Abstract
Management of perineal wounds can be very frustrating as these invariably get contaminated from the ano-genital tracts. Moreover, the apparent skin defect may be associated with a significant three dimensional dead space in the pelvic region. Such wounds are likely to become chronic and recalcitrant if appropriate wound management is not instituted in a timely manner. These wounds usually result after tumor excision, following trauma or as a result of infective pathologies like hideradenitis suppurativa or following thermal burns. Many options are available for management of perineal wounds and these have been discussed with illustrative case examples. A review of literature has been done for listing commonly instituted options for management of the wounds in perineum.
KEY WORDS: Muscle flaps, perforator flaps, perineum, reconstructive options, skin grafts, vacuum assisted
INTRODUCTION
The perineum includes the region between the pubic symphysis and the coccyx and is probably derived from the Greek words “peri+inan” meaning to “empty out”.[1] However for this presentation we have included the genitalia, the rectum, anus, the sacral, and the groin region for description of perineal wounds.
Plastic surgeons are involved in management of perineal wounds which may be of various etiologies and we may be teaming up with general surgeons, urologists, orthopedic surgeons, gynecologists, or even dermatologists. The reconstruction can be quite demanding as one has to preserve both the sexual as well as the excretory functions of the perineal area. The unhealed perineal wounds can lead to significant morbidity. Acute complications can occur in 25-60% cases.[2–5] About 25-45% of these patients can develop serious complications.[2–4]
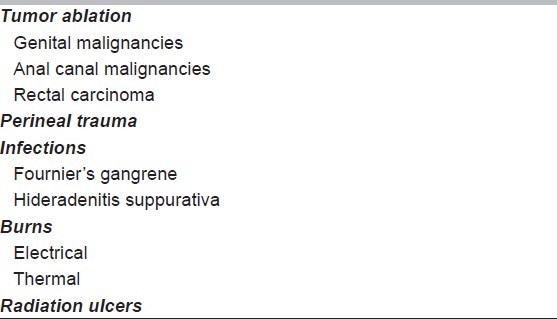
Perineal wounds usually occur following trauma, ablation of malignancy of the genitalia, low pelvic tumors, or following thermal or electrical injuries. [Table 1] These wounds remain a significant problem and can commonly present as wound infection, abscess, dehiscence, delayed healing, or persistent perineal sinuses. These wounds result in significant morbidity requiring prolonged hospital stay, hospital readmission, home-nursing wound care needs; all involving significant medical costs.[6] Perineal wounds can also occur following Fournier's gangrene, hideradenitis suppurativa, or in chronically bed ridden patients where pressure sores can extend into the perineal region. For the patient, these wounds are painful, malodorous lesions requiring constant care and adversely affecting the quality of life. This article aims to review the risk factors associated with development and delayed healing of perineal wounds. Commonly used operative techniques and other ancillary methods to optimize perineal wound healing are also discussed.
Table 1.
Etiology of perineal wounds
EVALUATION OF A PERINEAL WOUND
The wound may be a freshly created surgical wound or may be as a result of trauma or burns. A chronic perineal wound could result due to a variety of causes. Multiple discharging sinuses should be investigated to rule out Crohn's disease, tuberculosis, or even malignancy.
The perineal defects need to be assessed in three dimensions. The skin defects may be associated with a large dead space in the pelvis following surgical extirpation of tumors. The rigid bony pelvis does not allow the wound to collapse resulting in fluid collection.
It is important to know if the patient has received or is likely to receive radiotherapy. Provision of a well vascularised muscle cover is very important in such a situation.
Draining sinuses in a chronic wound need to be probed and may mandate radiological evaluation for assessment of cause and extent of lesion.
An algorithm is suggested to provide guidelines for management in a given defect.
ROLE OF FECAL DIVERSION
This is a controversial issue and there are no clear cut guidelines for the same. It has been suggested that a diversion colostomy helps in better wound management in patients with perineal wounds after trauma or burns. However, a large review of colostomy has highlighted the morbidity associated with this procedure. The most common early complications were skin irritation (12%), pain associated with poor stoma location (7%) and partial necrosis (5%).[7] Another plausible alternative to diversion colostomy is fecal diversion with a special rectal catheter.[8]
Surgical options
Like reconstruction anywhere there are several options available for managing the perineal wounds. These include direct closure of the wound, simple skin graft, or allowing the cavity to heal by secondary intention. However these simple techniques may lead to significant morbidity in majority of the situations. Large defects necessitate use of some kind of skin and/or muscle flap that can obliterate the cavity, resurface the skin defect, and restore the sexual and excretory functions of the region. The aims in a perineal wound reconstruction would be to:
Provide well vascularized tissue to fill the perineal defect. This would also avoid collection of fluid that can be a potential source of infection
Provide a skin paddle for closing the perineum.
Enable rapid healing
Improve patients quality of life
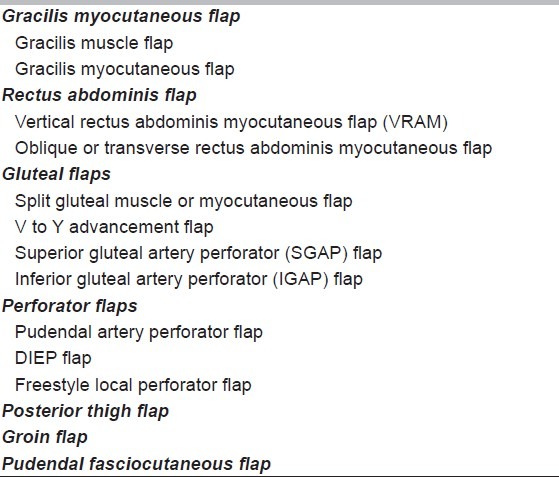
Various flaps which may be considered during the preoperative evaluation of the patient are listed in Table 2.
Table 2.
