Abstract
Importance of the field
Dystonia is a neurological syndrome characterized by involuntary twisting movements and unnatural postures. It has many different manifestations and causes, and many different treatment options are available. These options include physical and occupational therapy, oral medications, intramuscular injection of botulinum toxins, and neurosurgical interventions.
Areas covered in this review
In this review, we first summarize the treatment options available, then we provide suggestions from our own experience for how these can be applied in different types of dystonia. In preparing this review article, an extensive literature search was undertaken using PubMed. Only selected references from 1970 to 2008 are cited.
What the reader will gain
This review is intended to provide the clinician with a practical guide to the treatment of dystonia.
Take home message
Treatment of dystonia begins with proper diagnosis and classification, followed by an appropriate search for underlying etiology, and an assessment of the functional impairment associated with the dystonia. The therapeutic approach, which is usually limited to symptomatic therapy, must then be tailored to the individual needs of the patient.
Keywords: anticholinergics, baclofen, benzodiazepines, dopaminergics, dystonia, tetrabenazine, therapeutics
1. Introduction
Dystonia is a neurological condition with a broad range of clinical manifestations that can emerge at any age. It is defined as a syndrome of involuntary movement that manifests as excessive muscle contractions that frequently cause twisting and repetitive movements or abnormal postures.
The dystonias are classified according to the parts of the body affected (Table 1), the age at onset, and the underlying cause (Table 2). Focal dystonias involve an isolated body region. Examples include blepharospasm (periocular muscles), cervical dystonia (spasmodic torticollis), laryngeal (spasmodic dysphonia) dystonia, oromandibular dystonia, and limb dystonia. Segmental dystonias involve two or more contiguous regions. Meige’s syndrome (blepharospasm plus oromandibular dystonia) is one of the more common examples. Multifocal dystonias involve two or more non-contiguous regions, while hemidystonia involves one side of the body (e.g., ipsilateral arm and leg). Generalized dystonias are more widespread, involving both legs and at least one other body region. Classification by age of onset also is valuable because those with onset before age 30 (young-onset disease) are more likely to have a discoverable condition that often evolves into a more generalized form, while those with onset after age 30 (adult-onset disease) are more likely to have a more focal form with little progression but no discoverable cause. Lastly, the dystonias can be classified as primary or idiopathic, when accompanying features are absent, versus secondary, when dystonia is due to an identifiable cause (e.g., neurodegenerative diseases, stroke, trauma, demyelinating disease, drugs, etc).
Table 1.
Affected areas in dystonia.
| Focal Dystonia (isolated region) |
| Blepharospasm (periocular muscles only) |
| Cervical (torticollis) |
| Laryngeal (spasmodic dysphonia) |
| Oromandibular (jaw, tongue, or peri-oral) |
| Limb (writer’s cramp, foot dystonia) |
| Segmental dystonia (two contiguous regions) |
| Meige syndrome (blepharospasm + oromandibular dystonia) |
| Cervical dystonia + one arm |
| Multifocal dystonia |
| Two or more non-contiguous body areas |
| Hemidystonia |
| Ipsilateral arm and leg |
| Generalized (more extensive areas) |
| Both legs, another region, ± trunk |
| One leg, another region, + trunk |
Table 2.
Causes of dystonia.
