Abstract
We have previously reported on the development and assessment of the tetracycline inducible vector pMIND [1]. Here we report the development of improved pMIND vectors that exhibit both reduced basal transcription in the absence of inducer and increased fold induction in the presence of inducer. An amino acid change in the repressor protein, TetR(Z), produced a 6-fold reduction in basal transcription compared to the original pMIND-Lx and a 100-fold induction of LuxAB in the presence of tetracycline. An integration version of the improved vector (pMEND-Lx) was constructed which resulted in a 9-fold reduction in basal transcription compared to pMIND-Lx and a 17-fold induction of LuxAB in the presence of tetracycline. Further improvements were obtained by cloning the pMEND TetRO promoter into an alternative vector backbone. The resulting vector, pKW08-Lx, exhibited a 70-fold reduction in background compared to pMIND-Lx and a 230-fold induction of LuxAB in the presence of tetracycline. An integration version of pKW08-Lx was constructed and the basal transcription for this vector was zero; an 11-fold induction of LuxAB was observed in the presence of tetracycline. The construction of these improved mycobacterial vectors will prove extremely useful for genetic studies.
Keywords: Mycobacteria, conditional gene expression, tetracycline-inducible
1. Introduction
Mycobacterium smegmatis belongs to the Actinomycetes family, which contains the important human pathogen Mycobacterium tuberculosis, the causative agent of tuberculosis (TB). TB is the leading cause of death from an infectious disease worldwide and, although still primarily a disease of developing countries, the increased incidence of drug-resistant strains, increased immigration and the world-wide HIV epidemic means that developed countries are experiencing gradual increases in the number in TB cases [2]. It is estimated that as many as fifty million people worldwide may have been infected with resistant strains of M. tuberculosis [2]. There are also recent reports of totally drug-resistant (TDR) TB strains [3]. Therefore, there is an urgent need for new TB drugs with novel modes of action that are active against such resistant strains. Genes essential for growth in vitro, in vivo or both account for approximately 20% of the M. tuberculosis genome [4, 5]. Therefore, the development of a conditional gene expression system that would allow the characterisation of essential genes may facilitate the validation of up to 800 potential drug targets.
Conditional gene expression systems are powerful tools for studying essential genes in bacteria. Conditional gene regulation studies require a stable, tightly regulated, highly inducible promoter that does not require specialist growth medium for its activity and is induced by a non-toxic compound with good bio-availability. Tetracycline and its analogues are ideal inducer molecules as they regulate gene expression at very low concentrations (nM range), show good bio-availability (ie. they can penetrate both mycobacterial and animal cells), are non-toxic at the necessary levels and are stable over the required length of study. Repressor-regulated tetracycline efflux systems contain two divergent genes separated by an intergenic region. This intergenic region contains two divergent promoters (one driving the expression of the TetR repressor protein and the other driving the expression of the TetA efflux pump) and two tetO operator gene sequences which bind the TetR repressor in the absence of tetracycline preventing expression of TetA. Upon the addition of tetracycline the repressor protein binds the tetracycline, undergoes a conformational change and dissociates from the tetO operator sites and so releasing the repression on TetA expression [6, 7]. Inducible target gene expression can be achieved by replacing the efflux pump gene (tetA) with the gene under investigation. Several tetracycline-inducible systems have been described for use in mycobacteria, [1, 8-10], however all of these systems exhibit varying amounts of background transcription in the absence of inducer.
We have previously reported on the construction and validation of the tetracycline inducible vector pMIND in various models of TB infection [1] and have subsequently shown this system to be inducible in the Wayne model of hypoxia and in in vivo mouse studies (unpublished data). However, further studies using this vector have raised problems with the levels of background expression from the promoter in the absence of inducer. Not only do we require the inducible system to permit study of genes both in vitro and in vivo, we also require a system that allows us to study genes that are only required in very small amounts to be active. Therefore, as pMIND had all the required attributes to study gene function in mycobacteria, except for strong silencing in the absence of inducer, we sought to create an improved pMIND system. Here we describe the construction and validation of our improved tetracycline inducible systems in the non-pathogenic M. smegmatis.
