Abstract
The history of tissue banking is as old as the use of skin grafting for resurfacing of burn wounds. Beneficial effects of tissue grafts led to wide spread use of auto and allograft for management of varied clinical conditions like skin wounds, bone defects following trauma or tumor ablation. Availability of adequate amount of tissues at the time of requirement was the biggest challenge that forced clinicians to find out techniques to preserve the living tissue for prolonged period of time for later use and thus the foundation of tissue banking was started in early twentieth century. Harvesting, processing, storage and transportation of human tissues for clinical use is the major activity of tissue banks. Low temperature storage of processed tissue is the best preservation technique at present. Tissue banking organization is a very complex system and needs high technical expertise and skilled personnel for proper functioning in a dedicated facility. A small lapse/deviation from the established protocol leads to loss of precious tissues and or harm to recipients as well as the risk of transmission of deadly diseases and tumors. Strict tissue transplant acts and stringent regulations help to streamline the whole process of tissue banking safe for recipients and to community as whole.
KEY WORDS: Tissue banking, tissue preservation, tissue storage
The human body is the perfect example of an efficient machine. Every machine requires repair and replacement of defective components for proper functioning. Unlike machines, the spares for the human body are not available. Tissue engineering has tried to find out the answer to provide the spares but none can match the quality of natural tissues. Human tissues can be harvested from the dead, potentially dead and before the death of transplantable tissues has occurred and can be occurence of stored suitably to be used at the time of need later. This concept is the basic idea behind the development of tissue banks. Tissue banking is the activity of harvesting, processing, storage and distribution of transplantable human tissues. Tissues retrieved from the human body are used to repair and or replace the diseased or lost tissues of living human body and have saved many precious lives. The common tissues being harvested and used are cornea, skin,[1] bones, cartilage, joints, heart valves, fascia, tendons, and duramater from human cadaver.
Practically any human tissue can be harvested and banked for clinical use and research. Biobanking of normal and tumor cells,[2] stem cells from bone marrow, umbilical cord, and adipose tissue,[3] are increasingly used by pathologist to maintain cell lines and bioengineering.[4] Newer applications of autologus banked tissues for future use are being regularly reported in literature viz., blood vessels,[5] testicular tissue,[6] ovarian tissue,[7] nipple areola complex,[8] sperm, penile skin,[9] cord blood, Placenta[10] etc.
HISTORICAL BACKGROUND
History of tissue banking is as old as use of skin grafting. Reverdin in 1869 described skin graft in clinical practice for the first time.[11] In 1871, George Pollock used his own skin along with patient's skin for coverage of a burn wound.[12] Girdner in 1881, first reported successful use of Cadaver allograft in burn wound.[13] Wentscher in 1903, reported that skin graft stored in refrigerator after harvesting, retain their viability for 3-14 days.[14] Following these successes, clinicians started using more allografts for wound coverage to save major burns patients. With advent of blood bank establishments in 1930, the foundation of tissue banks also started taking shape. US navy established its first tissue bank in 1949. Research in prolonging the viability of graft, by storage below zero degree Celsius temperature, done by Baxter in 1948, Billingham and Medawar in 1952, further strengthened the roots of establishment of tissue bank in large number of institutions.[15,16]
As development in field of skin grafting was going on, use of bone graft also progressed. William Macewen in 1881 used bone allograft from the tibia of child suffering from rickets and used it for the reconstruction of a humeral shaft of another young boy.[17] Erich Lexer (1908) used large bone allografts harvested from amputees and used them for filling the bone gaps following osteomyelitis and tumors.[18,19] Bauer in 1910 reported that refrigerated allograft bone could be successfully transplanted after three weeks of storage.[20] Tuffier (1911) succesfully used thin refrigerated slices of bone for transplantation.[21] Trout utilized bone graft from a father to fill the defect of spina bifida of his son in 1915.[22] In 1947 Bush and Wilson preserved bone graft at minus twenty degree celsius temperatures and built a bone bank for storing smallbone pieces.[23,24] George Hyatt of US Navy in 1949 established the first tissue bank at Maryland. He also utilized freeze-drying for bone storage. By the later half of the twentieth century Rudolph Klen opened Hardec Kralove tissue bank Czechoslovakia in 1952. Leeds tissue bank was established in City of Leeds (U.K.) in 1955 followed by German Tissue Bank in Berlin and in Warsaw (Poland) in 1963. Burma tissue bank was established in 1981 at Rangoon and in 1984 Bangkok Biomaterial Centre at Thailand in Asia.[25]