| Inherited |
| Amino acid metabolism |
| Glutaric academia |
| GAMT deficiency |
| Hartnup disease |
| Homocystinuria |
| Methylmalonic acidemia |
| Propionic acidemia |
| Sulfite oxidase deficiency |
| Neurotransmitter metabolism |
| Aadc deficiency |
| Dihydropterin reductase deficiency |
| GTP cyclohydrolase deficiency |
| PTPS deficiency |
| Tyrosine hydroxylase deficiency |
| Lipid metabolism/storage |
| Gm1 or Gm2 gangliosidosis |
| Krabbe’s disease |
| Metachromatic leukodystrophy |
| Neuronal ceroid lipofuscinosis |
| Niemann-Pick type C |
| Pelizaeus-Merzbacher disease |
| Ion/metal homeostasis |
| Aceruloplasminemia |
| Cav2.1 calcium channel defects |
| Fahr’s disease |
| Neuroferritinopathy |
| Rapid-onset dystonia-Parkinsonism |
| Wilson’s disease |
| Polyglutamine expansions |
| Dentato-rubral-pallidoluysian atrophy |
| Huntington’s disease |
| Spinocerebellar ataxias (1, 2, 3, 6, 7, 17) |
| DNA handling/transcription |
| Ataxia-oculomotor apraxia |
| Ataxia telangiectasia |
| Cockayne’s disease |
| Lubag |
| Rett’s syndrome |
| Xeroderma pigmentosum |
| Mitochondrial function |
| Deafness-dystonia syndrome |
| Fumarase deficiency |
| Leber’s hereditary optic neuropathy |
| Leigh’s syndrome |
| MELAS |
| MERRF |
| Pyruvate dehydrogenase deficiency |
| Other |
| Ataxia with vitamin E deficiency |
| Biotin-responsive basal ganglia disease |
| Frontotemporal dementias |
| Lesch-Nyhan disease |
| Myoclonus dystonia |
| Neuroacanthocytosis |
| Neuronal intranuclear inclusion disease |
| Oppenheim dystonia |
| Pantothenate kinase neurodegeneration |
| Triosephosphate isomerase |
| Acquired/sporadic |
| Medications |
| Carbamazepine |
| Cinnarizine |
| Dopamine antagonists/agonists |
| Fenfluramine |
| Flunarizine |
| Levodopa |
| Phenytoin |
| Prochlorperazine |
| Metaclopramide |
| Serotonin uptake inhibitors |
| Tiagabine |
| Toxins |
| 3-Nitropropionic acid |
| Bilirubin (kernicterus) |
| Carbon disulfide |
| Carbon monoxide |
| Cyanide |
| Disulfiram |
| Manganese |
| Methanol |
| Vascular |
| Stroke (hemmorhagic or ischemic) |
| Vascular malformation |
| Vasculitis |
| Infection |
| Bacterial |
| Fungal |
| Prion |
| Protozoan |
| Viral |
| Autoimmune |
| Anti-phospholipid syndrome |
| Dystonia gravidarum |
| Hymenoptera stings |
| Multiple sclerosis |
| Reye’s syndrome |
| Sjögren’s syndrome |
| Systemic lupus erythematosis |
| Subacute sclerosis panencephalitis |
| Trauma |
| Head |
| Nerve |
| Spine |
| Structural |
| Abcess |
| Arnold-Chiari malformation |
| Atlanto-axial subluxation |
| Syringomyelia |
| Tumors (brain, spine) |
| Other |
| Cerebral palsy |
| Corticobasal ganglionic degeneration |
| Hypoparathyroidism |
| Multiple system atrophy |
| Parkinson’s disease |
| Progressive supranuclear palsy |
| Tic disorder |
Though previous tables listing dystonia causes are commonly organized according to mode of inheritance, here the causes are organized according to biochemical or functional defects to emphasize the concept of shared themes in pathogenesis.
AADC: Aromatic amino acid decarboxylase; Cav2.1: P/Q-Type voltage regulated calcium channel; GAMT: Guanidinoacetate methyltransferase; GTP: Guanosine triphosphate; MELAS: Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes; MERRF: Mitochondrial encephalopathy with ragged red fibers; PTPS: Pyruvoyltetrahydropterin synthase.
More than 3 million people worldwide suffer from dystonia. Despite the prevalence of dystonia, available treatments are often only modestly efficacious. The goals of therapy are to ameliorate involuntary movements, correct abnormal postures, reduce pain, prevent contractures, and improve overall function and quality of life. The therapeutic approach must be tailored to the individual patient.
Several recent reviews listing the many medical and surgical treatment options available for dystonia have been published [1–5]. This review provides an overview of these options, as well as some guidance on how the various options are best applied in different cases.
2. Oral medications
2.1 Anticholinergics
Anticholinergic agents are generally the most successful oral medications for the treatment of dystonia, with trihexyphenidyl being the most commonly used agent. In a prospective, double-blind trial of high-dose trihexyphenidyl, Burke and colleagues found a clinically significant improvement in 71% of 31 patients (mean age 19 years) on an average daily dose of 30 mg daily during a 36-week study period [6]. No prospective, double-blind, placebo-controlled trials have been conducted in older adults. Other anticholinergic agents have been used with variable success including benztropine, biperiden, atropine, procyclidine, orphenadrine, and scopolamine. Patients started on anticholinergic agents within 5 years of onset are more likely to experience therapeutic benefit [7].