2. Materials and Methods
2.1 Bacterial Strains and Growth Conditions
M. smegmatis mc2155 was grown in Middlebrook 7H9 liquid broth (supplemented with 0.2% glycerol, 0.05% Tween 80 and 10% OADC) at 37°C shaken at 180 r.p.m. in an orbital shaker or on Middlebrook 7H11 solid media supplemented with 0.5% glycerol and 10% OADC (Becton Dickinson). All E. coli strains were grown on LB-agar plates or in LB broth. Hygromycin (Invitrogen) was added as required at a concentration of 200 μg ml−1 for E. coli and 50 μg ml−1 for mycobacteria. Kanamycin (Sigma) was added at a concentration of 50 μg ml−1. Tetracycline (Tc), Anhydrotetracycline (Atc) and Doxycycline (Doxy) (Sigma) were added as stated.
2.2 Plasmids and DNA work
pMIND is an E. coli-mycobacteria shuttle vector containing the Tc-inducible TetRO promoter from the C. glutamicum plasmid pAG1 [11] upstream of a multiple cloning site, as described previously [1]. pSE100 is an E. coli-mycobacteria shuttle vector with a pMS2 vector backbone and pMC1m is a mycobacterial integration vector. All handling of DNA including restriction enzyme digestions, ligations, transformations and gel electrophoresis were performed using standard procedures unless otherwise stated.
2.3 Construction of transcription terminators
Transcription terminators were constructed by annealing two complementary oligonucleotide primers together producing Acc65 restriction site overhangs. The phosphorylated oligonucleotide primers used to create the rrnB2 terminators were 5′-GTACCAGAAGGCCATCCTGACGGATGGCCTTTTT (forward) and 5′-GTACAAAAAGGCCATCCGTCAGGATGGCCTTCTG (reverse) and the oligonucleotide primers used to create T7 terminators were 5′-GTACTAGCATAACCCCTTGGGGCCTCTAAACGGGTCTTGAGGGGTTTTTTG (forward) and 5′-GTACCAAAAAACCCCTCAAGACCCGTTTAGAGGCCCCAAGGGGTTATGCT A (reverse). An equal concentration of each oligonucleotide were mixed together in annealing buffer (10 mM Tris, pH 7.5–8.0, 50 mM NaCl, 1 mM EDTA), denatured at 95°C for 5 minutes then left at room temperature for at least an hour. The DNA was concentrated by ethanol precipitation.
2.4 Site-Directed Mutagenesis of TetR
In order to create the required amino acid change of threonine to glycine in position 40 of the TetR repressor protein the QuikChange II XL SDM kit (Qiagen) was used according to manufacturer’s instructions. Oligonucleotide primers used to create the amino acid change were 5′-CAGGTACAGCAGCCCACCCTCTACTGGCACTT (forward) and 5′-AAGTGCCAGTAGAGGGTGGGCTGCTGTACCTG (reverse). The required base pair changes are underlined.
2.5 Integration Vector Construction
The L5 integrase gene and phage attachment attP sequences were amplified by PCR from the integration vector pMC1m using 5′-TATCTAACCGGTGTACGATCGACTGCCAGG (forward) and either 5′-TATACAATTGTTGCTAGCTGGCGGGAACGG (reverse) or 5′-TATAAAGCTTGTACGATCGACTGCCAG (reverse) and cloned into the AgeI and MfeI sites of pMEND-Lx or the HindIII and MfeI sites of pKW08-Lx creating pMEND-Lx-Int and pKW08-Lx-Int, respectively.
2.6 Luciferase Assay
Bioluminescence of M. smegmatis was measured in a Berthold Mithras LB940 Plate Reader. M. smegmatis cells were grown to mid-log phase (OD600 of 1-1.2) and induced with 0 or 20 ng ml−1 tetracycline for 2 hours, previously shown to be the optimal concentration for TetRO induction [1]. Samples in triplicate were analysed for their luciferase activity and each experiment was performed three times. To measure Relative Light Units (RLU), 0.01 ml of the substrate, 1% n-decyl aldehyde (Sigma) in ethanol, was injected automatically into wells containing cells in a final volume of 0.1 ml in phosphate-buffered saline (PBS). Raw data were collected in triplicate over a 20 s period and the mean and standard deviation values calculated. The luminescent output was normalized to the cell density and expressed as RLU per OD. Samples were diluted 10-fold in PBS to minimize quenching of luminescence. Each experiment was performed three times.