Commonly used flaps for perineal defects
PERINEAL WOUNDS FOLLOWING TUMOR ABLATION
Resection of tumor along with anatomic structures like rectum and anal canal from the pelvis creates a large cavity that is fixed by surrounding pelvic bony structures. This pelvic dead space results in accumulation of fluid and blood clot that increases the risk of developing a pelvic abscess, a wound infection, and perineal wound sinus tracts. In addition, the rigidity of the surrounding structures of the pelvis makes the perineum a difficult wound to close. Primary closure is frequently under tension and is a significant factor in wound breakdown. Specific risk factors such as operative perineal wound management, the use of preoperative radiation therapy (XRT), and indications for surgery (e.g., rectal cancer, anal cancer, or inflammatory bowel disease) have been shown to influence perineal wound healing.[9] Preoperative XRT is routinely used for low rectal and anal cancer, and significantly increases the risk for perineal wound complication. Although preoperative XRT may offer benefit in terms of recurrence and local control of these cancers, there is significant postoperative morbidity associated with this therapy. The adverse effects of XRT on wound healing are directly related to normal tissue injury through progressive occlusive vasculitis and fibrosis.[10] In the pelvis, radiation-induced fibrosis likely limits the ability to close the perineum and pelvic sidewall increasing the risk for wound complication. Other factors may include obliteration of lymphatics and alteration of fibroblast function that is required for wound healing. Other associated medical co-morbidities have been studied and shown to increase the risk of perineal wound complications. Diabetes, low preoperative hematocrit, tumor size, and obesity have all been shown to be significant predictors of perineal wound complications.[11] Interestingly, in obese patients this study indicated that for every point the body mass index (BMI) increased, there was a 10% increase in odds of developing wound complications.
Whenever there is soft tissue loss from the perineum there are many options for reconstruction. These include allowing the wound to heal by secondary intention and the use of local random or axial pattern flaps, regional flaps, or free flaps. The perineum has a rich blood supply with multiple perforating vessels, and the vascular network of the perineum is similar to that of the head and neck. Anatomically, there exist circles of anastomosis around any orifice or joint.[12]
TISSUE TRANSFER AFTER ABLATION OF PELVIC TUMORS
Preoperative consultation with the plastic surgeon is sought most often when the ablative surgeon is not confident that he or she can achieve a closed wound primarily. The oncologic team principally is concerned with separating the pelvic and abdominal cavities, protecting the small bowel from postoperative enteritis problems, preventing postoperative perineal herniation, and obtaining a healed wound primarily. Because this anatomic site is particularly prone to wound healing problems, the cancer surgeon often is concerned with bringing fresh, nonirradiated, vascularized tissue into the region. Small studies have attempted to address the problem of perineal wound complications by using muscle or myocutaneous flap reconstruction.[13–15] Suggested indications for tissue transfer include patients with large perineal soft tissue defects, the need for posterior vaginal wall reconstruction, the need to fill a large pelvic dead space, and the reconstruction of a large perineal defect especially in the setting of preoperative radiation.[14,16] These flaps represent a partial list of those that should be considered preoperatively. Certain flaps may be favored because of positioning considerations; for example, the split gluteus myocutaneous flap can be performed with the patient in the prone position. If the defect is limited to the perianal region, these flaps are robust and may provide adequate closure. If the defect is anticipated to be superficial only, then a groin flap, pudendal flap, or posterior thigh flap may be preferred. If significant dead space requires obliteration, a rectus abdominis myocutaneous flap may be indicated.[17] Lower extremity flaps may be preferred if abdominal donor sites are unavailable.
THE GRACILIS MUSCLE AND MYOCUTANEOUS FLAP
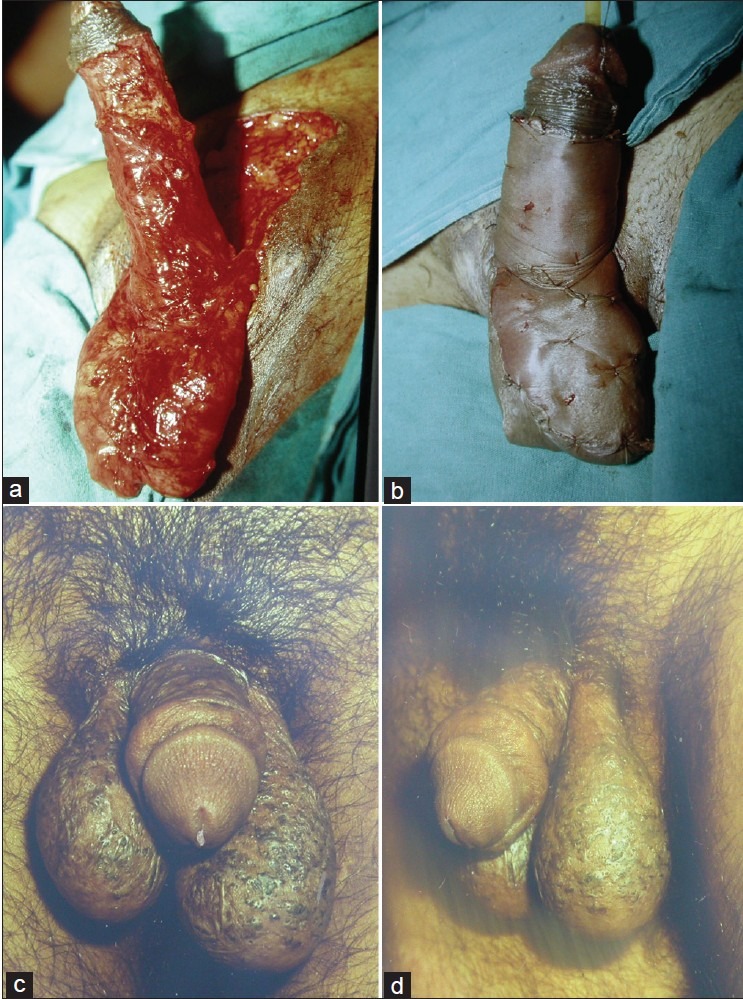
Gracilis flap remains one of the commonest flaps being done for perineal reconstruction. The major blood supply to the gracilis myocutaneous or gracilis muscle flap is derived from the medial femoral circumflex artery. Additional minor perforators originate proximally from the obturator artery and may supply a short gracilis. Bilateral flaps can be used to restore the pelvic cavity and the cutaneous islands can also be tubed to create a neovagina. For eliminating pelvic dead space, the flaps may be de-epithelialized if necessary. Use of this flap has shown promising results in delayed reconstruction of persistent perineal sinus tracts after APR for inflammatory bowel disease.[18–20] Advantages of the gracilis flap in the setting of APR are primarily related to its avoidance of interfering with the creation of a colostomy site. The flap is particularly useful in small defects that are relatively narrow and distal in the pelvis. Disadvantages include smaller muscle mass with decreased effectiveness in large perineal defects and pelvic dead space, and high susceptibility to vascular spasm and cutaneous skin paddle ischemia. The muscle can also be used for reconstruction of major urethral losses following trauma or electrical burns [Figures 1 and 2]. The urethra can be made from the available urethral lining and covered with gracilis muscle flap that is then split skin grafted.[21]
Figure 1.