Unorganized tissue and skin banks on individual department basis are in existence in major Plastic Surgery and Burns department of this country since a long time where surplus skin grafts/ bone grafts from patient is stored for later use in the patients. A similar skin bank is in operation at Safdarjang Hospital, New Delhi since 1964 for storage of Skin and amnion. Freeze dried skin grafts and amniotic membranes were also used in patients after installation of freeze dryer in 1976, which was later discontinued due to unfavorable results. The department has low temperature deep freezers for long term storage of tissues at minus eighty degree Celsius. In 1988 Tata Memorial Hospital Tissue Bank was setup in Mumbai. It was truly multi tissue bank wherein amnion, dura mater, skin and bones were harvested and stored. All tissues were terminally sterilized by ionising radiation for the first time in the country. The bank supplies tissues to other centres also.[26]
DEVELOPMENT OF TISSUE BANK
Research in the field of tissue preservation at low temperature and high clinical success of transplanted tissue led to increase in the use of stored tissue in major burn and trauma patients. Increased demand of allografts resulted in the establishment of in house tissue banking activity at major burns center(s). Large numbers of skin banks were opened near major institutions handling burns patients in US. There are more than fifty operational skin and tissue banks in the US at present.[27]
ACTIVITY OF SKIN BANKS
Skin banking is a process in which tissue is removed from a donor's body, tested for suitability as a graft material, packaged and stored and finally reused as a graft. The tissue intended to be used may be harvested from living, brain dead and dead donor. The harvested tissue is processed and needs to be used immediately or stored for future use. Strict adherence of protocols, procedures and code of practice provide safe tissue of reliable quality fit for human use.
Preparation of donor
Tissues are collected from human cadavers within 12 hours of death for non- refrigerated bodies and within 24 hours for refrigerated bodies.[28] Upon arrival the body is thoroughly washed with water and Povidone Iodine Scrub twice. Body hair are shaved off and the body is examined in details and is wrapped in clean sheets. Blood is collected from femoral vein or ventricle puncture. Microbiological surface swabs are collected from four or five skin donor sites. The body is stored in mortuary cabinets at 4°C. All the legal formalities and paper work are completed, medical history and records are checked and suitability of donation is explored. Consent as per local regulation is obtained. A unique identification number is generated and henceforth the same number is used to identify the source and harvested tissues to maintain confidentiality. After the exclusion and inclusion criteria have been fulfilled and it has been decided to harvest tissues, the body is shifted to the operation theatre for tissue retrieval.
It is beyond the scope of this article to discuss the process and preservation of variety of tissues harvested, processed and stored in tissue banks; however the processing and preservation of two most common tissues which are increasingly used by plastic surgeons are discussed here.
TISSUE RETRIEVAL
Same protocol is observed in the operation theatre for harvesting body tissues as is done in living patients under full aseptic precaution. Skin preparation is done by using povidone iodine and isopropopyl alcohol and area is draped with sterile sheets. Sheets of split thickness skin are removed by electric dermatome. Harvested sheets of skin are washed with saline and immediately transferred into sterile containers containing Eagle's Minimal Essential Media and sealed. Skin harvested from different body area is stored in different containers to prevent cross contamination. 1-2 Square cms of skin piece from each area is collected separately, properly labelled and sent to laboratory for determination of viability. All containers are properly labelled mentioning time/date, site of harvest and approximate size and unique ID. The containers are stored at four degree Celsius in refrigerator.
Majority of bones grafts for tissue banking are obtained from femoral head, removed from living donors, being operated for femoral neck fracture and hip transplant procedures. Large segments of bone with or without joints are removed from cadaver limbs under sterile conditions.