3. Results and discussion
3.1 Insertion of alternative transcription terminators upstream of the TetRO promoter
Firstly, we investigated whether the addition of alternative transcription terminators upstream of the TetRO promoter reduced background transcription from exogenous promoters within the vector backbone. Two copies of either the E. coli rrnB2 or T7 terminator sequences were inserted into the Acc65 site downstream of the trpA terminators but upstream of the TetRO promoter. The vectors were then transformed into M. smegmatis for assessment in the luciferase assay. However, insertion of either rrnB4 or T7 terminators upstream had no effect on background luciferase activity in the absence of tetracycline (n=3; data not shown). This suggests that the leakiness of the TetRO promoter may be due to incomplete repression of the TetRO promoter system, rather than read-through from other promoters within the vector.
3.2 Mutation of an amino acid in the repressor protein
A single amino acid change of threonine to glycine in position 40 of the TetR(Z) repressor protein had previously been reported to give a 40-fold increase in repression in E. coli [12]. This amino acid is in the DNA binding α2 helix-turn-helix motif of the repressor which contacts position 6 of the operator. Site directed mutagenesis (SDM) was performed on the TetR(Z) protein in pMIND as described to give the required amino acid change at position 40 to create pMEND. This change, and the absence of other changes incorporated accidentally during the SDM procedure within the TetRO region, was confirmed by DNA sequencing. The LuxAB genes were then cloned from pMIND-Lx into pMEND using the unique BamHI and SpeI sites to create pMEND-Lx. Attempts to directly create the desired amino acid change in TetR(Z) were unsuccessful, possibly due to the increased size of the pMIND-Lx plasmid (2 Kb larger than pMIND). An integration version of pMEND-Lx was also constructed as described in Section 2.2 and the three vectors were transformed into M. smegmatis for assessment in the luciferase assay.
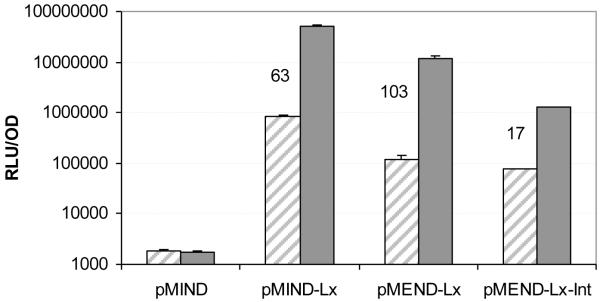
Data from one representative experiment is presented (n=3). Figure 1 shows that in the absence of tetracycline the original pMIND-Lx vector had a background luminescence of 819,844 RLU/OD, pMEND-Lx had a background luminescence of 117,236 RLU/OD and pMEND-Lx-Int had a background luminescence of 75,289 RLU/OD. pMIND control vector had a background luminescence of 3,396 RLU/OD. The amino acid change in the repressor protein gave on average a 6-fold reduction in basal transcription, and integration of the improved vector onto the chromosome gave on average a 9-fold reduction in basal transcription compared to the original pMIND-Lx vector. Upon addition of 20 ng ml−1 tetracycline for 2 hours, pMIND-Lx was induced 63-fold, pMEND-Lx was induced 103-fold and pMEND-Lx-Int was induced 17-fold (Figure 1).
Fig 1. Effect of an amino acid change in the repressor protein.
A representative result (n=3) for background transcription levels and Tc-induction of pMIND-Lx, pMEND-Lx and pMEND-Lx-Int. pMIND without the LuxAB genes is included as control. Induction of LuxAB with 0 ng ml−1 (diagonal shading) or 20 ng ml−1 (solid grey) tetracycline for 2 hours (fold induction values are shown on the graph).
