Abstract
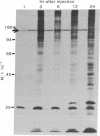
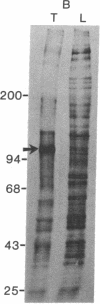
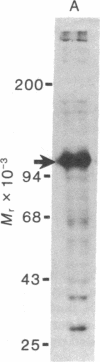
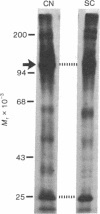
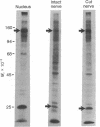
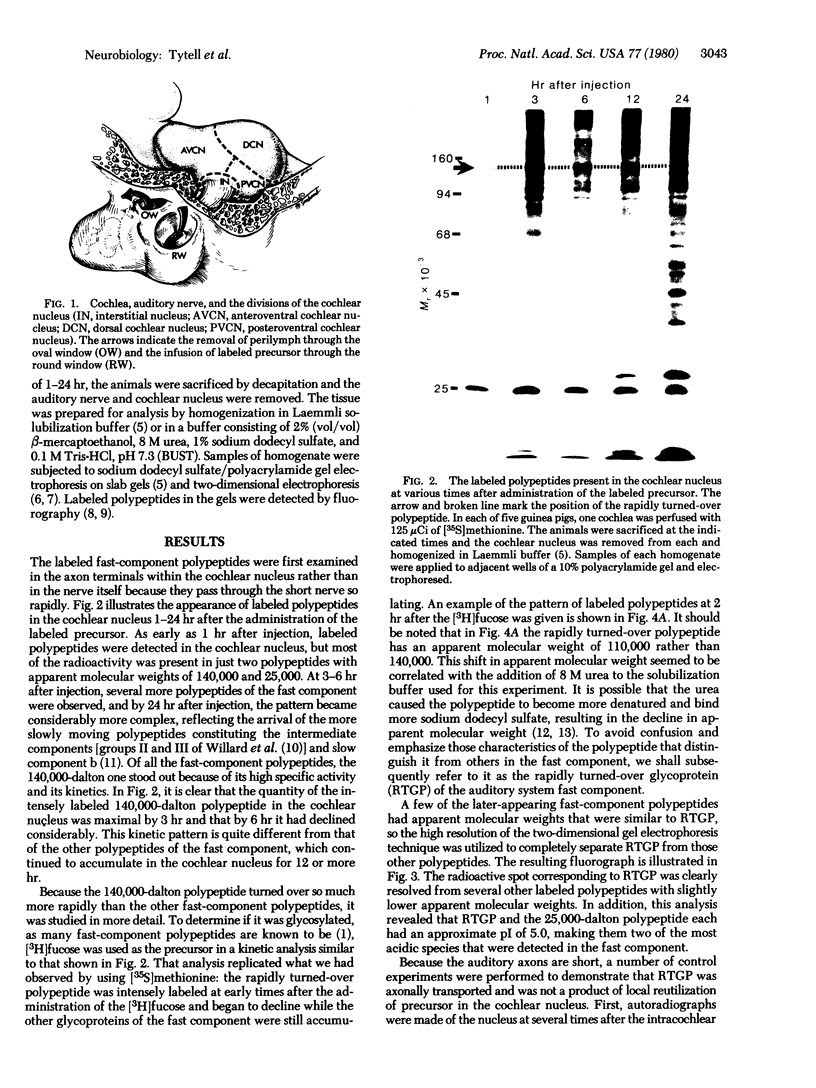
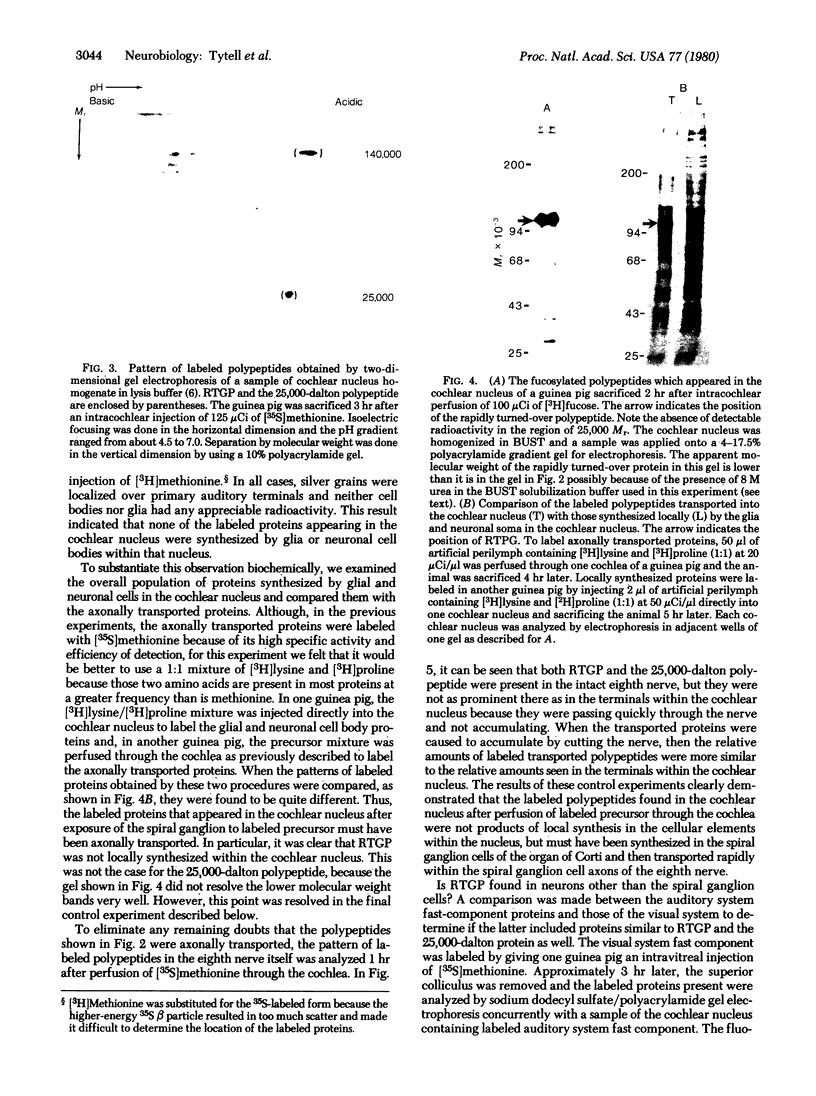
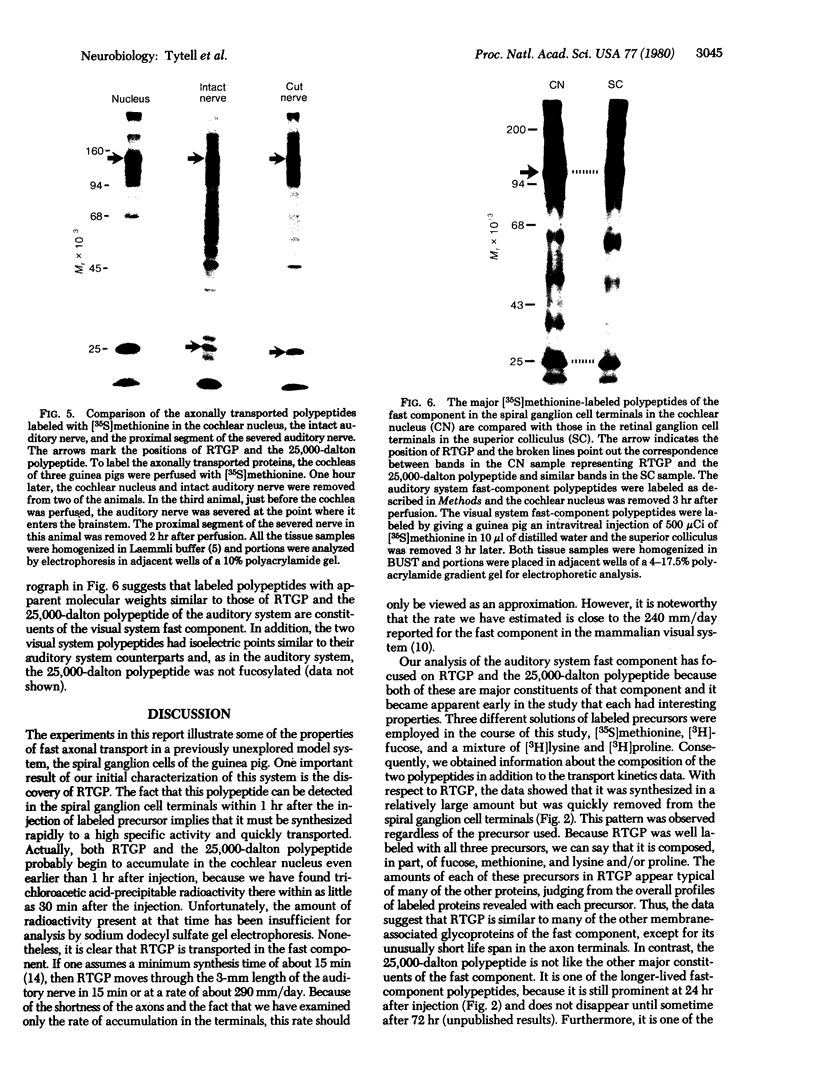
Proteins of the fast component of axonal transport were analyzed by one- and two-dimensional polyacrylamide gel electrophoresis in the guinea pig spiral ganglion, which has its cell bodies in the cochlea and its axons in the eighth cranial nerve projecting to the ipsilateral cochlear nucleus. We found that we could easily identify the proteins of the fast component even though these axons are only about 3 mm long because the cochlea minimized diffusion of labeled precursor into the cochlear nucleus. The composition of the fast component of the spiral ganglion cells was similar, but not identical, to the fast component of guinea pig retinal ganglion cells. One difference was the predominance in the spiral ganglion cell fast component of a rapidly turned-over glycoprotein (RTGP) with a molecular weight of 110,000-140,000 and an isoelectric point of 5.0 RTGP accumulated in the cochlear nucleus for just the first 3 hr after the application of the labeled precursor and then rapidly disappeared, whereas the other major fast component polypeptides continued to accumulate for 12-24 hr. RTGP was also tentatively identified