TISSUE PROCESSING
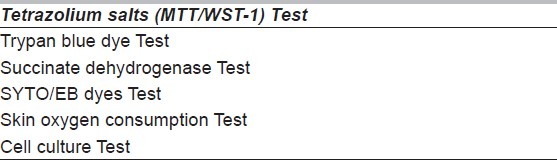
Tissue Processing and storage temperatures differ for different Tissues. All tissues are processed under aseptic and sterile conditions and in clean room of class 10000 under laminar air flow cabinet. Processing of only skin and bones are described below. Once the microbiological and serological samples collected at the time of storage of cadaver are reported negative and found suitable for transplantation, tissues are processed further for storage, otherwise tissues are destroyed. Skin graft is tested for viability as well. There is progressive loss of surviving cells in skin graft following death. The success of transplant depends on the number of living cells in the graft. A number of tests [Table 1] are available to find out viability of skin. Tetrazolium salt test give quick and fairly predictable results.[29] Skin grafts showing viability below 80% are not suitable for storage. The tissues are removed from quarantine fridge and transferred to tissue processing laboratory having clean room of class 10000.
Table 1.
Test for viability of skin
Skin
Processing of Skin for storage at above zero degree Celsius and below zero degree Celsius is different.
Storage at above zero degree celsius (Four degree)
Skin grafts are taken out from storage media and put into fresh Eagle's Minimal Essential Media in sterile container. The media is changed after every third day.[30]
Storage at sub zero degree celsius
The storage media is discarded and the grafts are soaked in Eagle's Minimal Essential Media with 15 % glycerol (Cryo protective agent) for thirty minutes.[31] The grafts are spread on a fine gauze piece soaked in media with glycerol. Folded grafts are transferred to sterile flat packets and sealed. Addition of glycerol prevents intracellular ice crystal formation and cell death (Cryo preservation).
Irradiation, Freeze drying (lyophilisation),[32] Glutaraldehyde,[33] and glycerol immersion[34] of skin grafts are other preservation methods. These processes make the skin graft non-viable but prolongs its shelf life. Freeze drying also compromises the advantages of skin cover like poor adherence on wound bed and barrier to microbes.[35]
Bones
Harvested bones are cleaned by fluid pressurization which eliminates bone marrow and cellular debris.[36,37] Ethanol, ether, acetone and hydrogen peroxide are commonly used clearing agents. Washing with hydrogen peroxide for less than 60 minutes the retains osteoinductive properties of bones.[38] Bones are shaped the into large blocks, small blocks, granules and powder. No special processing like addition of cryoprotective agent is needed for storage of bones at below zero degrees Celsius. Bones are preserved by freezing at zero degrees Celsius in liquid nitrogen at minus one hundred ninety six degree Celsius. Bones can be freeze dried (lyophilised) as well.[39] Sometimes, bones are irradiated with 25 KGy for sterilization. It is a very effective sterilization method for majority of microbes except HIV.[40].
Storage
Proper storage of processed tissue is the key to the success of tissue transplant. Maintenance of temperature during storage is crucial. Deep freezers fitted with temperature backup and alarm system during power failure are a must. The inner chamber temperature of each deep freezer is monitored and recorded round the clock. There should be alternative arrangements to store tissues during major breakdown/ disasters. Different tissues should be stored in different freezers at pre designated temperatures.
Processed tissues are maintained at following temperature zones-
Four degree celsius
Tissues stored at four degree Celsius temperature retain their viability for only two to three weeks. Frequent change of storage media (every third day) is required for the preservation of skin graft.[30] This carries a high risk of contamination, and is labour intensive and only suited for storing small quantity of grafts.
Minus seventy degree celsius
Tissues stored at minus seventy to minus eighty degree Celsius retain their viability for more than six months.[41,42] Skin grafts are preserved in cryo protective agent to prevent cell death. Tissues are pre cooled in controlled rate freezer preset at the rate of one to two degree Celsius temperature drop every minute to prevent cryo injury to cells.[31,43] Once the desired drop in temperature is achieved, packets containing tissues are transferred to a deep freezer. Deep freezers providing minus eighty degree Celsius are readily available and economical to run. In case of a power failure, the temperature can be maintained by Carbon dioxide temperature backup system.
Minus one hundred eighty to minus one hundred ninety six degree celsius temperature
This temperature offers the longest viability for stored tissues. Skin allografts are reported to maintain viability for up to ten years.[27] Tissues stored in cryo protective agents are pre cooled to minus eighty degree Celsius and then transferred to the liquid nitrogen containers. Ultra low temperature deep freezers are other alternatives which can be used.
