structures.[3,11]
Non-healing wounds where HBOT has been successfully used include diabetic foot ulcers, venous and arterial insufficiency ulcers. Diabetic lower extremity ulcers have been the focus of most wound research in hyperbaric medicine, since the aetiology of these wounds is multifactorial, and HBOT can address many of these factors. Several randomised controlled clinical trials have proven the beneficial effects of HBOT for the treatment of diabetic lower extremity wounds, apart from many prospective, non-controlled clinical and retrospective studies.[1,3,14]
HBOT in diabetic wounds is used in conjunction with other wound management techniques including wound debridement, dressings, pressure-relieving strategies, appropriate glycaemic control, and nutrition and antibiotic management.
Patient selection and follow-up is usually done objectively with transcutaneous oximetry (TcPO2) [Figure 1].
Figure 1.
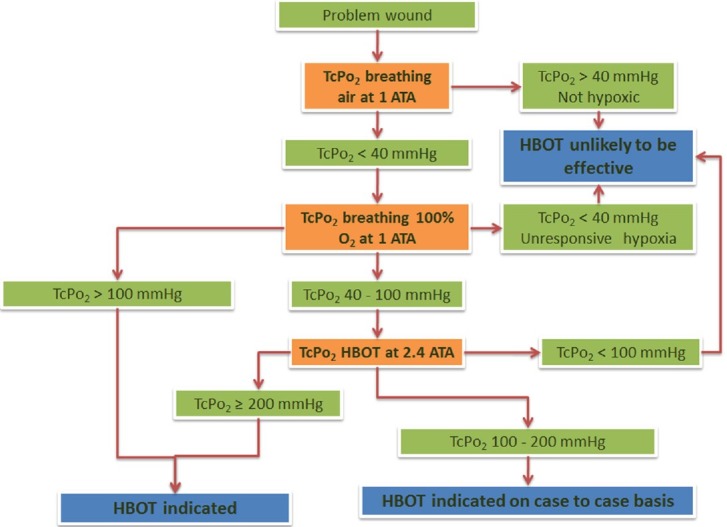
Flow chart for patient selection and predictor of outcomes in patients managed with HBOT based on transcutaneous oximetry measurements[15,16]
Patient selection for HBOT is usually based on measurement of oxygen tension around the wound using TcPO2. TcPO2 can be successfully used to predict patient outcome in wound management with HBOT. It has been proven to be a better predictor of healing outcome compared to Ankle Brachial Index (ABI) and Toe Blood Pressure (TBP).[15] Also, in-chamber TcPO2 >200 mmHg with patient breathing 100% O2 inside the hyperbaric chamber is the single best discriminator between success and failure with HBOT.[16]
A flow chart for patient selection and suitability for HBOT using TcPO2 is shown in Figure 1.[16,17]
In case of nonavailability of TcPO2, Modified Wagner's grading for wounds can also be used for patient selection. Grades IIIb and above are generally regarded as ideal candidates for HBOT.[17]
Figure 2 shows sequential photographs of a 53-year-old male diabetic suffering from spontaneous gangrene of the thigh who was managed successfully with HBOT at a tertiary care hospital of the Armed Forces.
Figure 2.