in the fast component of retinal ganglion cells, but was not as prominently labeled relative to the other fast-component proteins in those cells. The rapid disappearance of RTGP from spiral ganglion cell terminals in the cochlear nucleus may be a result of secretion, perhaps as part of a synaptic vesicle, or retrograde transport as a feedback signal. The difference in the relative amounts of RTGP found in spiral ganglion and retinal ganglion cell terminals may reflect differences in the fundamental properties of the two groups of neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Black M. M., Lasek R. J. A difference between the proteins conveyed in the fast component of axonal transport in guinea pig hypoglossal and vagus motor neurons. J Neurobiol. 1978 Nov;9(6):433–443. doi: 10.1002/neu.480090603. [DOI] [PubMed] [Google Scholar]
- Black M. M., Lasek R. J. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 1979 Aug 10;171(3):401–413. doi: 10.1016/0006-8993(79)91045-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Di Giamberardino L. D., Bennett G., Koenig H. L., Droz B. Axonal migration of protein and glycoprotein to nerve endings. 3. Cell fraction analysis of chicken ciliary ganglion after intracerebral injection of labeled precursors of proteins and glycoproteins. Brain Res. 1973 Sep 28;60(1):147–159. doi: 10.1016/0006-8993(73)90854-8. [DOI] [PubMed] [Google Scholar]