Bones are also preserved by freezing at zero degree Celsius, in liquid nitrogen at minus one hundred ninety six degree Celsius.
Amnion
Amnion is extensively used as a biological dressing in burns in developing countries due to ease of availability.[44] Amnion is collected from placenta following delivery. The membrane is washed thoroughly by 0.25% Sodium hypochlorite solution.[45] The membrane is air dried and lyophilised. The lyophilised amnion is sealed in packet and gamma irradiated by 25KGy.[46] The lyophilised amnion has a very long shelf life of five years and stored at room temperature. Amnion can also be stored in 85% glycerol after proper washing, up to two years at four degree Celsius temperature.[47]
Transportation
Banked tissues are transported under strict temperature control in special containers to prevent cell death. Tissue being transported to short distances can be thawed at the bank itself and then can be transported at 4 -5 degree Celsius temperatures if the time interval between thawing and application on the patient is less than 24 hours. In other situations, frozen tissues are put into insulated container with dry ice (Solid CO2) to maintain temperature of less than minus fifty degrees Celsius. Frozen tissues stored in liquid nitrogen are transported in cryo containers containing Liquid nitrogen. Tissues stored above zero degree are transported at four to five degree Celsius. Before tissues are dispatched, the label of each package is checked, their suitability for transplantation is ensured from records and they are dispatched along with detailed instruction of thawing and clinical application.
Rewarming
Cryopreserved tissues are re-warmed to room temperature in two to four minutes to decrease the damage to the cells.[48,49] Heating of tissues by microwave energy is not recommended.
Clinical application of banked tissue
Banked tissue has reconstructive, restorative, therapeutic and academic uses [Table 2]. The list of application and uses is getting longer day by day with advancement in technology.
Table 2.
Application and uses of Banked Tissue

Regulation and quality control
Transmission of deadly diseases is one of the major concerns following tissue transplantation. To eliminate this risk, every tissue bank follows a code of conduct which is strictly governed by the Tissue Banking Associations of their countries (e.g. American Association of Tissue banks, British Association for Tissue Banking, European Association of Tissue Banks etc.). Each country has its own standard operating procedure, guidelines and regulations to control the selection of donors, microbiological and serological tests, viability of tissues and ethical considerations.
Donor selection
All cadavers should be thoroughly examined and all available medical records are screened. Close friends and family members are interviewed. American Association of Tissue banks has specified about the donors to be excluded from donation [Table 3].[50,51] A donor should also be serologically negative for HIV I/II, Hepatitis B and C, Human T lymphotrophic virus HTLV-1, Cyto Megalo Virus (CMV) and syphilis. Presence of Group A, beta-hemolytic Streptococci,Clostridia sp., Coagulase-positive Staphylococci, Enterococci, Gram-negative organisms and yeast or fungi on the skin surface of donor make them unsuitable for skin donation.[50,51]
Table 3.
Conditions debarring tissue donation

Infrastructure of tissue bank
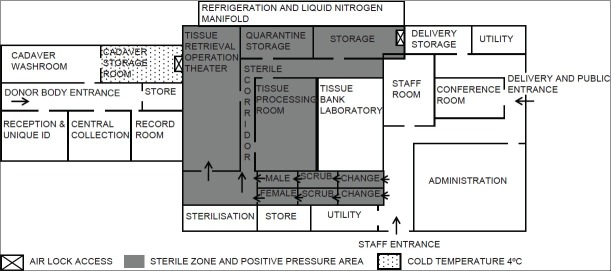
A dedicated building accommodating all the activities of tissue banking as detailed above is ideal. Adequate floor space for every area and provisions for future expansion is always kept in mind. About twenty percent of floor area should be reserved as storage space to avoid clutter. There should be adequate segregation of non-sterile, clean and sterile zones with separate access. Movement in sterile zone should be unidirectional. There should be separate air conditioning for all three zones without air mixing. Sterile area should be equipped with high efficiency particulate air filter and positive air pressure ventilation and preferably should have class 10000 clean room.. An in-house microbiology, serology, tissue typing and cell culture laboratory is an asset which provides timely and accurate result as well as obviates the need of out sourcing these services. A general layout plan of tissue bank is shown in Figure 1.
Figure 1.