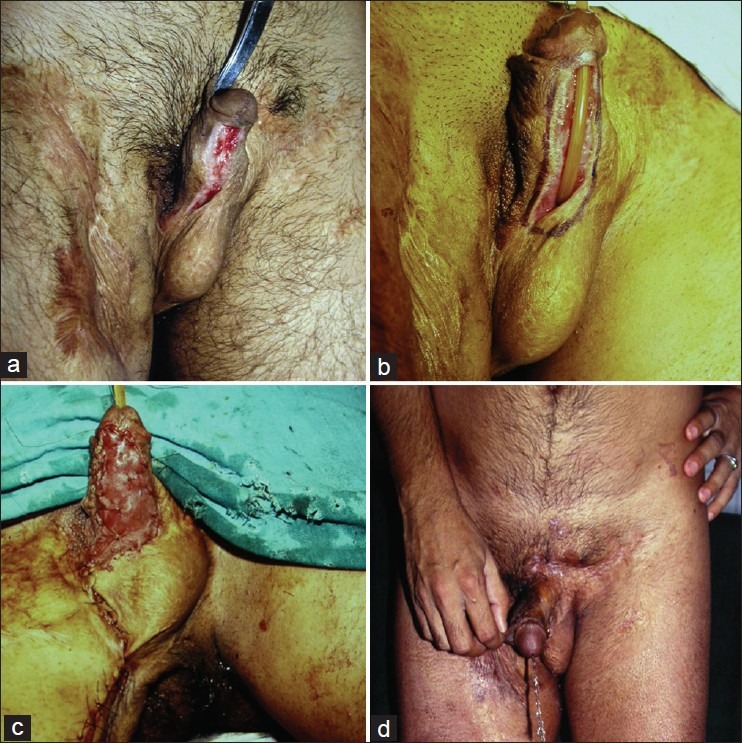
(a) Urethral loss after road side trauma (b) The urethra is planned to be made from the scarred epithelium (c) The reconstructed urethra has been covered with gracilis muscle flap covered with split skin graft (d) Patient passing urine normally from the tip
Figure 2.
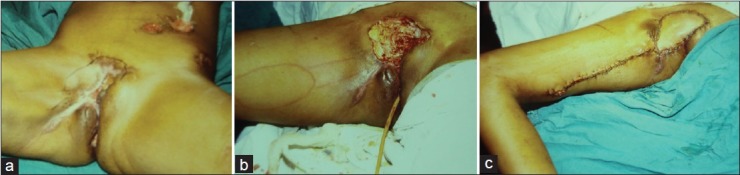
(a) Chronic non healing wound perineum following trauma to rectum and anal region. A colostomy can be seen. (b) The defect has been recreated and skin island is marked on the gracilis muscle. (c) Appearance on the third postoperative day
The myocutaneous flap can also be used to provide the bulk and skin resurfacing for the large perineal defects as shown in Figure 3.
Figure 3.
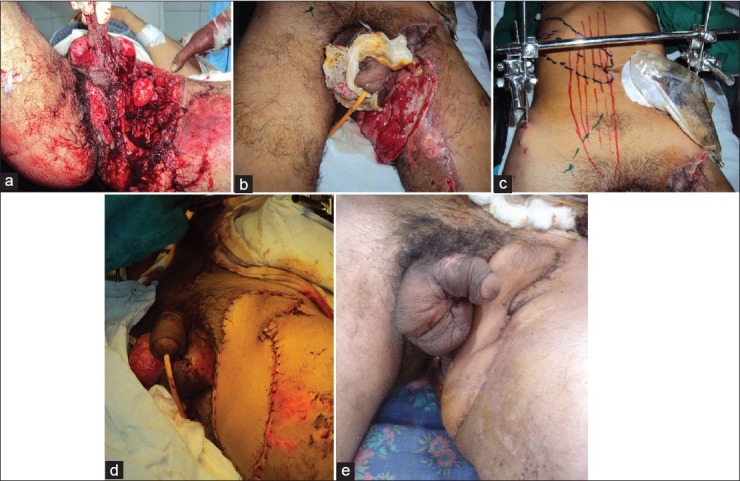
(a) Extensive injury to the anorectal, penoscrotal, groin, and upper thigh in a road accident, (b) The penoscrotal region has been grafted; appearance on day 7 after injury showing necrotic wound in groin with exposed femoral vessels. (c) A contra-lateral rectus musculocutaneous island flap with an oblique skin paddle designed. (d) Flap transferred into the defect (e) Well healed wound at 1 month
RECTUS ABDOMINIS FLAP
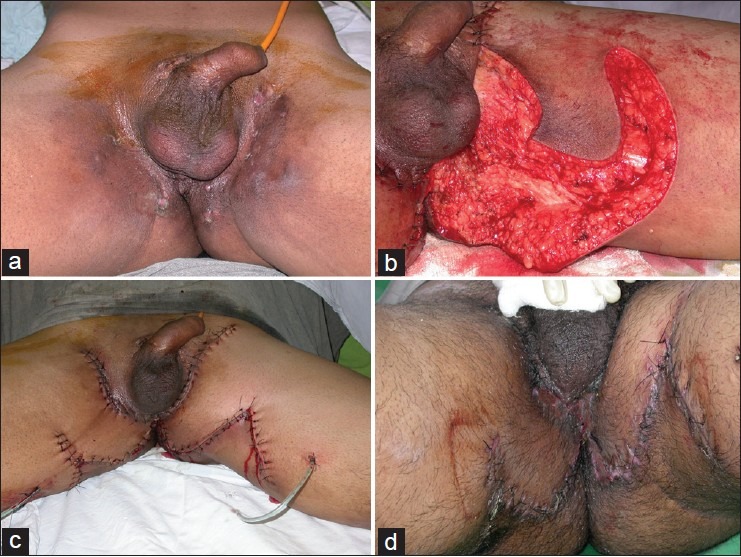
The rectus abdominis flap based on the deep inferior epigastric vessels provides several distinct advantages over bilateral gracilis flap reconstruction. Its most obvious advantage is the robust skin paddle that can be de-epithelialized for bulk or tubed for neovaginal reconstruction.[22] For large pelvic exenteration defects, the rectus abdominis muscle can be used alone or in combination with the de-epithelialized skin paddle. This flap is well perfused by the robust dominant pedicle and the deep inferior gastric artery and vein. In addition, this flap provides adequate muscle bulk to obliterate pelvic dead space. The skin island can be used for resurfacing the perineal region, including the vaginal wall, and provides versatility for all patterns of resection.[23,24] The cutaneous paddle can be designed in various ways along the epigastric region using the superior subcostal musculocutaneous perforators of the upper abdominal wall. The well-perfused region extends from below the costal margin to below the umbilicus and is approximately 20-25 cm long, directly overlying the rectus muscle. The skin paddle of 8-10 cm width usually can be closed primarily. However, if a larger paddle is desired, skin grafting usually is necessary. Alternative skin paddle designs to the longitudinally based pattern include a transverse orientation. If the pattern is to be de-epithelialized, this can be performed to resurface a large perineal defect. As the flap is elevated, the anterior fascia sacrifice remains somewhat narrower than the skin paddle. Thus, a strip of anterior rectus sheath, both lateral and medial, can be closed primarily. Despite its advantages in pelvic and vaginal reconstruction there are several disadvantages associated with rectus abdominis flaps. These include lack of sensation to the cutaneous portion