Abstract
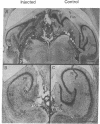
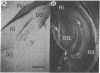
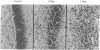
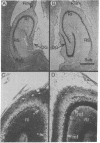
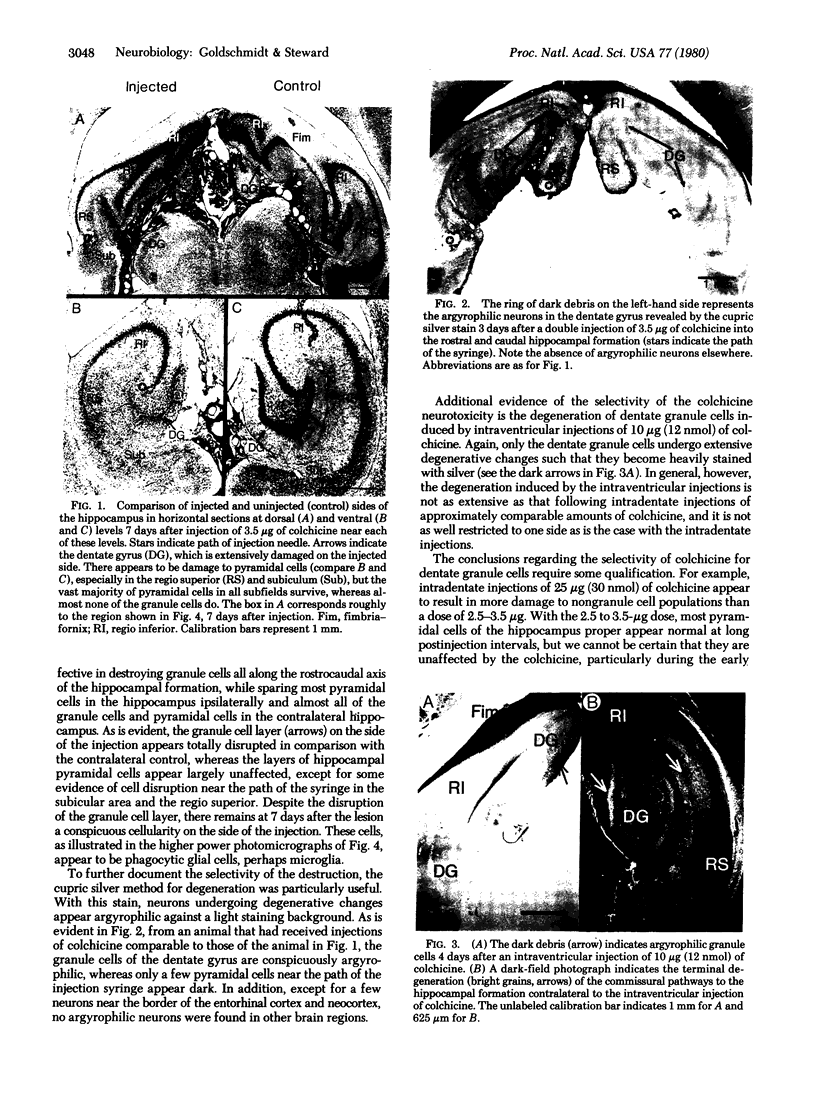
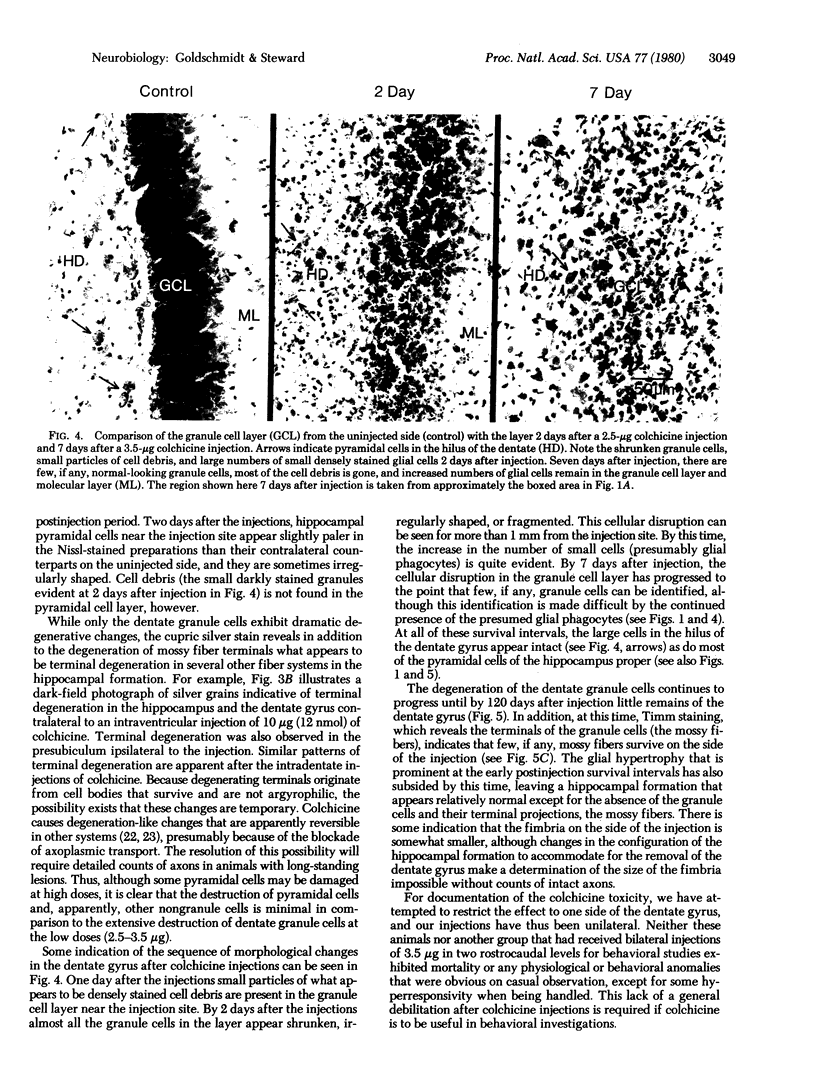
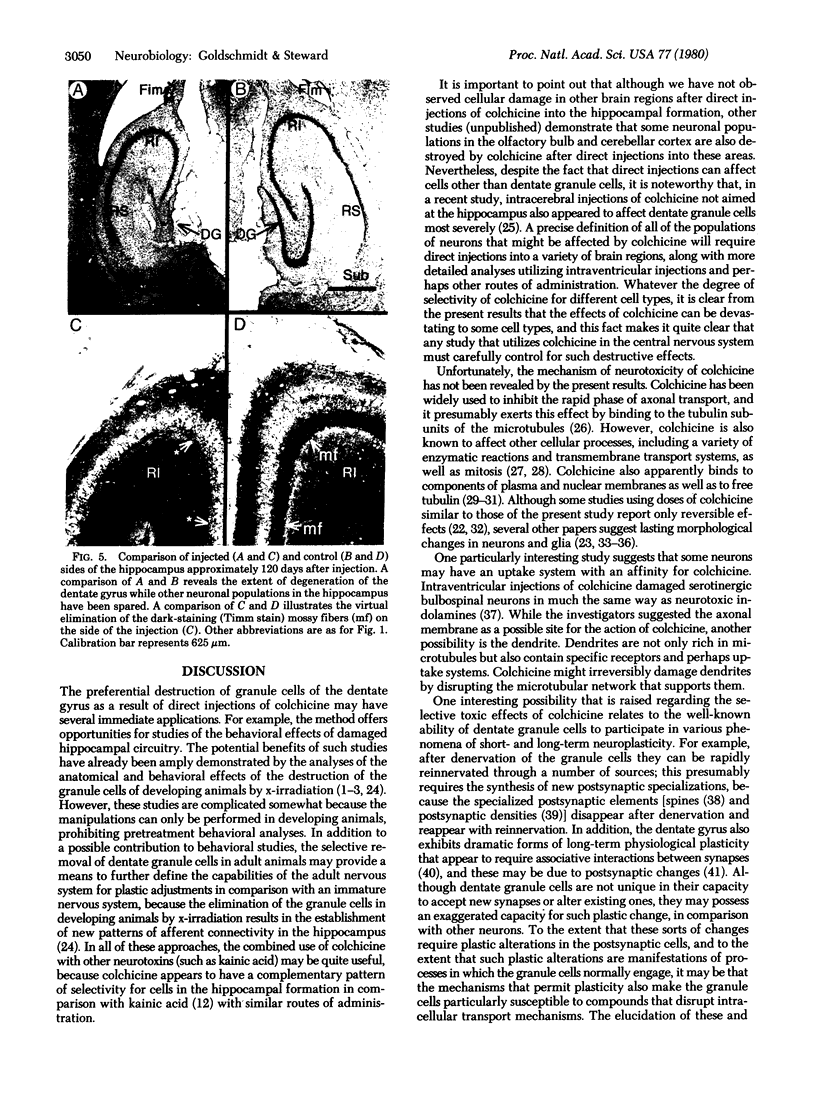
Injections of 5-7 microgram (6-9 nmol) of colchicine into the dentate gyrus of the hippocampus of mature rats result in widespread destruction of dentate granule cells with little, if any, damage to other cell populations, including hippocampal pyramidal cells. Selective destruction of dentate granule cells is also observed after intraventricular injections. The destructive effects of colchicine appear as soon as 12 hr after the injection and lead to the disappearance of the granule cells over a period of days. Whereas the effects on nongranule cell populations in the hippocampus appear to be reversed by approximately 11 days after injection, the granule cells are almost completely absent at long intervals after injection. At the long postinjection survival intervals the disappearance of the granule cells is accompanied by elimination of their terminal projections, the mossy fibers, as revealed by Timm staining for heavy metals. Because the preferential neurotoxic effects of colchicine do not result in morbidity or obvious behavioral debilitation, the toxicity may prove useful for studying the functional consequences of removing specific cell populations in the central nervous system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGEVINE J. B., Jr Nerve destruction by colchicine in mice and golden hamsters. J Exp Zool. 1957 Nov;136(2):363–391. doi: 10.1002/jez.1401360209. [DOI] [PubMed] [Google Scholar]
- Altman J., Anderson W. J. Irradiation of the cerebellum in infant rats with low-level x-ray: histological and cytological effects during infancy and adulthood. Exp Neurol. 1971 Mar;30(3):492–509. doi: 10.1016/0014-4886(71)90150-6. [DOI] [PubMed] [Google Scholar]
- Avrith D., Mogenson G. J. Reversible hyperphagia and obesity following intracerebral microinjections of colchicine into the ventromedial hypothalamus of the rat. Brain Res. 1978 Sep 15;153(1):99–107. doi: 10.1016/0006-8993(78)91131-9. [DOI] [PubMed] [Google Scholar]