General Layout of Tissue Bank
Manpower for tissue banking
Tissue banking is a labor-intensive job, which requires devoted work force and high work ethics. Tissue banking activity employs varied categories of staff from non-skilled to highly skilled technical personnel. Since this specialty is new in our country, the availability of trained staff for each job description would be unavailable. The banks have added responsibility of training their own workforce for specific area. Cadavers reception, cadaver preparation, documentation, and tissue retrieval area operates round the clock throughout. Monitoring of different preset temperature of stored tissues in each freezer is a major responsibility. Specialized Cryo technicians to handle break down of deep freezer should be available at short notice.
Social activity and responsibility
Success of tissue bank is judged by the number of tissues harvested from donor cadaver. Availability of suitable donor cadaver is a major limiting factor. Tissue harvesting from deceased person is a relatively new concept in our country. Though the eye donation campaign has been in existence for last few decades, but families are reluctant to allow the retrieval of tissues from deceased person. Ignorance, misconception, fear of mutilation of body, religious belief and bereavement etc. are generally the causes. To promote active participation from community, mass awareness campaigns regarding tissue donation and its life saving potential, should be done at regular intervals. Participation of print and electronic media, community leaders, religious preachers, elected representatives and social organisations can mobilise massive support for this noble cause. A chapter highlighting the need and benefit of organ donation can be introduced in middle level school curriculum. Public felicitation, eulogising the noble gesture and conferring public awards to the families who have donated tissues of their deceased relatives will go a long way in mobilising the masses. Recently, Govt of India has amended[52] the existing Transplantation of Human Organs Act 1994[53] wherein the medical personnel have been given the responsibility to discuss and work out the feasibility with family members of terminally ill patient or potentially dead individual about tissue and organ donation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bosco F, Governa M, Rossati L, Vigato E, Vassanelli A, Aprili G, et al. The use of banked skin in the Burns Centre of Verona. Blood Transfus. 2011;9:156–61. doi: 10.2450/2011.0107-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw PM, Patterson SD. The value of banked samples for oncology drug discovery and development. J Natl Cancer InstMonogr. 2011:46–9. doi: 10.1093/jncimonographs/lgr004. [DOI] [PubMed] [Google Scholar]
- 3.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularizedtissue-engineered blood vessel as an arterial conduit. ProcNatlAcadSci U S A. 2011;108:9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CW, Corselli M, Péault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy TF. Parents’ choices in banking boys’ testicular tissue. J Med Ethics. 2010;36:806–9. doi: 10.1136/jme.2010.037192. [DOI] [PubMed] [Google Scholar]
- 7.Akar ME, Carrillo AJ, Jennell JL, Yalcinkaya TM. Robotic-assisted laparoscopic ovarian tissue transplantation. FertilSteril. 2011;95:1120.e5–8. doi: 10.1016/j.fertnstert.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed AK, Hahn DE, Hage JJ, Bleiker EM, Woerdeman LA. Temporary banking of the nipple-areola complex in 97 skin-sparing mastectomies. PlastReconstrSurg. 2011;127:531–9. doi: 10.1097/PRS.0b013e3181fed578. [DOI] [PubMed] [Google Scholar]