of the flap (vagina and perineum), abdominal weakness, and a risk of fascial dehiscence and hernia formation. Furthermore, use of the VRAM flap limits colostomy placement or re-siting in the future should be the primary site suffer from a significant complication. Figure 4 shows a case of post-traumatic penoscrotal avulsion injury and a big defect in the perineal region and exposed femoral vessels with a large defect in the groin region. The exposed penis and testis were covered with split skin graft and the defect in the groin region was resurfaced with an contralateral rectus abdominis flap based upon deep inferior epigastric vessels. Figure 5 shows a patient who had radical vulvovaginectomy done for cancer vulva. The pelvic dead space was filled with the rectus muscle and the skin island of the same musculocutaneous flap resurfaced the skin defect. This defect in the skin also necessitated another flap in the adjacent region based upon a perforator that was mapped using Doppler signal.
Figure 4.
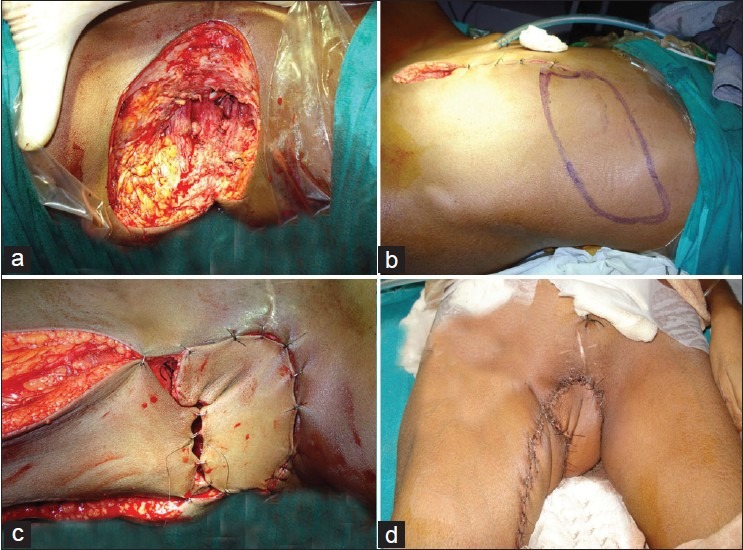
(a) Radical vulvo-vaginectomy defect. There was a large dead space in the pelvis. (b) Design of rectus musculocutaneous island flap with and oblique skin design. (c) The flap was tunneled though the pelvis to obliterate the dead space. The skin island resurfaced the skin defect in the vulval region. A perforator based V-Y advancement flap from the medial thigh has also been used to resurface the skin defect. (d) The flaps on 5th postoperative day.
Figure 5.
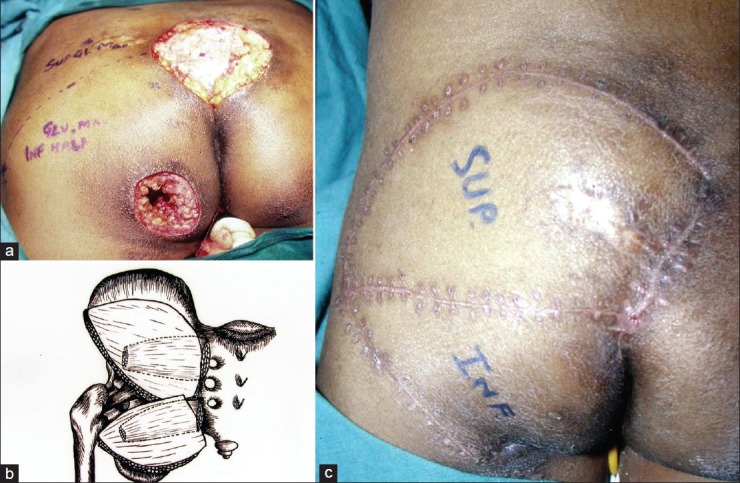
(a) Sacral and ischial pressure sores in a paraplegic patient. The superior and inferior half of gluteus maximus muscle has been marked. (b) schematic description of the superior and inferior musculocutaneous units based upon superior and inferior gluteal artery. (c) well healed flaps at 1 month period
POSTERIOR THIGH FLAP
The posterior thigh flap may provide a reliable, versatile reconstruction of perineal defects, with low donor site morbidity. This flap includes the inferior portion of the gluteus maximus muscle and encompasses the territory of the posterior thigh, directly supplied by the descending branch of the inferior gluteal artery. The design of the flap is centered on the descending branch of the inferior gluteal artery along the central axis of the posterior thigh, perpendicular to the gluteal crease, with the rotation point 5 cm above the ischial tuberosity. It extends to 5-7 cm above the popliteal fascia. The flap is elevated with the fascia overlying the hamstring musculature along with the posterior cutaneous nerve of the thigh. Dissection proceeds proximally to elevate the inferior portion of the gluteus maximus muscle, with the flap up to the lower border of the piriformis muscle. The flap may remain sensate and provides excellent cover for the perineal region.[25] It may be de-epithelialized in its distal portion and tubed for distal vaginal reconstruction. A disadvantage includes a “dog ear” formation at the medial rotation point, which may require secondary revision.