- Baumgarten H. G., Björklund A., Lachenmayer L., Rensch A., Rosengren E. De- and regeneration of the bulbospinal serotonin neurons in the rat following 5,6-or 5,7-dihydroxytryptamine treatment. Cell Tissue Res. 1974;152(3):271–281. doi: 10.1007/BF00223949. [DOI] [PubMed] [Google Scholar]
- Bayer S. A., Brunner R. L., Hine R., Altman J. Behavioural effects of interference with the postnatal acquisition of hippocampal granule cells. Nat New Biol. 1973 Apr 18;242(120):222–224. doi: 10.1038/newbio242222a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Coyle J. T., Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature. 1976 Sep 16;263(5574):244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Csillik B., Knyihár E., Elshiekh A. A. Degenerative atrophy of central terminals of primary sensory neurons induced by blockade of axoplasmic transport in peripheral nerves. Experientia. 1977 May 15;33(5):656–657. doi: 10.1007/BF01946557. [DOI] [PubMed] [Google Scholar]
- Danscher G., Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry. 1978 Feb 3;55(1):27–40. doi: 10.1007/BF00496691. [DOI] [PubMed] [Google Scholar]
- Estridge M. Polypeptides similar to the alpha and beta subunits of tubulin are exposed on the neuronal surface. Nature. 1977 Jul 7;268(5615):60–63. doi: 10.1038/268060a0. [DOI] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Fink B. R., Byers M. R., Middaugh M. E. Dynamics of colchicine effects on rapid axonal transport and axonal morphology. Brain Res. 1973 Jun 29;56:299–311. doi: 10.1016/0006-8993(73)90343-0. [DOI] [PubMed] [Google Scholar]
- Gerbrandt L. K., Rose G., Wheeler R. L., Lynch G. Distribution of the perforant path following selective elimination of granule cells. Exp Neurol. 1978 Oct;62(1):122–132. doi: 10.1016/0014-4886(78)90045-6. [DOI] [PubMed] [Google Scholar]
- Grossman S. P., Dacey D., Halaris A. E., Collier T., Routtenberg A. Aphagia and adipsia after preferential destruction of nerve cell bodies in hypothalamus. Science. 1978 Nov 3;202(4367):537–539. doi: 10.1126/science.705344. [DOI] [PubMed] [Google Scholar]
- Hanson M., Edström A. Mitosis inhibitors and axonal transport. Int Rev Cytol Suppl. 1978;(7):373–402. [PubMed] [Google Scholar]
- Hansson H. A. A histochemical study of retinal changes induced by treatment with colchicine. Exp Eye Res. 1971 Sep;12(2):198–205. doi: 10.1016/0014-4835(71)90091-1. [DOI] [PubMed] [Google Scholar]