- 9.Massanyi EZ, McMahon DR. Technique for preservation of penile skin in genital reconstruction: Free graft to the scrotum. Urology. 2011;78:659–61. doi: 10.1016/j.urology.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Bárcena A, Muench MO, Kapidzic M, Gormley M, Goldfien GA, Fisher SJ. Human placenta and chorion: Potential additional sources of hematopoietic stem cells for transplantation. Transfusion. 2011;51(Suppl 4):94S–105S. doi: 10.1111/j.1537-2995.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reverdin JL. Greffeepidermique, experiencefaitedans le service de M le docteurGuyon, a l’hopitalnecker. Bull Imp SocChir Paris. 1869;10:511–5. [Google Scholar]
- 12.Pollock GD. Cases of skin grafting and skin transplantation. Trans ClinSocLond. 1871;4:37–54. [Google Scholar]
- 13.Girdner JH. Skin-grafting with grafts taken from the dead subject. Med Record NY. 1881;20:119–20. [Google Scholar]
- 14.Wentscher J. A further contribution about the survivability of human epidermal cells. Dtsch Z Chir. 1903;70:21–44. [Google Scholar]
- 15.Baxter H, Entin MA. Experimental and clinical studies of reduced temperatures in injury and repair in man. III. Direct effect of cooling and freezing on various elements of the human skin. PlastReconstrSurg. 1948;3:303–34. doi: 10.1097/00006534-194805000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Billingham RE, Medawar PB. The freezing, drying, and storage of mammalian skin. J ExpBiol. 1952;19:454–68. [Google Scholar]
- 17.Macewen W. Observations concerning transplantation on bone. Proc R SocLond B BiolSci. 1881;32:232–47. [Google Scholar]
- 18.Lexer E. Ueberglenktransplantation. Med Klin. 1908;4:817. [Google Scholar]
- 19.Lexer E. Die verwendung der freienknochenplastiknebstversuchenubergelenkversteifung und gelenktransplantation. Arch KlinChir. 1908;86:939–54. [Google Scholar]
- 20.BauerH Ueberknochentransplantation. ZentralblChir. 1910;37:20–1. [Google Scholar]
- 21.Tuffier T. Des greffes de cartilage etd’oshumaindans les resections articulaires. Bull et MemSoc de Chir de Paris. 1911;37:278. [Google Scholar]
- 22.Trout HH. Spina bifida, tibial transplant, father to child. SurgGynecolObstet. 1915;22:523. [Google Scholar]
- 23.Bush LF. The use of homogenous bone grafts. J Bone Joint Surg Am. 1947;29:620–8. [PubMed] [Google Scholar]
- 24.Wilson PD. Experiences with a bone bank. Ann Surg. 1947;126:932–46. [PubMed] [Google Scholar]
- 25.Nather A, Zheng S. Evolution of Allograft Transplantation A Comprehensive guide for tissue banks [Internet] 2010:3–19. Available from: http://www.worldscibooks.com/etextbook/7539/7539_chap01.pdf . [Google Scholar]
- 26.Gajiwala AL. Setting up a Tissue Bank in India: The Tata Memorial Hospital Experience. Cell Tissue Bank. 2003;4:193–201. doi: 10.1023/B:CATB.0000007026.00604.97. [DOI] [PubMed] [Google Scholar]
- 27.Kagan RJ, Robb EC, Plessinger RT. Human skin banking. Clin Lab Med. 2005;25:587–605. doi: 10.1016/j.cll.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Robert MC. The Skin Bank. In: Herndon DN, editor. Total Burn Care. London: WB Saunders; 1996. pp. 159–63. [Google Scholar]
- 29.Castagnoli C, Alotto D, Cambieri I, Casimiri R, Aluffi M, Stella M, et al. Evaluation of donor skin viability: Fresh and cryopreserved skin using tetrazolioum salt assay. Burns. 2003;29:759–67. doi: 10.1016/j.burns.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Robb EC, Bechmann N, Plessinger RT, et al. A comparison of changed vs.unchanged media for viability testing of banked allograft skin. Proc Am Assoc Tissue Banks. 1997;21:42. [Google Scholar]
- 31.Aggarwal SJ, Baxter CR, Diller KR. Cryopreservation of skin: An assessment of current clinical applicability. J Burn Care Rehabil. 1985;6:469–76. doi: 10.1097/00004630-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Brown JB, Fryer MP, Zaydon TJ. A skin bank for postmortem homografts. SurgGynecolObstet. 1955;101:401–12. [PubMed] [Google Scholar]