GLUTEAL FLAPS
Gluteal flaps can be used as split gluteal flaps,[26] V to Y advancement gluteal perforator flaps. In split gluteal flap, only the superficial 1-1.5 cm of gluteal muscle is harvested, supplied by the proximal parasacral perforators. This allows elevation of the gluteal region primarily as a musculofascial cutaneous flap. Sharma -[27] also described splitting the gluteus muscle into superior and inferior halves each based upon superior and inferior gluteal artery to have independent movements of each musculocutaneous unit. This allows to simultaneously covering both sacral and ischial sores in paraplegic patients [Figure 6]. Other commonly used variations of gluteal flaps is their use as V to Y advancement flaps either as musculocutaneous or perforator flaps.[28,29] The perforator flaps can be based on superior or inferior gluteal artery. The advantage of using perforator based flaps is that the muscle is spared and the movement of the skin flap can be increased by dissection of the perforator length [Figure 7]. The inferior gluteal artery perforator flap, which can be harvested in dorsal lithotomy position, is specially suited for APR defects without requiring any position change. These perforator flaps have the advantage of providing adequate coverage and bulk for perineal reconstruction without affecting muscle function. These also recreate a vertical gluteal cleft.[30] However, their use is limited to perineal defects without the need of extensive dead space obliteration or neovagina reconstruction.
Figure 6.
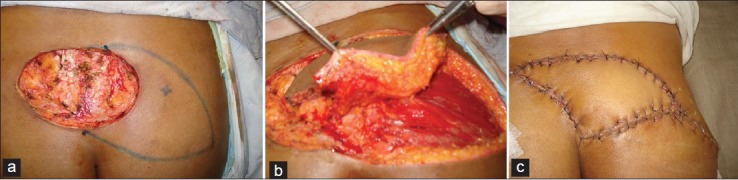
(a) Post tumor excision defect lower sacrum. A perforator has been identified by Doppler. (b) The skin island has been isolated on a perforator (c) Healed flap at 10th days postoperative
Figure 7.
(a) Fournier's gangrene after debridement. (b) Split skin graft applied. (c-d) Healed wound at 6 months postoperative
MANAGEMENT OF PERINEAL WOUND COMPLICATIONS AFTER TUMOR ABLATION
Perineal wound breakdown can occur in setting of pelvic tumors even after following meticulous surgical technique, hemostasis and tissue transfer for pelvic dead space as mentioned previously. Minor wound complications include superficial skin separation, granulation tissue, and chronic perineal sinus. Major wound complications include deep tissue and pelvic abscess and perineal wound dehiscence. Superficial wounds are common and are managed with routine wound care. Patients presenting with fever and pelvic pain should raise the suspicion of a pelvic abscess. Chronic draining sinus tracts are easily found in the perineum, and may represent a communication to the pelvic dead space. Radiographic imaging is useful and should include a computed tomography (CT) scan. In the presence of pelvic sepsis and abscess, admission and broad spectrum antibiotics are recommended. In patients with perineal sinus tracts, a CT scan may show an incompletely drained fluid collection in the pelvis. In patients with persistent perineal sinus tracts, examination under anesthesia may provide a valuable diagnostic and therapeutic approach through the probing and unroofing of simple tracts and the debridement and curettage of devitalized tissue. Opening the wound enough to provide adequate drainage and access for wound dressing changes is important when healing by secondary intention is anticipated. Perineal wound dehiscence is an acute complication and is easily identified during examination. In the absence of small bowel evisceration, wound management with wet to dry dressing changes and sharp debridement of devitalized tissue will promote wound healing. Management of perineal wounds with damp to dry dressings is a well-established and effective means of promoting wound healing. Frequent dressing changes result in serial debridement of the wound and decreases bacterial counts. Over time, the wound begins to heal by secondary intention. Several adjuvant therapies have been introduced to promote healing in these difficult perineal wounds. These include the addition of hydrotherapy, enzymatic debridement, growth factors, and VAC therapy. Hydrotherapy through pulsed lavage or immersion techniques can be helpful in initial cleansing of the perineal wound and can promote debridement, but long-term use is not practical and not routinely used in the outpatient setting at our institution. Enzymatic debridement and growth factor therapies are used to promote wound healing; however, no study till date has examined the effects on management of the perineal wound. Indirect evidence may suggest a benefit. The use of recombinant human platelet-derived growth factor- BB has been reported to promote rapid healing in skin ulcerations associated with perineal hemangiomas of infancy,[31] and chronic neck wounds following XRT.[32] Enzymatic products containing papain are routinely used for the debridement of infected wounds and chronic skin ulcers, and may play a role in the management of perineal wound complications. There have also been several reports of utility of VAC therapy in management of perineal wounds[33,34] The VAC effectively removes excess wound fluid and may reduce levels of the inflammatory mediators improving wound healing. Other benefits of the VAC include effective reduction of wound bacterial counts, increasing oxygen tension in healing wound, and assistance in mechanical approximation of the wound edges. However, further prospective randomized studies are necessary to determine the effects of enzymatic debridement, growth factors, and VAC as adjuvant therapy to promote healing of the perineal wounds. Wound failure after 6-8 weeks of conservative management is likely mandate surgical intervention and may require placement of well-vascularized, non-irradiated tissue flap for a large defect, or skin grafting to clean granulating wounds.