Trihexyphenidyl is a muscarinic acetylcholine receptor antagonist. The therapeutic dose varies considerably. Average daily doses of 41 mg for children and 24 mg for adults have been reported to be effective [8]. Although high doses sometimes are required, children occasionally may respond to doses as low as 4 mg/day [9]. Adults are less likely to tolerate high doses of trihexyphenidyl, as they are more sensitive to its side effects [8,10]. Central side effects include memory loss, confusion, restlessness, insomnia, and nightmares. Children may experience chorea, or exacerbation of a pre-existing tic disorder. Peripheral side effects include blurred vision, dry mouth, constipation, and urinary retention. Eye drops and oral pyridostigmine can be used to combat peripheral side effects. Acute anticholinergic side effects parallel the rise and fall of serum drug levels, but the therapeutic response of dystonia does not [11]. Acute narrow-angle glaucoma is the only absolute contraindication to its use, although there are several relative contraindications including urinary hesitancy and dementia.
Trihexyphenidyl is started at 1 mg daily and increased by 1 mg every 3 – 5 days over a period of 1 month to a goal dose of 2 mg t.i.d.. It can then be increased by 2-mg increments every week until side effects emerge or a dose of 30 mg t.i.d. is reached.
2.2 Baclofen
Baclofen is a presynaptic GABA receptor agonist primarily used to treat spasticity. The exact mechanism by which it helps in dystonia is not known. No controlled studies have evaluated the efficacy of baclofen in dystonia; however, a number of case reports and retrospective studies support its utility, particularly in children [12–17]. In retrospective studies, 20% of patients with various forms of dystonia had a good response to oral baclofen [7]. Those with mild to moderate dystonia, and age < 20 years, were more likely to have a good response than those with severe dystonia and those beyond age 20 [7]. Significant clinical improvement was demonstrated in 30% of 31 children with primary idiopathic dystonia who were treated with an average daily dose of 79 mg [12]. In children with DYT1 dystonia (an early-onset primary dystonia caused by mutations in the DYT1 gene), oral baclofen improved leg dystonia and gait in 14 of 33 patients with a dose of > 50 mg daily, with nine reporting prolonged benefit [17]. Adults with focal dystonia typically do not respond as well [7]. The combination of baclofen and valproic acid was found to be helpful in a small number of patients with various focal dystonias [14–16].
Baclofen should be administered in 3 – 4 divided daily doses. A typical starting dose is 5 mg daily. Increasing by 5 mg/day every 3 – 5 days is generally reasonable until side effects or benefits are achieved. Side effects of baclofen include sedation, dizziness, dry mouth, and urinary urgency or hesitation. Baclofen may increase blood glucose, requiring adjustment of diabetes medications. Abrupt withdrawal can cause psychosis or seizures, so tapering is required if it is discontinued.
2.3 Benzodiazepines
Benzodiazepines are often used in the dystonias, despite the fact that their efficacy has not been evaluated in any large, controlled studies. Clonazepam is the most frequently used. In an open study, clonazepam and other benzodiazepines were found to be beneficial in 16% of patients with various types of dystonia [7]. Other open and retrospective studies have subsequently shown efficacy in treating generalized and focal dystonias, with up to 23% of patients demonstrating a good clinical response [18,19]. Benzodiazepines may be particularly useful in blepharospasm [20] and cervical dystonia with predominant head tremor [19,21].
A typical starting dose is 0.5 mg of clonazepam in the evening. The dose is slowly increased to an average daily dose of 1 – 4 mg divided t.i.d. Side effects include sedation, confusion, impaired coordination, and depression. There is also potential for dependence and negative interaction with alcohol. Abrupt withdrawal can cause seizures, so the dose must be tapered when discontinuation is desired. Use of benzodiazepines is contraindicated in severe hepatic disease.
2.4 Dopaminergics
Levodopa was used to treat generalized dystonia in several early studies, but results were contradictory [22–25]. Some reported improvement [22,24] while others reported worsening [23,25]. Other dopaminergic agents including bromocriptine, apomorphine, and lisuride have been studied, with inconclusive results [26,27]. The varied responses to dopaminergics probably reflects etiological heterogeneity in dystonia, yielding inconsistent results when mixed patient populations are studied [28].