- 33.Schechter I. Prolonged retention of glutaraldehyde-treated skin allografts and xenografts: Immunological and histological studies. Ann Surg. 1975;182:699–704. doi: 10.1097/00000658-197512000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Q, Pegg DE, Kearney JN. Banking of non-viable skin allografts using high concentrations of glycerol or propylene glycol. Cell Tissue Bank. 2004;5:3–21. doi: 10.1023/b:catb.0000022234.02322.13. [DOI] [PubMed] [Google Scholar]
- 35.Pruitt BA, Levine NS. Characteristics and uses of biologic dressings and skin substitutes. Arch Surg. 1984;119:312–22. doi: 10.1001/archsurg.1984.01390150050013. [DOI] [PubMed] [Google Scholar]
- 36.Aspenberg P. A new bone chamber used for measuring osteoconduction in rats. Eur J Exp Musculoskeletal Res. 1993;2:69–74. [Google Scholar]
- 37.Yates P, Thomson J, Galea G. Processing of whole femoral head allografts: Validation methodology for the reliable removal of nucleated cells, lipid and soluble proteins using a multi-step washing procedure. Cell Tissue Bank. 2005;6:277–85. doi: 10.1007/s10561-005-1235-z. [DOI] [PubMed] [Google Scholar]
- 38.DePaula CA, Truncale KG, Gertzman AA, Sunwoo MH, Dunn MG. Effects of hydrogen per- oxide cleaning procedure on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank. 2005;6:287–98. doi: 10.1007/s10561-005-3148-2. [DOI] [PubMed] [Google Scholar]
- 39.Gajiwala K, Lobo GajiwalaA. Use of banked tissue in plastic surgery. Cell Tissue Bank. 2003;4:141–6. doi: 10.1023/B:CATB.0000007023.85139.c5. [DOI] [PubMed] [Google Scholar]
- 40.Pruss A, Kao M, Gohs U, Koscielny J, von Versen R, Pauli G. Effect of gamma irradiation on human cortical bon trans- plants contaminated with enveloped and non-enveloped viruses. Biologicals. 2002;30:125–33. doi: 10.1006/biol.2002.0326. [DOI] [PubMed] [Google Scholar]
- 41.May SR, Guttman RM, Wainwright JF. Cryopreservation of skin using an insulated heat sink box stored at -70°C. Cryobiology. 1985;22:205–14. doi: 10.1016/0011-2240(85)90142-7. [DOI] [PubMed] [Google Scholar]
- 42.May SR, Roberts DP. Development of a passive device for freezing large amounts of transplantable skin at one time in a-70 degree C mechanical refrigerator. Cryobiology. 1988;25:186–96. doi: 10.1016/0011-2240(88)90025-9. [DOI] [PubMed] [Google Scholar]
- 43.Cuono CB, Langdon R, Birchall N. Viability and functional performance of allograft skin preserved by slow, controlled, non-programmed freezing. Proc Am Burn Assoc. 1988;20:55. [Google Scholar]
- 44.Mostaque AK, Rahman KB. Comparisons of the effects of biological membrane (amnion) and silver sulfadiazine in the management of burn wounds in children. J Burn Care Res. 2011;32:200–9. doi: 10.1097/BCR.0b013e31820aad94. [DOI] [PubMed] [Google Scholar]
- 45.athangi R, Jayaraman V, Babu M. Biological Wound Coverings. In: Hanumadass ML, Ramakrishnan KM, editors. PaediatricBurns. Hyderabad: Paras Medical Publisher; 2011. pp. 161–76. [Google Scholar]
- 46.Gajiwala K, Gajiwala AL. Evaluation of lyophilised, gamma-irradiated amnion as a biological dressing. Cell Tissue Bank. 2004;5:73–80. doi: 10.1023/B:CATB.0000034076.16744.4b. [DOI] [PubMed] [Google Scholar]
- 47.Ravishanker R, Bath AS, Roy R. “Amnion Bank”--the use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns. 2003;29:369–74. doi: 10.1016/s0305-4179(02)00304-2. [DOI] [PubMed] [Google Scholar]
- 48.May SR, DeClement FA. Skin banking. Part II. Low contamination cadaveric allograft skin for temporary burn wound coverage. J Burn Care Rehabil. 1981;2:64–76. [Google Scholar]
- 49.May SR, DeClement FA. Skin banking. Part III. Cadaveric allograft skin viability. J Burn Care Rehabil. 1981;19:362–71. [Google Scholar]
- 50.Standards for Tissue Banking. Arlington: VirginiaAATB; 1984. A merican Association of Tissue Bank. [Google Scholar]
- 51.Information Alert. Vol. 3. Arlington, VA: AATB; 1993. A merican Association of Tissue Bank; pp. 1–8. [Google Scholar]
- 52.Government of India. The Gazette of India, Extraordinary Part II-Section I. 1994;42:1–13. [Google Scholar]
- 53.Government of India. The Gazette of India, Extraordinary Part II-Section I. 2011;22:1–9. [Google Scholar]