Foam elastomere dressings have been suggested for the management of deep seated wounds in the perineal region and elsewhere. These help in decreasing the wound discharge and promote granulations.[35,36]
PERINEAL WOUNDS FOLLOWING FOURNIER'S GANGRENE
Fournier's gangrene is a potentially fatal disease characterized by necrotizing fasciitis of the perineal and genital regions.[37] The typical therapeutic approach is prompt aggressive surgical debridement of the necrotic tissue.[37,38] Nevertheless, the Fournier's gangrene wound remains open, sometimes for an extended period of time, so multiple regular wound dressing changes are needed. The localization and extent of the wounds associated with Fournier's gangrene usually necessitate analgesia or the use of operating rooms for changing the dressing. Since the dressing must sometimes be changed more than once per day, this has a large negative impact on the patient's quality of life as well as on the physician's quality of care. There have been many reports of use of VAC therapy for management of these wounds.[39,40] Ozturk et al.[39] found VAC therapy to be a cost effective, patient and physician friendly method of managing Fournier's gangrene before contemplating wound closure with STSG . However, the distinct benefits of VAC therapy over conventional wound dressing protocol in Fournier's gangrene are yet to be established in a prospective trial. The split skin grafting in such patients gives an excellent functional and aesthetic results [Figure 8].
Figure 8.
(a) Extensive hideradenitis suppurativa. (b) Defect after excision. A local perforator based flap planned. (c) Flaps sutured into the defect. (c) Appearance at 2 weeks
PERINEAL WOUNDS FOLLOWING HIDERADENITIS SUPPURATIVA AND TRAUMA
Perineal wounds following excision of Hideradenitis suppurativa are usually surface wounds. These can be primarily resurfaced with perforator flaps available locally. Post traumatic perineal wounds require adequate debridement followed by wound closure usually by skin grafting. In grossly contaminated perineal wounds, use of damp to dry dressings is an effective method to achieve a clean granulating wound. These wounds can then be closed by split thickness skin grafts. However, local perforator flaps if available can also be used. Figure 9 shows use of such flaps in extensive bilateral perineal hideradenitis.
Figure 9.
(a-b) Post burn extensive perineal contracture. (c) Perforator based flaps transferred into the defect after release. (d-e) Healed wound at 1 month
LOCAL PERFORATOR BASED FLAPS FOR PERINEAL DEFECTS
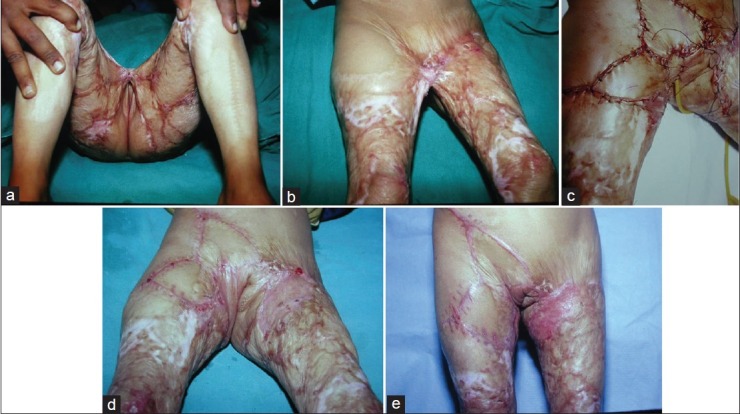
Many local flaps can be used based upon abundant perforators in the region to resurface much variety of perineal defects. Some examples are shown in figures. The perineum has a rich blood supply with multiple perforating vessels, and the vascular network of the perineum is similar to that of the head and neck. Anatomically, there exist circles of anastomosis around any orifice or joint.[6] Figure 10 shows use of multiple flaps based upon Doppler marked perforators for extensive perineal contractures following healing of burns. The flaps may be moved either as transposition or advancement flaps. Such flaps are quite useful for moderate defects following excision of vulval carcinomas [Figure 10]. Many flaps based upon the local perforators have been described and these have proved to be very handy in management of a variety of perineal defects.[41–47]
Figure 10.
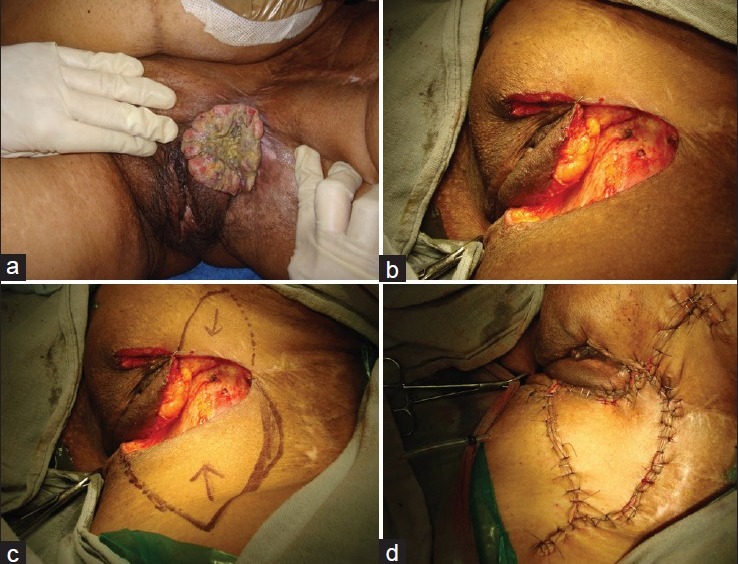
(a) Vulval carcinoma. (b) Defect after excision. (c) Perforator based flaps planned. (d) Flaps sutured into the defect
An algorithm has been suggested for management of perineal wounds [Figure 11] that can help us plan an appropriate treatment modality in a given situation.
Figure 11.