One small subset of patients with substantial improvement with levodopa is doparesponsive dystonia (DRD), which may represent up to 5% of childhood dystonias [29]. DRD is characterized by childhood onset, gait disturbance, Parkinsonism, female predominance, and diurnal fluctuations [30]. It is often misdiagnosed as cerebral palsy. It usually results from mutations in the GCH1 gene encoding GTP cyclohydrolase 1 on chromosome 14q [30,31], although other mutations have been reported [32–34]. Patients with DRD typically show dramatic, sustained improvement with low doses of levodopa. Thus, a trial of levodopa is mandatory in any patient with young-onset dystonia. Because limb dystonia can be a presenting feature of Parkinson’s disease, a trial of levodopa in adult-onset limb dystonia is also appropriate.
Levodopa is administered in combination with the decarboxylase inhibitor carbidopa. Side effects include nausea, orthostasis, and constipation. Unlike patients with Parkinson’s disease, people with dystonia rarely develop confusion, hallucinations, or dyskinesias. Dosing typically begins with one tablet of carbidopa/levodopa 25/100 mg per day, and increases by one tablet every 3 – 5 days. Most children with DRD will respond to doses as low as 25 – 100 mg levodopa, though some may require doses as high as 1200 mg daily (20 mg/kg for children).
2.5 Dopamine receptor antagonists and dopamine depletion
Given that dopamine-blocking agents (antipsychotics) have the potential to induce tardive dyskinesia and tardive dystonia [35], it is paradoxical that they may be useful in the treatment of dystonia. Although some case reports and case series have supported the utility of typical antipsychotics in the treatment of certain types of dystonia, most clinical trials have yielded mixed results [26,7]. This fact, coupled with their side-effect profile that includes sedation, parkinsonism, and tardive dyskinesia, has generally discouraged the use of typical antipsychotics in the treatment of dystonia.
Some of the atypical antipsychotics (e.g., quetiapine and risperidone) have also been reported to be effective in treating primary dystonia [36,37]. Clozapine is an atypical antipsychotic that deserves special mention. Clozapine’s mechanism of action in dystonia is not known. It has been reported to be moderately effective in a small number of open trials for the treatment of segmental, axial, and generalized dystonia, with mixed results in cervical dystonia [38–41]. A typical starting dose of clozapine is 12.5 mg daily followed by increases of 12.5 – 25 mg per week until benefit or side effects are achieved. Doses as high as 900 mg daily may be required in patients with axial or generalized dystonia [39,40]. Unlike other dopamine blockers, clozapine does not cause tardive dyskinesia, and it rarely causes other extrapyramidal side effects. Though it may be useful in some patients, clozapine is not routinely used in dystonia, due to the risk of seizures, severe cardiac side effects, fatal agranulocytosis and the need for weekly blood tests.
Tetrabenazine (TBZ) depletes vesicular stores of dopamine by inhibiting the monoamine transporter 2 (VMAT-2). TBZ is effective for the treatment of a variety of hyperkinetic movement disorders, including chorea, tics, tardive dyskinesia, myoclonus, and dystonia [42]. TBZ was found effective for various types of focal and generalized dystonia in a small, double-blind, crossover study [43]. These results have been confirmed in retrospective data analyses and large open studies [44,45]. It may be particularly useful in the treatment of tardive dystonia, as one study reported that 80% of patients with tardive dystonia and 63% with idiopathic dystonia showed significant improvement [44,46].
It can be started as a single 12.5 mg dose once daily, and titrated up by 12.5 mg every 3 – 5 days, to a target of 25 – 100 mg daily. Side effects include drowsiness, parkinsonism, depression, insomnia, nervousness, anxiety, and akathisia. Its primary advantage is that it does not cause tardive dyskinesia. Lithium can be used as an adjunct to augment the therapeutic effect of TBZ or to enable the use of lower doses in patients who are experiencing side effects on higher doses of TBZ [45,47,48].
2.6 Other agents
Multiple other agents have been used to treat dystonia in small, open trials. These agents include oral and intravenous lidocaine [49], riluzole [50], lithium [51,52], carbamazepine [53], alcohol [54], tizanidine [55], and nabilone [56]. At present, there is insufficient evidence to support the routine use of any of these agents for dystonia.