3.3 Alternative Vector Backbone
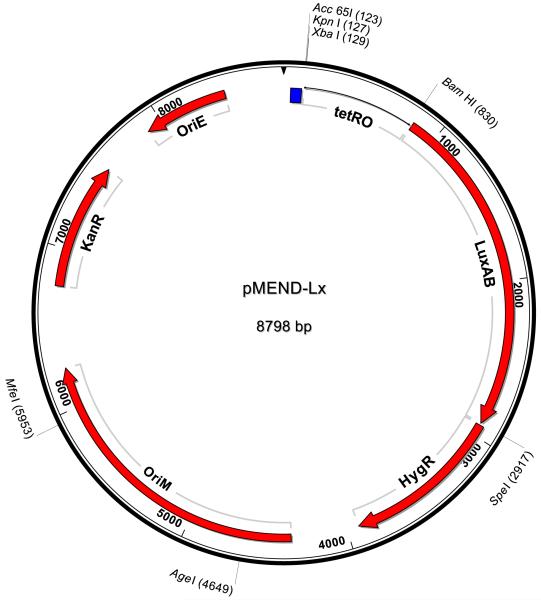
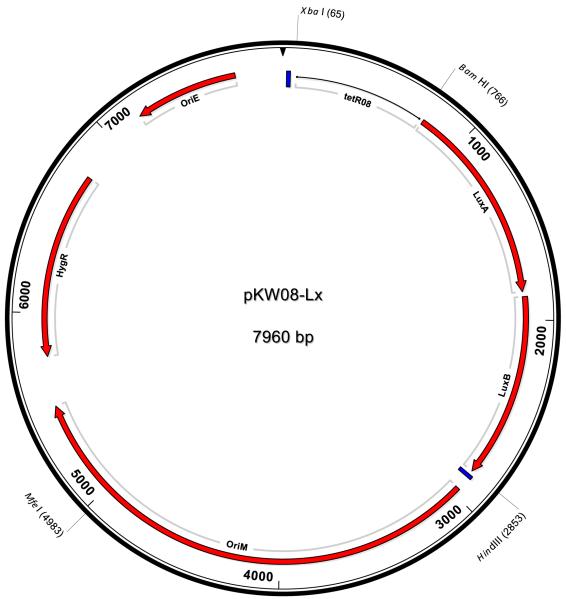
Although the background levels had been reduced by changing an amino acid in the repressor protein, the background luminescence remained high and did not significantly drop when integrated onto the chromosome as a single copy. However upon closer analysis of the pMIND-Lx DNA sequence, the hygromycin resistance gene start codon was found to be just 6bp downstream of the luxB stop codon and therefore the hygromycin gene did not appear to have its own promoter (Figure 4a). Therefore, we reasoned that the background luminescence observed with this vector may be due to a minimum required read-through of the luxAB genes for propagation of the plasmid. Therefore we cloned the BamHI and HindIII TetRO-Lx fragment from pMIND-Lx into a multiple cloning site (mcs) on an alternative mycobacteria-E. coli shuttle vector, pSE100, which has the hygromycin cassette in the opposite direction to the mcs and at a different location in the plasmid, to make pKW08-Lx (Figure 4b). An integration version, pKW08-Lx-Int, was also constructed as described previously. Both vectors were transformed into M. smegmatis for assessment in the luciferase assay.
Fig 4a. Plasmid map for pMEND-Lx.
The Integration version is identical except that the mycobacterial origin of replication (oriM) has been replaced with the attP and int genes of mycobacteriophage L5. Transcription terminators are represented by a blue box. Restriction sites used in this study are shown.
Fig 4b. Plasmid map for pKW08-Lx.
The Integration version is identical except that the mycobacterial origin of replication (oriM) has been replaced with the attP and int genes of mycobacteriophage L5. Transcription terminators are represented by a blue box. Restriction sites used in this study are shown.