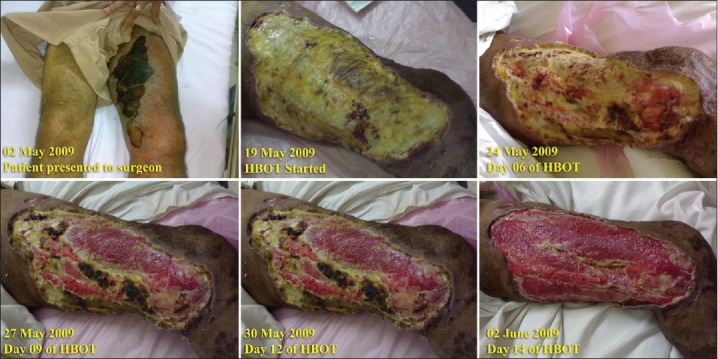
A combination picture showing sequential progress of spontaneous gangrene in a 53-year-old male diabetic managed with HBOT
Infected wounds: Clostridial myonecrosis, necrotising soft tissue infections, Fournier's gangrene
HBOT has direct effect in inhibiting the production of clostridial alpha toxin and the high concentration of oxygen induces greater oxidative free radical mediated microbicidal killing by polymorphonuclear lymphocytes. These mechanisms help in faster and better management of necrotising soft tissue infections and gas gangrene. Multiple studies have proven the beneficial effect of, and justified the use of adjunctive HBOT, in addition to aggressive surgical debridement and aggressive antibiotic therapy which remain the cornerstones of treatment. Studies have also demonstrated significantly reduced mortality rates in patients of necrotising fasciitis and Fournier's gangrene managed with adjunctive HBOT compared to patients managed without HBOT.[4]
HBOT in these cases is given aggressively, twice a day initially, and is best initiated as quickly as possible.[1,3]
Traumatic wounds: Crush injury, compartment syndrome
Hyperbaric oxygen ameliorates the effects of acute traumatic ischaemia through four mechanisms: hyperoxygenation, vasoconstriction, and influence on reperfusion and host factors.[18] HBOT also decreases neutrophil activation, preventing margination, rolling and accumulation of WBCs, thereby reducing the production of free radicals by neutrophils and preventing reperfusion injury.[3] HBOT is also seen to reduce sludging of RBCs.[1]
Besides adequate shock management, direct surgical intervention with debridement and repair of soft tissues and of any damaged vessels and stabilisation of bony elements are of paramount importance. Adjuvant HBOT should be administered as soon as possible; when it is given early it can prevent large expanses of ischaemic necrosis, minimise the frequency and extent of amputations, reduce oedema, control infection, support healing and prevent reperfusion injury.[18]
Gustilo classification of open fractures is commonly used for objective assessment in crush injuries to determine whether HBOT is indicated or not. For the uncompromised host, HBOT is recommended for all Gustilo Grade III B and III C fractures. In the compromised host, the indication for using adjunctive HBOT should start at Grade II.[18]
HBOT should be started as soon as is feasible, ideally within 4–6 h from the time of injury. After emergent surgical intervention, the patient should undergo HBOT at 2–2.5 ATA for 60–90 min. For the next 2–3 days, perform HBOT 3 times daily, then twice daily for 2–3 days, and then daily for the next 2–3 days.[3]
Compromised skin grafts and flaps
Most skin grafts and flaps in normal hosts heal well. In patients with compromised circulation, this may not be the case. The leading pathophysiological factor of compromised grafts and flaps is hypoxia. HBOT benefits patients by reducing the oxygen deficit. The mechanism of improvement of survival of skin grafts may be twofold, by effects on the bed and by effects on the graft. As discussed earlier, wound beds are improved by the hyperoxia, angiogenesis, leukocyte function enhancement and antimicrobial actions of HBOT.[3,11,19] The effects on the graft are principally the improvement of oxygenation. In the first 48 h of grafting, the graft survives by “plasmatic imbibition.” As discussed earlier, oxygen is the most critical needs of tissues. Improved oxygen availability contributes to better graft survival and engraftment. In the usual circumstances, this should not be necessary. However, in the compromised graft/bed, it may be invaluable. The benefit has been established in several animal and clinical studies. HBOT is indicated when the TcPO2 in the wound is less than 40 mmHg and compromised wounds may reveal values as low as 15 mmHg.[19] The benefit of HBOT is expected to be maximal in cases where a relatively large bulk of tissue is transferred as graft, i.e. full-thickness skin grafts and particularly composite grafts which in the process of “take” regularly show a cyanotic period before successful engraftment.
In compromised flaps also, HBOT is found to be of value. Again, the benefits may accrue partly from improvement of the bed and by effects on the flap. An acutely elevated flap is known to be hypoxic and ischaemic. With passage of time, there is an improvement and reorientation in the circulation within the surviving flap. Mechanisms of action on the flap include hyperoxia and anti-oedema effect which improves microcirculation. Further, as reperfusion occurs, HBOT is seen to reduce reperfusion injury and decreases the “no flow” phenomenon. This last action may be due to the effects of HBOT on neutrophils, endothelium and free radicals.[19] A unique mechanism of action of HBOT for preserving compromised flaps is the possibility of closing arteriovenous shunts, decreasing non-nutritive blood flow. Additionally, the same mechanisms of action that improve wound healing, namely, improved fibroblast and collagen synthesis and angiogenesis, also are likely to reduce flap dehiscence.[3]
In clinical practice, improvement in the surviving length of a compromised flap is seen with the use of HBOT.