3. Botulinum toxin
Since its introduction in the 1980s, botulinum toxin has revolutionized the treatment of dystonia. Botulinum toxin is a toxic protein produced by the bacterium Clostridium botulinum, which exists in seven different serotypes (toxins A – G). Botulinum toxins exert their therapeutic benefit by blocking the release of acetylcholine into the neuromuscular junction [57]. The toxin is injected into dystonic muscles, thereby weakening the muscles and ameliorating dystonic symptoms. Effects generally take effect in the first 2 weeks and last for 3 – 4 months.
Botulinum toxin has become the treatment of choice for most patients with focal or segmental dystonia, including those with blepharospasm, spasmodic dysphonia, cervical, oromandibular, and lingual dystonia. It can also be used to treat writer’s cramp and other occupational dystonias. The safety and efficacy of botulinum toxin to treat focal dystonia have been documented in several open-label and controlled studies [58–63].
Worldwide, three formulations of botulinum toxin type A (Botox™ [Allergan, CA, USA], Dysport™ [Tercica, CA, USA], and NT 201 [Merz, Germany]) and one type of botulinum toxin type B (Myobloc™ [Solstice, CA, USA]) are commercially available. The doses of the different botulinum toxin formulations differ significantly, requiring injectors to be familiar with the various dosage schemata.
The major side effects are weakness of the injected muscles or weakness of nearby muscles due to local diffusion of the toxin. Spread can cause other side effects, depending on the site of injection (e.g., ptosis or diplopia after eyelid injections or dysphagia after neck injections). Systemic symptoms are uncommon. With repeated use, neutralized antibodies can develop, rendering the toxin ineffective [64,65]. To avoid developing resistance, injections are best performed at intervals of ≥3 months and the lowest possible doses that are effective should be used.
The use of botulinum toxins is contraindicated during pregnancy and lactation. The presence of neuromuscular disease, such as myasthenia gravis or amyotrophic lateral sclerosis, is a relative contraindication.
4. Surgical treatment
A variety of neurosurgical procedures exist for the treatment of dystonia, including peripheral denervation (mainly for cervical dystonia), intrathecal baclofen (ITB), ablative procedures (pallidotomy and thalamotomy), and deep brain stimulation (DBS) [4,5,66]. Currently, pallidal DBS is the most widely used surgical procedure for dystonia.
4.1 Selective denervation
Selective denervation requires correct identification of the muscles involved in cervical dystonia, followed by performance of a peripheral or extraspinal selective denervation of only those muscles. Patient selection is critical for success with this procedure. Patients who have developed a fixed posture as a result of either muscle fibrosis or spinal column degeneration do not respond well [66]. The presence of more than one primary movement (e.g., torticollis and laterocollis) also limits the success of selective denervation [66]. Optimal results are obtained in patients with pure rotational torticollis, laterocollis, retrorotational torticollis, and retrocollis [66]. In Bertrand’s series of 260 patients, 89% had very good results [67]. Several other series report success rates of 68 – 88% [68–71]. No major morbidity is associated with this procedure, though dysesthesia, dysphagia, and trapezius weakness are sometimes seen temporarily after surgery [66].
In summary, selective peripheral denervation is a safe and effective procedure for a select group of cervical dystonia patients. It is probably underutilized secondary to the limited number of centers with experience required to perform it.
4.2 Intrathecal baclofen
ITB was first used to treat dystonia in 1991 [72]. Theoretically, it can reduce the central side effects associated with oral baclofen administration. Though shown to be effective in treatment of both primary and secondary dystonia in some reports [73,74], the available data is inconsistent. This inconsistency again may reflect etiological heterogeneity in dystonia, with some subtypes responding better than others. Currently, it is used most often in patients with dystonia combined with spasticity of the lower limbs, as seen in cerebral palsy. Infection, CSF leak, catheter and pump malfunction (which can lead to withdrawal reactions), and overdose are among the possible complications [75].