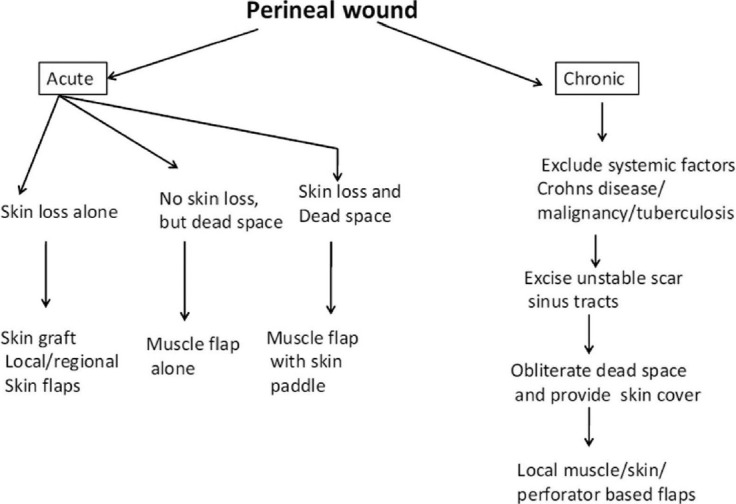
Algorithm for perineal reconstruction
CONCLUSIONS
The perineal wounds continue to be a significant challenge especially those after pelvic tumor ablation. Tissue transfer techniques are effective methods for preventing wound failure, especially in the setting of preoperative XRT and large perineal defects. When perineal wound complications do occur, local wound management through careful debridement of devitalized tissue, effective drainage of pelvic fluid collections and wound dressing is successful in achieving healing in majority of cases. Alternative measures to hasten local wound healing include the use of adjuvant therapies such as hydrotherapy, enzymatic debridement, growth factors, and VAC therapy. Persistent wound failure likely mandates surgical intervention and may require wound coverage with a flap. The choice of the flap is dictated both by the depth and size of the wound, as well as the donor site morbidity associated with the available reconstructive options.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1. [visited on 18 04 2012 at 7pm]. http://www.merriam-webster.com/dictionary/perineum .
- 2.McAllister E, Wells K, Chaet M, Norman J, Cruse W. Perineal reconstruction after surgical extirpation of pelvic malignancies using the transpelvic transverse rectus abdominus musculocutaneous flap. Ann Surg Oncol. 1994;1:164–8. doi: 10.1007/BF02303561. [DOI] [PubMed] [Google Scholar]
- 3.Yeh KA, Hoffman JP, Kusiak JE, Litwin S, Sigurdson ER, Eisenberg BL. Reconstruction with musculocutaneous flaps following resection of locally recurrent rectal cancer. Am Surg. 1995;61:581–9. [PubMed] [Google Scholar]
- 4.Touran T, Frost DB, O’Connell TX. Sacral resection: Operative technique and outcome. Arch Surg. 1990;125:911–3. doi: 10.1001/archsurg.1990.01410190109017. [DOI] [PubMed] [Google Scholar]
- 5.McCraw JB, Massey FM, Shanklin KD, Horton CE. Vaginal reconstruction with gracilis musculocutaneous flaps. Plast Reconstr Surg. 1976;58:176–83. doi: 10.1097/00006534-197608000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Wiatrek RL, Thomas JS, Papaconstantinou HT. Perineal wound complications after abdominoperineal resection. Clin Col Rect Surg. 2008;21:76–85. doi: 10.1055/s-2008-1055325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JJ, Del Pino A, Orsay CP, Nelson RL, Pearl RK, Cintron JR, et al. Stoma complications: The Cook County Hospital experience. Dis Colon Rectum. 1999;42:1575–80. doi: 10.1007/BF02236210. [DOI] [PubMed] [Google Scholar]
- 8.Bordes J, Goutorbe P, Asencio Y, Meaudre E, Dantzer E. A non-surgical device for faecal diversion in the management of perineal burns. Burns. 2008;34:840–4. doi: 10.1016/j.burns.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Artioukh DY, Smith RA, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2006;9:362–7. doi: 10.1111/j.1463-1318.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 10.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 11.Christian CK, Kwaan MR, Betensky RA, Breen EM, Zinner MJ, Bleday R. Risk factors for perineal wound complications following abdominoperineal resection. Dis Colon Rectum. 2005;48:43–8. doi: 10.1007/s10350-004-0855-x. [DOI] [PubMed] [Google Scholar]
- 12.Niranjan NS. Perforator flaps for perineal reconstructions. Semin Plast Surg. 2006;20:133–44. [Google Scholar]
- 13.Shibata D, Hyland W, Busse P, Kim HK, Sentovich SM, Steele G, Jr, et al. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33–7. doi: 10.1007/s10434-999-0033-4. [DOI] [PubMed] [Google Scholar]
- 14.Tei TM, Stolzeburg T, Buntzen S, Laurberg S, Kjeldsen H. Use of transpelvic rectus abdominis musculocutaneous flap for anal cancer salvage surgery. Br J Surg. 2003;90:575–80. doi: 10.1002/bjs.4073. [DOI] [PubMed] [Google Scholar]
- 15.Bakx R, Van Lanschot JJB, Zoetmulder FAN. Inferiorly based rectus abdominis myocutaneous flaps in surgical oncology: Indications, technique, and experience in 37 patients. J Surg Oncol. 2004;85:93–7. doi: 10.1002/jso.20014. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor V, Cole J, Isik FF, Sinanan M, Flum D. Does the use of a flap during abdominoperineal resection decrease pelvic wound morbidity? Am Surg. 2005;71:117–22. [PubMed] [Google Scholar]
- 17.Buchel EW, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominis musculocutaneous flaps. Ann Plast Surg. 2004;52:22–6. doi: 10.1097/01.sap.0000099820.10065.2a. [DOI] [PubMed] [Google Scholar]
- 18.Woods JE, Beart RW., Jr Reconstruction of nonhealing perineal wounds with gracilis muscle flaps. Ann Plast Surg. 1983;11:513–6. doi: 10.1097/00000637-198312000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Bartholdson L, Hulten L. Repair of persistent perineal sinuses by means of a pedicle flap of musculus gracilis.Case report. Scand J Plast Reconstr Surg. 1975;9:74–6. doi: 10.3109/02844317509022861. [DOI] [PubMed] [Google Scholar]
- 20.Baek SM, Greenstein A, McElhinney AJ, Aufses AH., Jr The gracilis myocutaneous flap for persistent perineal sinus after proctocolectomy. Surg Gynecol Obstet. 1981;153:713–6. [PubMed] [Google Scholar]
- 21.Sharma RK, Biswas G. Repair of a urethral fistula following electrical burns using a gracilis muscle flap. Burns. 1990;16:467–70. doi: 10.1016/0305-4179(90)90079-c. [DOI] [PubMed] [Google Scholar]
- 22.Bell SW, Dehni N, Chaouat M, Lifante JC, Parc R, Tiret E. Primary rectus abdominis myocutaneous flap for repair of perineal and vaginal defects after extended abdominoperineal resection. Br J Surg. 2005;92:482–6. doi: 10.1002/bjs.4857. [DOI] [PubMed] [Google Scholar]