HBOT treatments are performed at a pressure of 2.0–2.4 ATA for duration of 90 min twice daily for 2–3 days and then, as soon clinical stabilisation occurs, once per day to a total of 20–30 treatments. In case of total venous or arterial occlusion, specifically in free tissue transfer, treatments are given thrice daily on the first day, twice daily for the next 2 days, followed by once daily thereafter along with appropriate surgical therapy.[19]
The action of HBOT on the flap can be quantified by TcPO2 measurements. If intra-chamber (at 2.4 ATA on 100% O2) TcPO2 remains below 50 mmHg, flap survival is unlikely despite the use of HBOT.[19]
Radiation-induced wounds
Radiation injury alters the normal tissue physiology and anatomy. Radiation-induced wounds are typically hypocellular, hypovascular, and hypoxic due to an occlusive endarteritis caused by radiation injury. Hyperbaric oxygen promotes angiogenesis and hyperoxygenation to the irradiated tissues. Increasing the oxygen content to the surrounding tissues markedly increases the overall oxygen gradient between these tissues and the central hypoxic area. The increased oxygen gradient is the essential catalytic factor for angiogenesis.[11]
Typically, radiation-induced wounds are chronic and non-healing and show poor skin graft take. HBOT has been a very successful adjunct in the management of late complications of radiotherapy.[20] Typically, in mandibular reconstruction, in an irradiated field, HBOT has been found to markedly improve outcomes after composite reconstruction. HBOT has been particularly beneficial in the management of mandibular osteoradionecrosis. Similarly, complications of radiotherapy in the pelvic region (cystitis, proctitis) are also benefited by adjunctive HBOT.[3,4]
Multiple hyperbaric treatments, sometimes as many as 50–60,[1] are usually required to significantly increase the capillary density in the affected tissues and promote healing. Prophylactic hyperbaric oxygen is also recommended for procedures (e.g. tooth extraction) on irradiated mandibles.[11]
Thermal burns
Although burns have been mentioned as one of the approved indications for HBOT by UHMS, there is lack of universal consensus on the issue.[21] The mechanisms of action at play in improving the outcome in a burnt patient may involve the following. Hyperoxia produces pre-capillary vasoconstriction. This results in reduced plasma exudation while preserving and enhancing tissue oxygenation. The resultant reduction in oedema and fluid loss cause a marked reduction in the amount of fluids required for resuscitation. Hart,[22] in a prospective study on burn patients, found that HBOT treated patients required 2.2 ml/kg/% total body surface area (TBSA) of fluids as opposed to 3.4 ml/kg/% TBSA in the control group.
Burn wounds typically have a central zone of coagulation surrounded by a zone of stasis, in turn surrounded by a zone of hyperaemia. HBOT was noted to reduce the capillary stasis in the zone of stasis and reduce the increase in size of the zone of coagulation as occurs in burns. Thus, HBOT assists in tissue preservation. This mechanism might be of particular value in the case of burns in aesthetically or functionally important zones (face, hands, perineum) or with delicate vascularisation (cartilaginous - ears, nose). Further, HBOT may exert beneficial effects by way of its anti-sludging effect in the microcirculation and prevention of injury by oxygen free radicals.[21]
Many cases of burns will have associated smoke inhalation with concomitant carbon monoxide poisoning which is a universally accepted leading indication for the use of HBOT. HBOT will thus be invaluable in these cases.
Patients who are likely to benefit most from HBOT are those with a 20–80% TBSA mixed second/third degree burns. In these patients, the first HBOT session should be given within 6 h of the burn injury, followed by two sessions per day, at a pressure of 2.0 ATA, for the first 4–5 days only. Volume of fluids required to be administered is likely to be less than calculated, and hence should be closely monitored so as to prevent pulmonary overload.[21]
Treatment must be in a multiplace chamber equipped for intensive care treatments, preferably with optimal bacteriological isolation and provision for mechanical ventilation and continuous (invasive) monitoring of haemodynamic parameters as may be required.
HBOT in the later stages of management of burns may be useful by its antibacterial action (thus reducing sepsis) and by improved take of skin grafts.
CONTRAINDICATIONS
The absolute contraindication for HBOT is the presence of an untreated pneumothorax as compression and decompression during HBOT could lead to development of tension pneumothorax and gas emboli. Concurrent administration of some medication is also seen as an absolute contraindication. Bleomycin could cause interstitial pneumonitis; Disulfiram blocks superoxide dismutase, and thus reduces body's protection against oxygen toxicity; Cisplatin and Sulfamylon have a mechanism of action opposite to that of HBOT in wound healing, and thus could nullify any benefit from HBOT, and Doxorubicin could cause cardiotoxicity.[3,11]
Relative contraindications include claustrophobia, asthma, chronic obstructive pulmonary disease (COPD), pregnancy, congenital spherocytosis, upper respiratory tract infection or any other Eustachian tube dysfunction, fever, pacemaker in situ and seizures/epilepsy.