4.3 Brain surgery
The use of ablative procedures, such as pallidotomy and thalamotomy, to treat movement disorders dates back to the 1940s. Though these procedures can be effective in treating different types of dystonia, they have largely been replaced by DBS and will not be further discussed. DBS has several advantages over the ablative procedures. Because of its non-destructive nature, DBS is reversible and adjustable [4]. It can also be safely performed bilaterally without the risk of permanent speech, swallowing, or cognitive deficits that have been reported with bilateral ablative procedures [76]. Patients with an unequivocal diagnosis of dystonia who are significantly disabled despite optimal medical treatment should be considered for DBS. Potential candidates should undergo surgery prior to the development of contractures and fixed deformities that may limit functional improvement as the dystonia improves. The most extensively studied target for treatment of dystonia is the internal segment of the globus pallidus. Although the mechanism of action of stimulation is not well understood, it has been used effectively to treat various types of dystonia. The benefits of pallidal DBS often are delayed by several weeks or months after surgery, and patients should be advised accordingly [4].
Patients with primary generalized dystonia are the most extensively studied group. Several small, open, non-blinded studies reported improvement in dystonia after DBS [77–87], with the degree of improvement in the range of 21–95% [78,82]. The first prospective, controlled trial evaluating bilateral pallidal DBS for treatment of dystonia came from Vidailhet et al in 2005 [80]. They performed a prospective, controlled, multicenter study that evaluated the efficacy and safety of bilateral pallidal DBS in 22 patients with primary generalized dystonia. Using the movement and disability subscores of the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS), they evaluated subjects preoperatively and again at 3, 6, and 12 months postoperatively during stimulation. Movement scores were assigned by review of a videotaped examination by a blinded observer. A mean improvement of 54% in the BFMDRS movement score and 44% in the BFMDRS disability score was observed after 12 months of chronic stimulation. At 3 months, subjects underwent a double-blind assessment in the presence and absence of stimulation. Subjects showed a 29% improvement in the BFMDRS movement scale in the stimulated state. Subsequently, other evidence from randomized, controlled trials has been published, confirming the safety and efficacy of bilateral pallidal DBS in the treatment of primary generalized dystonia [81].
Pallidal DBS can also been used to treat medication-refractory cervical dystonia [88,89], Meige’s syndrome [90–92], and tardive dystonia [93]. Clinical improvement after pallidal DBS for the secondary dystonias seems less consistent, with some forms responding well and others responding poorly. The tardive dystonias and pantothenate kinase-associated neurodegeneration respond very well, while many others do not [78]. This inconsistency probably again reflects etiological heterogeneity and the different mechanisms responsible for dystonia.
5. Physical and occupational therapy
Physical and occupational therapists can help to mobilize frozen joints, limit mounting contractures, establish appropriate exercise programs, and provide assistive devices to those who need them. Therapists with knowledge of dystonia also can maximize the use of sensory tricks (geste antagonist) to ameliorate dystonic symptoms. Examples include ankle-foot orthotics, dental devices for oromandibular dystonia, and various writing devices for writer’s cramp.
Sensory motor retuning, also known as constraint-induced movement therapy, may be useful in hand dystonias. The technique involves splinting the non-dystonic fingers, thereby forcing the dystonic finger to work in concert with the other fingers to complete the desired task. This technique has shown promise in small studies of task-specific hand dystonias [94]. The opposite technique, where the dystonic arm is immobilized by splinting for several weeks, followed by a period of retraining, has also been tried in subjects with occupational hand dystonia, musician’s dystonia, and writer’s cramp [95,96]. Results of these small, open trials were also promising, but further studies are needed for confirmation.
6. Conclusion
Treatment of dystonia begins with proper diagnosis and classification, followed by an appropriate search for underlying etiology, and an assessment of the functional impairment associated with the dystonia. The therapeutic approach, which is usually limited to symptomatic therapy, must then be tailored to the individual needs of the patient. Physical therapy and occupational therapy can be useful in many patients. Botulinum toxin is currently the mainstay of treatment for focal and segmental dystonia, while oral medications and DBS are the mainstays of therapy for generalized dystonia. In the future, mechanism-directed therapy will be feasible as our growing knowledge of the pathogenesis of dystonia enables rational drug design [28].