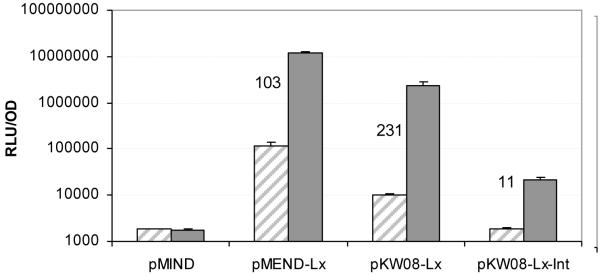
Figure 2 shows a representative result from three experiments. Changing the vector backbone on average gave a 73-fold reduction in basal transcription. The integration version of this vector had on average a 357-fold reduction in basal transcription, which gave comparable background levels similar to control vector containing no LuxAB genes (Figure 2). Upon addition of 20 ng ml−1 Tc for 2 hours, a 231-fold induction was observed for pKW08-Lx and an 11-fold induction was observed for pKW08-Lx-I (Figure 2). Therefore, changing the vector backbone greatly reduced basal transcription and also produced a vector with un-detectable basal transcription. As with all induction systems, there is a balance of fold induction required and levels of repression tolerated. Although the induction of the fully repressed vector pKW08-Lx-I was only 11 fold, this vector may be extremely useful for studying genes that only require low levels of expression.
Fig 2. Effect of changing vector backbone.
A representative result (n=3) for background transcription levels and Tc induction of pMEND-Lx, pKW08-Lx and pKW08-Lx-Int. pMIND without the LuxAB genes is included as control. Induction of LuxAB with 0 ng ml−1 (diagonal shading) or 20 ng ml−1 (solid grey) tetracycline for 2 hours (fold induction values are shown on the graph).
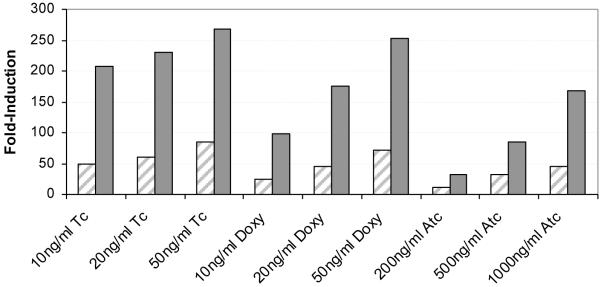
3.4 Dose-dependent induction by Doxy, Tc and Atc
To determine if the Tc induction observed was dose-dependent and if the vectors could be induced by other tetracyclines, the vectors pMEND-Lx and pKW08-Lx were induced with Tc, Atc and Doxy for 2 hours. The MIC values were determined (data not shown) and the concentrations of tetracyclines used for induction were 5 to 10-fold less than the MIC values. There was no inhibitory effect on growth over the induction period for the range of tetracyclines tested except for slight inhibition of growth with both Doxy and Tc at 50 ng ml−1. Induction was dose-dependent for both pMEND-Lx and pSE08-Lx over the concentration range 0-50 ng ml−1 for Tc and Doxy and 0-1000 ng ml−1 for Atc (Figure 3). Previously it was reported that pMIND-Lx could not be induced by other tetracyclines [1], however in this study the concentration of Atc used was much higher than previously tested, and at 20 ng ml−1 Atc we also saw no induction as previously reported (data not shown). It is not clear why no induction was observed previously for Doxy. The fold-induction levels obtained with Doxy were slightly lower than Tc in this study, whereas Atc gave much lower fold-inductions than both Doxy and Tc (Figure 3). Therefore the best inducer of these vectors is Tc, but Doxy can also be used which may be useful for in vivo studies. As observed previously in this study, fold induction levels for all tetracyclines tested were higher with pKW08-Lx compared to pMEND-Lx (Figure 3).
Fig 3. Dose-dependent induction of pMEND-Lx and pKW08-Lx by tetracycline related compounds.
A representative result showing dose-dependent fold induction (compared to no tetracycline control) of luciferase in pMEND-Lx (diagonal shading) and pKW08-Lx (solid grey) after 2 hours.
4. Conclusion
There is a lack of genetic tools available to researchers to study the function of essential mycobacterial genes [2, 13], we have therefore constructed a range of improved tetracycline inducible vectors. We have shown that changing the terminators upstream of the inducible promoter does not affect background transcription, and that repressor binding to the operator is a more critical step in controlling regulation. We have eliminated further read-through problems by changing the backbone vector used. The TetRO promoter can be induced in a dose-dependent manner by tetracycline, anhydrotetracycline and doxycycline. These vectors offer considerable improvements on the previous pMIND system. The episomal and integration Tc-inducible vector systems with varying levels of background gene expression and varying levels of induction developed in this study will be extremely useful for mycobacterial genetic research and their application in slow growing mycobacteria, such as M. bovis BCG and M. tuberculosis, now merits further investigation.