COMPLICATIONS
When used in standard protocols, HBOT is safe.[1]
The most common complication during HBOT is barotrauma (injury caused by pressure as a result of an inability to equalise pressure from an air-containing space and the surrounding environment) usually of the middle ear. For an unconscious patient, myringotomy may be done to prevent middle ear barotrauma. Other organs affected by barotrauma are external and inner ear, air sinuses, GI tract and tooth cavities.
Other complications include pulmonary barotrauma which could arise as a result of cavitary or fibrotic lesions in the lung parenchyma, and development of acute CNS oxygen toxicity (known as Paul Bert effect and caused by acute exposure to high partial pressures of oxygen) leading to lowering of seizure threshold and precipitation of seizures inside the chamber. Prolonged exposure to lower pressures of oxygen could cause pulmonary oxygen toxicity (known as Loraine Smith effect) leading to reversible pulmonary restrictive changes. At our centre, as part of a protocol, patients are given a break of about a week from hyperbaric oxygen after 2 weeks of daily sessions in order to prevent the development of pulmonary oxygen toxicity.[3,4,11]
Development of reversible myopia and clouding of pre-existing cataracts are other complications of HBOT.
HBOT: RESULTS OF TREATMENT
Non-traumatic wounds form a major clientele for any HBOT centre. Data from an Armed Forces HBOT centre in a tertiary care hospital for the year 2008 and part of 2009 (January–September) showed that of all the patients who were administered HBOT in these years, non-traumatic wounds formed 40% and 36% of the total HBOT workload, respectively.[23] In this group of patients, significant healing of wounds was seen in 87%. Another study from the same centre, which discussed the healing rates of non-traumatic wounds, found that 84.7% of the cases managed during the period were diabetics with only one patient not having a lower extremity ulcer.[13] Satisfactory healing was seen in 88.37% of cases in this study.
Doctor et al.[14] did a prospective controlled trial to study the effect of HBOT in chronic diabetic foot lesions. Thirty diabetics with chronic foot lesions were randomised to study and control groups and were assessed for average hospital stay, control of infection and wound healing. In the study group managed with HBOT, positive cultures decreased significantly compared to the control group (most pronounced for Escherichia coli) and the need for major amputation was significantly less. The average hospital stay was not affected. They concluded that HBOT could be safely used and would be beneficial as an adjuvant therapy in chronic diabetic foot lesions.
CONCLUSION
HBOT was started as a treatment modality for management of decompression sickness and, with the passage of time, its scope has gradually increased to include numerous indications. Indications of particular interest to the plastic surgeon include ischaemic wounds, diabetic ulcers, traumatic wounds, necrotising infections, failing grafts and flaps, radiation wounds, and thermal burns, and the use of HBOT must be considered by the managing surgeon.
HBOT administration was once the sole purview of the Armed Forces hospitals, that too, in only two centres across the country, namely, Institute of Aerospace Medicine, Bangalore, and Institute of Naval Medicine, Mumbai [Figures 3–5]. Today, HBOT is available at 10 hospitals in the country. These chambers are located in Mumbai (four hospitals), New Delhi, Ahmedabad (two each), Pune and Trichur (one hospital each). Further, it is believed that there are 10 more hospitals in the country where installation of hyperbaric chambers is being planned.
Figure 3.

A multiplace hyperbaric chamber installed at INHS Asvini
Figure 5.

Control panel of the same hyperbaric chamber
Figure 4.

A combination photograph showing auxiliary units of a hyperbaric chamber
HBOT is witnessing a phase of phenomenal growth in the country, something which took place much earlier in other countries. It is time that we recognise the benefits of this treatment modality so that our patients are benefited by the advantages that HBOT has to offer both in terms of speedier recovery as well as cost benefits.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sahni T, Hukku S, Jain M, Prasad A, Prasad R, Singh K. Recent advances in hyperbaric oxygen therapy. Medicine update. Assoc Physicians India. 2004;14:632–9. [Google Scholar]
- 2.Neubauer RA, Maxfield WS. The polemics of hyperbaric medicine. J P and S. 2005;10:15–7. [Google Scholar]
- 3.Latham E, Hare MA, Neumeister M. Schraga BD, Windle ML, Mosenifar Z, editors. Hyperbaric oxygen therapy. eMedicine. Medscape. [Last accessed on 2009 Oct 02, Last updated on 2008 Nov 7]. Available from: http://emedicine.medscape.com/article/1464149-overview .