- Hansson H. A. Glial reactions induced by treatment with colchicine of the central nervous system of rabbits. Acta Neuropathol. 1972;22(2):145–157. doi: 10.1007/BF00688781. [DOI] [PubMed] [Google Scholar]
- Hedreen J. C., Chalmers J. P. Neuronal degeneration in rat brain induced by 6-hydroxydopamine; a histological and biochemical study. Brain Res. 1972 Nov 27;47(1):1–36. doi: 10.1016/0006-8993(72)90248-x. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Effects of colchicine and cytochalasin B on labelling and enzyme activities of brain plasma membranes. Int J Neurosci. 1973;6(3):159–163. doi: 10.3109/00207457309147659. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Hansson H. A., Sjöstrand J. Effect of colchicine on axonal transport and morphology of retinal ganglion cells. Z Zellforsch Mikrosk Anat. 1971;115(2):265–283. doi: 10.1007/BF00391128. [DOI] [PubMed] [Google Scholar]
- Laguzzi R. F., Adrien J., Bourgoin S., Hamon M. Effects of intraventricular injection of 6-hydroxydopamine in the developing kitten. 1. On the sleepwaking cycles. Brain Res. 1979 Jan 19;160(3):445–459. doi: 10.1016/0006-8993(79)91072-2. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Cotman C., Lynch G. An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. II. Reappearance of morphologically normal synaptic contacts. Brain Res. 1976 Oct 8;115(1):23–41. doi: 10.1016/0006-8993(76)90820-9. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., McGeer P. L. Duplication of biochemical changes of Huntington's chorea by intrastriatal injections of glutamic and kainic acids. Nature. 1976 Oct 7;263(5577):517–519. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., McGeer P. L., Singh K. Kainate-induced degeneration of neostriatal neurons: dependency upon corticostriatal tract. Brain Res. 1978 Jan 13;139(2):381–383. doi: 10.1016/0006-8993(78)90941-1. [DOI] [PubMed] [Google Scholar]
- McMartin D. N., Schedlbauer L. M. Effect of experimental colchicine encephalopathy on brain protein synthesis and tubulin metabolism. J Neurobiol. 1978 Nov;9(6):453–463. doi: 10.1002/neu.480090605. [DOI] [PubMed] [Google Scholar]