Table 1.
Plasmids used in this study.
| Plasmid | Description | Reference or source |
|---|---|---|
| pMIND | Original mycobacterial Tc-inducible vector | [1] |
| pSE100 | Mycobacterial shuttle vector; source of alternative vector backbone |
[14] |
| pMClm | Mycobacterial integration vector; source of integrase cassette |
[14] |
| pMIND-Lx | Original mycobacterial Tc inducible vector containing LuxAB genes |
[1] |
| pMEND-Lx | pMIND-Lx with Gly40 to Thr40 change in TetR protein; episomal |
This study Accession no. HM002749 |
| pMEND-Lx-Int | Integration version of pMEND-Lx | This study |
| pKW08-Lx | TetRO promoter from pMEND-Lx inserted into the backbone of pSE100; episomal |
This study Accession no. HM002748 |
| pKW08-Lx-Int | Integration version of pKW08-Lx | This study |
Acknowledgments
KW and BR are funded by the Bill & Melinda Gates Foundation and the Wellcome Trust through the Grand Challenges in Global Health Initiative GC11 and GJ is funded by a BBSRC studentship. The authors wish to thank Sabine Ehrt and Dirk Schnappinger for the gifts of pSE100 and pMC1m. The authors also acknowledge Joseph Xhuxhi and Cristina Sanchez-Blanco for technical help.
References
- 1.Blokpoel MC, et al. Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res. 2005;33(2):e22. doi: 10.1093/nar/gni023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KJ, Duncan K. Current strategies for identifying and validating targets for new treatment-shortening drugs for TB. Curr Mol Med. 2007;7(3):297–307. doi: 10.2174/156652407780598575. [DOI] [PubMed] [Google Scholar]
- 3.Velayati AA, et al. Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J. 2009;34(5):1202–3. doi: 10.1183/09031936.00081909. [DOI] [PubMed] [Google Scholar]
- 4.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proceedings of the National Academy of Sciences. 2003;100(22):12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 6.Kamionka A, et al. Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor. Nucl. Acids Res. 2004;32(2):842–847. doi: 10.1093/nar/gkh200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillen W, Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu Rev Microbiol. 1994;48:345–69. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- 8.Ehrt S, et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005;33(2):e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Abanto S, et al. Tetracycline-inducible gene expression in mycobacteria within an animal host using modified Streptomyces tcp830 regulatory elements. Archives of Microbiology. 2006;186(6):459–464. doi: 10.1007/s00203-006-0160-2. [DOI] [PubMed] [Google Scholar]
- 10.Carroll P, Muttucumaru DG, Parish T. Use of a tetracycline-inducible system for conditional expression in Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl Environ Microbiol. 2005;71(6):3077–84. doi: 10.1128/AEM.71.6.3077-3084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tauch A, et al. TetZ, a new tetracycline resistance determinant discovered in gram-positive bacteria, shows high homology to gram-negative regulated efflux systems. Plasmid. 2000;44(3):285–91. doi: 10.1006/plas.2000.1489. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister R, Helbl V, Hillen W. Contacts between Tet repressor and tet operator revealed by new recognition specificities of single amino acid replacement mutants. Journal of Molecular Biology. 1992;226(4):1257–1270. doi: 10.1016/0022-2836(92)91065-w. [DOI] [PubMed] [Google Scholar]
- 13.Ehrt S, Schnappinger D. Controlling gene expression in mycobacteria. Future Microbiology. 2006;1(2):177–184. doi: 10.2217/17460913.1.2.177. [DOI] [PubMed] [Google Scholar]
- 14.Guo XV, et al. Silencing Mycobacterium smegmatis by using tetracycline repressors. J Bacteriol. 2007;189(13):4614–23. doi: 10.1128/JB.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]