- 4.Sharkey S. Current indications for hyperbaric oxygen therapy. Journal of the Australian Defence Health Service (ADF Health) 2000;1:64–72. [Google Scholar]
- 5.Indications for hyperbaric oxygen therapy. Definition of Hyperbaric Oxygen Therapy. UHMS. [Last accessed on 2012 Jan 10]. Available from: http://membership.uhms.org/?page=Indications .
- 6.Camporesi EC, editor. Current diseases approved for treatment, hyperbaric oxygen therapy: A Committee Report. UHMS. [Last cited in 1996]. Available from: http://hyperbaric.org.uk/conditionsTreatment.htm .
- 7.European committee for hyperbaric medicine. Lille, France: 2004. Proceedings of the 7th European consensus conference on hyperbaric medicine. [Google Scholar]
- 8.Sahni T, Singh P, John MJ. Hyperbaric oxygen therapy: Current trends and applications. J Assoc Physicians India. 2003;51:280–4. [PubMed] [Google Scholar]
- 9.Bhutani S. HBOT reduces fatigue in HIV positive patients. Jour Marine Medical Society. 2011;13:57–9. [Google Scholar]
- 10.Vishwanath G. Hyperbaric oxygen therapy in free flap surgery: Is it meaningful? Medical journal armed forces India (MJAFI) 2011;67:253–6. doi: 10.1016/S0377-1237(11)60052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael N. Cram AE, Talavera F, Newsome RE, Slenkovich NG, Torre JT, editors. Hyperbaric oxygen therapy. eMedicine. [Last accessed on 2005 May 20]. Available from: http://emedicine.com/plastic/topic526.htm .
- 12.Leach RM, Rees PJ, Wilmshurst P. ABC of oxygen, hyperbaric oxygen therapy. BMJ. 1998;317:1140–3. doi: 10.1136/bmj.317.7166.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhutani S, Verma R. Hyperbaric oxygen therapy in non-healing wounds. Jour Marine Medical Society. 2010;12:89–92. [Google Scholar]
- 14.Doctor N, Pandya S, Supe A. Hyperbaric oxygen therapy in diabetic foot. J Postgrad Med. 1992;38:112–4. [PubMed] [Google Scholar]
- 15.Löndahl M, Katzman P, Hammarlund C, Nilsson A, Landin-Olsson M. Relationship between ulcer healing after hyperbaric oxygen therapy and transcutaneous oximetry, toe blood pressure and ankle-brachial index in patients with diabetes and chronic foot ulcers. Diabetologia. 2011;54:65–8. doi: 10.1007/s00125-010-1946-y. [DOI] [PubMed] [Google Scholar]
- 16.Smart DR, Bennett MH, Mitchell SJ. Transcutaneous oximetry, problem wounds and hyperbaric oxygen therapy. Diving Hyperb Med. 2006;36:72–86. [Google Scholar]
- 17.Matos L, Nunez A. Enhancement of healing in selected problem wounds. In: Kindwall EP, Wheelan HT, editors. Hyperbaric Medicine Practice. 2nd ed. Florida: Best Publishing; 1999. pp. 813–49. [Google Scholar]
- 18.Kemmer A. Crush injury and other traumatic ischemia. In: Mathieu D, editor. Handbook on hyperbaric medicine. Netherlands: Springer; 2006. pp. 311–2. [Google Scholar]
- 19.Mesimeris TA. Compromised skin graft and flap. In: Mathieu D, editor. Handbook on hyperbaric medicine. Netherlands: Springer; 2006. pp. 329–61. [Google Scholar]
- 20.Pasquier D, Schmutz J, Lartigau E. Radio-induced lesion in normal tissues. In: Mathieu D, editor. Handbook on hyperbaric medicine. Netherlands: Springer; 2006. pp. 363–99. [Google Scholar]
- 21.Germonpre P. Burns. In: Mathieu D, editor. Handbook on Hyperbaric Medicine. Netherlands: Springer; 2006. pp. 479–94. [Google Scholar]
- 22.Hart GB, O’Reilly RR, Broussard ND. Treatment of burns with hyperbaric oxygen. Surg Gynecol Obstet. 1974;139:693–6. [PubMed] [Google Scholar]
- 23.Bhutani S. Poster presentation at: XXIV Annual Conference of the Marine Medical Society. Mumbai: Marine Medical Society; 2009. Hyperbaric oxygen therapy; pp. 24–5. [Google Scholar]