- Meibach R. C., Brown L., Brooks F. H. Histofluorescence of kainic acid-induced striatal lesions. Brain Res. 1978 Jun 9;148(1):219–223. doi: 10.1016/0006-8993(78)90393-1. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Vincent S. R., Lehmann J., Fibiger H. C., McGeer E. G. The use of kainic acid in the localization of enzymes in the substantia nigra. Brain Res. 1978 Jun 30;149(2):431–441. doi: 10.1016/0006-8993(78)90485-7. [DOI] [PubMed] [Google Scholar]
- Parnavelas J. G., Lynch G., Brecha N., Cotman C. W., Globus A. Spine loss and regrowth in hippocampus following deafferentation. Nature. 1974 Mar 1;248(5443):71–73. doi: 10.1038/248071a0. [DOI] [PubMed] [Google Scholar]
- Pettibone D. J., Kaufman N., Scally M. C., Meyer E., Jr, Ulus I., Lytle L. D. Striatal nondopaminergic neurons: possible involvement in feeding and drinking behavior. Science. 1978 Jun 9;200(4346):1175–1177. doi: 10.1126/science.653362. [DOI] [PubMed] [Google Scholar]
- Rensch A. Intracerebroventrikuläre Injektion von Colchicin: Präterminale Axonläsion mit konsekutiver Degeneration indolaminerger Terminals -- Folge direkter Membranotoxizität oder Axoplasmaflusshemmung. Verh Anat Ges. 1976;(70 Pt 1):363–368. [PubMed] [Google Scholar]
- Sanberg P. R., Lehmann J., Fibiger H. C. Impaired learning and memory after kainic acid lesions of the striatum: a behavioral model of Huntington's disease. Brain Res. 1978 Jun 30;149(2):546–551. doi: 10.1016/0006-8993(78)90502-4. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Coyle J. T. Striatal lesions with kainic acid: neurochemical characteristics. Brain Res. 1977 May 27;127(2):235–249. doi: 10.1016/0006-8993(77)90538-8. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Cooper B. R., Breese G. R. Growth and behavioral changes in developing rats treated intracisternally with 6-hydroxydopamine: evidence for involvement of brain dopamine. J Pharmacol Exp Ther. 1973 Jun;185(3):609–619. [PubMed] [Google Scholar]
- Stadler J., Franke W. W. Colchicine-binding proteins in chromatin and membranes. Nat New Biol. 1972 Jun 21;237(77):237–238. doi: 10.1038/newbio237237a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Tranzer J. P. The pharmacology of 6-hydroxydopamine. Annu Rev Pharmacol. 1973;13:169–180. doi: 10.1146/annurev.pa.13.040173.001125. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Kaplan R. F., Werboff J. Behavioral correlates of focal hippocampal x-irradiation in rats. Exp Brain Res. 1976 Feb 26;24(4):343–349. doi: 10.1007/BF00235002. [DOI] [PubMed] [Google Scholar]