7. Expert opinion
7.1 General approach
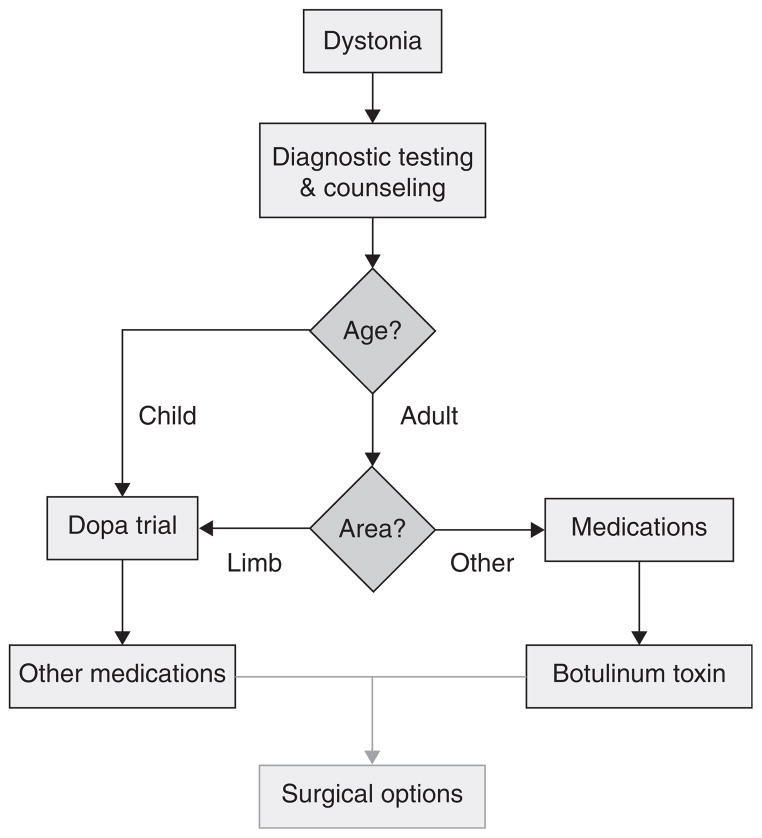
Due to the broad range of clinical manifestations and underlying causes, it is challenging to devise a universal algorithm that is appropriate for the management of all dystonias. Many treatments are available, each with varying applicability for different types of dystonia. Since there are few formal studies comparing treatments to guide recommendations, most recommended treatment strategies are based on personal experiences and preferences of providers who manage dystonia patients frequently. The approach outlined in Figure 1 provides general guidelines, but many exceptions exist and therapy must be customized according to each patient’s needs.
Figure 1. Schematic approach to the treatment of dystonia.
Other medications may include anticholinergics, baclofen, benzodiazepines, and others. Only a small proportion of patients proceed through the surgical options, which vary according to the type of dystonia.
The first step is directed towards diagnosis, and particularly the discovery of a treatable cause. The second step involves counseling regarding prognosis and treatment options, often combined with consultation requests for physical and occupational therapy. The third step involves medical therapy, which varies according to the type of dystonia. Finally, surgical options are reserved for those in whom medical therapy provides inadequate relief.
7.2 Diagnostic testing
Diagnostic testing plays an integral role in selecting the best treatment strategies, since some dystonias have specific treatments. Although the list of potential causes to investigate is enormous (Table 2), it is generally not useful to perform a large battery of tests to exclude them all. Instead, it is more useful to divide diagnostic tests into those that are essential because they may reveal a treatable cause with a specific therapy, and those that are optional because no specific treatments exist and therapy is largely symptomatic. The essential and optional tests vary according to patient age.
Among the essential tests, we consider MRI of the brain to be warranted for all ages. It can identify a causal structural defect, or provide clues to some degenerative disorders in adults. In younger individuals, unique MRI abnormalities also may provide clues for specific treatable developmental or metabolic disorders (e.g., Wilson’s disease, glutaric aciduria, biotin-responsive basal ganglia disease, etc).
Beyond brain MRI, extensive testing of adults with primary focal or segmental dystonia is rarely revealing, unless the history or examination provide clues for a specific cause. For adults with generalized dystonia and children with all forms of dystonia, several additional tests are essential. These include tests for Wilson’s disease, glutaric aciduria, vitamin E deficiency, GLUT1 deficiency, and autoimmune disease. Although these disorders are each quite rare, specific and highly effective treatments are available.