- 23.Hui K, Zhang F, Pickus E, Rodriguez LF, Teng N, Lineaweaver WC. Modification of the vertical rectus abdominis musculocutaneous (VRAM) flap for functional reconstruction of complex vulvoperineal defects. Ann Plast Surg. 2003;51:556–60. doi: 10.1097/01.sap.0000096444.59573.87. [DOI] [PubMed] [Google Scholar]
- 24.Sunesen KG, Buntzen S, Tei T, Lindegaard JC, Norgaard M, Laurberg S. Perineal healing and survival after anal cancer salvage surgery: 10-year experience with primary perineal reconstruction using the vertical rectus abdominis myocutaneous (VRAM) flap. Ann Surg Oncol. 2009;16:68–77. doi: 10.1245/s10434-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 25.Hurwitz DJ, Walton RL. Closure of chronic wounds of the perineal and sacral regions using the gluteal thigh flap. Ann Plast Surg. 1982;8:375–86. doi: 10.1097/00000637-198205000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gould WL, Montero N, Cukic J, Hagerty RC, Hester TR. The “split” gluteus maximus musculocutaneous flap. Plast Reconstr Surg. 1994;93:330–6. doi: 10.1097/00006534-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RK. Split gluteus maximus island flaps for concomitant closure of ischial and sacral pressure sores. Ann Plast Surg. 2001;46:52–4. doi: 10.1097/00000637-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Benito P, De Juan A, Cano M, Elena E. Reconstruction of an extensive perineal defect using two modified V-Y flaps based on perforators from the gluteus maximus muscle. J Plast Reconstr Aesthet Surg. 2008;61:e1–4. doi: 10.1016/j.bjps.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Blondeel PN, Van Landuyt K, Hamdi M, Monstrey SJ. Soft tissue reconstruction with the superior gluteal artery perforator flap. Clin Plast Surg. 2003;30:371–82. doi: 10.1016/s0094-1298(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 30.Arnold PB, Lahr CJ, Mitchell ME, Griffith JL, Salloum N, Walker MR, et al. Predictable Closure of the Abdominoperineal Resection Defect: A Novel Two-Team Approach. J Am Coll Surg. 2012;214:726–32. doi: 10.1016/j.jamcollsurg.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Metz BJ, Rubenstein MC, Levy ML, Metry DW. Response of ulcerated perineal hemangiomas of infancy to becaplermin gel, a recombinant human platelet-derived growth factor. Arch Dermatol. 2004;140:867–70. doi: 10.1001/archderm.140.7.867. [DOI] [PubMed] [Google Scholar]
- 32.Hom DB, Manivel JC. Promoting healing with recombinant human platelet-derived growth factor-BB in a previously irradiated problem wound. Laryngoscope. 2003;113:1566–71. doi: 10.1097/00005537-200309000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Papaconstantinou HT, Bullard KM, Rothenberger DA, Madoff RD. Salvage abdominoperineal resection after failed Nigro protocol: Modest success, major morbidity. Colorectal Dis. 2006;8:124–129. doi: 10.1111/j.1463-1318.2005.00911.x. [DOI] [PubMed] [Google Scholar]
- 34.Opelka FG. Unhealed perineal wound. Clin Colon Rectal Surg. 2001;14:65–8. [Google Scholar]
- 35.Ramakant SA, Bhattacharya S, Sinha KN, Dubey PC. Silastic foam elastomer for dressing open granulating wounds-a preliminary report. Indian J Plast Surg. 1987;20:56–60. [Google Scholar]
- 36.Wood RAB, Williams RHP, Hughes LE. Foam elastomer dressing in the management of open granulating wounds: Experience with 250 patients. Br J Surg. 1977;64:554–7. doi: 10.1002/bjs.1800640808. [DOI] [PubMed] [Google Scholar]
- 37.Morpurgo E, Galandiuk S. Fournier's gangrene. Surg Clin North Am. 2002;82:1213–24. doi: 10.1016/s0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 38.Eke N. Fournier's gangrene: A review of 1726 cases. Br J Surg. 2000;87:718–28. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 39.Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg. 2009;197:660–5. doi: 10.1016/j.amjsurg.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Czymek R, Schmidt A, Eckmann C, Bouchard R, Wulff B, Laubert T, et al. Fournier's gangrene: Vacuum-assisted closure versus conventional dressings. Am J Surg. 2009;197:168–76. doi: 10.1016/j.amjsurg.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 41.Sinna R, Qassemyar Q, Benhaim T, Lauzanne P, Sabbagh C, Regimbeau JM, et al. Perforator flaps: A new option in perineal reconstruction. J Plast Reconstr Aesthet Surg. 2010;63:e766–74. doi: 10.1016/j.bjps.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Wang TN, Whetzel T, Mathes SJ, Vasconez LO. A fasciocutaneous flap for vaginal and perineal reconstruction. Plast Reconstr Surg. 1987;80:95–102. doi: 10.1097/00006534-198707000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Wee JT, Joseph VT. A new technique of vaginal reconstruction using neurovascular pudendal thigh flaps: A preliminary report. Plast Reconstr Surg. 1989;83:701–19. doi: 10.1097/00006534-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Woods JE, Alter G, Meland B, Podratz K. Experience with vaginal reconstruction utilising the modified Singapore flap. Plast Reconstr Surg. 1992;90:95–102. [PubMed] [Google Scholar]
- 45.Spear S, Pellegrino CJ, Attinger CE, Potkul RK. Vulvar reconstruction using a mons pubis flap. Ann Plast Surg. 1994;32:602–5. doi: 10.1097/00000637-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Bertani A, Riccio M, Belligolli A. Vulval reconstruction after cancer excision: The island groin flap technique. Br J Plast Surg. 1990;43:159–61. doi: 10.1016/0007-1226(90)90155-s. [DOI] [PubMed] [Google Scholar]
- 47.Yii NW, Niranjan NS. Lotus petal flaps in vulvo-vaginal reconstruction. Br J Plast Surg. 1996;49:547–54. doi: 10.1016/s0007-1226(96)90132-0. [DOI] [PubMed] [Google Scholar]