- Droz B., Koenig H. L., Biamberardino L. D., Di Giamberardino L. Axonal migration of protein and glycoprotein to nerve endings. I. Radioautographic analysis of the renewal of protein in nerve endings of chicken ciliary ganglion after intracerebral injection of (3H)lysine. Brain Res. 1973 Sep 28;60(1):93–127. doi: 10.1016/0006-8993(73)90852-4. [DOI] [PubMed] [Google Scholar]
- Konishi T., Kelsey E. Effect of potassium deficiency on cochlear potentials and cation contents of the endolymph. Acta Otolaryngol. 1973 Dec;76(6):410–418. doi: 10.3109/00016487309121529. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P., Lux H. D., Kreutzberg G. W. Single cell isotope injection technique, a tool for studying axonal and dendritic transport. Acta Neuropathol. 1971;5(Suppl):179–186. doi: 10.1007/978-3-642-47449-1_23. [DOI] [PubMed] [Google Scholar]
- Stone G. C., Wilson D. L., Hall M. E. Two-dimensional gel electrophoresis of proteins in rapid axoplasmic transport. Brain Res. 1978 Apr 14;144(2):287–302. doi: 10.1016/0006-8993(78)90155-5. [DOI] [PubMed] [Google Scholar]
- Willard M., Cowan W. M., Vagelos P. R. The polypeptide composition of intra-axonally transported proteins: evidence for four transport velocities. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2183–2187. doi: 10.1073/pnas.71.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. L., Stone G. C. Axoplasmic transport of proteins. Annu Rev Biophys Bioeng. 1979;8:27–45. doi: 10.1146/annurev.bb.08.060179.000331. [DOI] [PubMed] [Google Scholar]