The optional tests include most disorders for which disease-specific treatments are not available. Many are simple blood tests, while others can be discomforting, such as bone-marrow or skin biopsy. We prefer to allow the history and exam to guide the selection of optional tests. It is also useful to consider patient preferences. Some patients take the pragmatic view that extensive testing is unwarranted, since reaching a specific diagnosis may not change the therapeutic approach. Other patients need a definitive answer for closure, and will tolerate extensive testing, even though specific treatments are absent. Such tests should be arranged in consultation with specialists in neurogenetics.
7.3 Counseling and adjunctive services
The best patient is an educated patient, so treatment should begin with counseling. Patients with dystonia often go misdiagnosed for years, which can cause some to become frustrated and even frankly depressed. The goals of counseling are to help patients understand their diagnosis and the available treatments, to restore hope while also setting realistic expectations for treatment, and to empower them to seek help from support groups and online resources.
An initial consultation with a physical or occupational therapist can be valuable to mobilize frozen joints, limit mounting contractures, establish appropriate exercise programs, and provide assistive devices to those who need them. Periodic visits with a physical or occupational therapist over the course of the disease may be helpful, even though they may not alter the long-term disease course.
7.4 Medications
All children with dystonia, as well as some adults with certain types of dystonia, should first be treated with levodopa. Levodopa is dramatically effective in children with dopa-responsive dystonia, enabling an essentially normal life in an otherwise severely debilitating condition. A common error is to provide an inadequate levodopa trial. While most children with dopa-responsive dystonia respond to very low doses, some require higher doses. A trial of up to 20 mg/kg divided into three daily doses for 1 month is needed to assess responsiveness adequately.
Adults with prominent limb dystonia also deserve a treatment trial with levodopa. This is because the clinical manifestations of dopa-responsive dystonia may sometimes be delayed until adulthood, and because dystonia may sometimes be the presenting manifestation of Parkinson’s disease. In both of these situations, dystonia most often affects the limbs, and it responds well to levodopa. Good responses to levodopa rarely are seen in adults with dystonia affecting the face, neck, or trunk. As a result, levodopa trials are not needed in patients with otherwise typical blepharospasm, craniofacial dystonia or cervical dystonia.
Beyond levodopa, the decision to proceed with trials of other medications must be accompanied by counseling regarding treatment expectations and side effects. All drugs should be given in divided doses throughout the day and at the lowest possible doses in order to minimize side effects. Medication side effects are summarized in Table 3. We typically provide all children with dystonia who do not respond to levodopa further trials of anticholinergics, benzodiazepines, and/or baclofen. We usually also provide all adults with trials of these agents, including those who receive botulinum toxins.
Table 3.
Oral medications and their side effects.
| Trihexyphenidyl | Memory loss, confusion, insomnia, blurry vision, dry mouth, constipation, urinary retention |
| Baclofen | Sedation, dizziness, dry mouth, urinary urgency or hesitancy, increased blood glucose |
| Clonazepam | Sedation, confusion, depression, impaired coordination, dependence |
| Carbidopa/levodopa | Nausea, orthostasis, constipation |
| Clozapine | Agranulocytosis (requires weekly CBC monitoring), sedation, hypotension, myocarditis, cardiomyopathy, sialorrhea, arrhythmia, seizures, diabetes mellitus |
| Tetrabenazine | Drowsiness, Parkinsonism, depression, insomnia, anxiety, akathisia |
CBC: Complete blood count.
7.5 Botulinum toxin
Multiple prior reviews describe the use of botulinum toxins for dystonia in great detail. We will not repeat these details here, but instead provide some general principles and strategies for their use.
Although the botulinum toxins are the treatment of choice for most adults with focal and segmental dystonia, we typically do not provide them on the initial visit. The first visit is devoted to diagnostic testing, counseling, planning subsequent treatments with botulinum toxin, and obtaining insurance approval for the procedure. A trial of one or more oral agents can usually be accomplished in the interval between the initial and a subsequent visit for botulinum toxin. This initial trial serves two purposes. First, a trial with an oral agent is sometimes required by insurance providers. Second, an oral agent with even partial efficacy can be useful as an adjunctive therapy as needed for unexpected flares or in those in whom botulinum toxin wears off early.
The botulinum toxins are routinely used in the focal dystonias, but it is useful to remember that they sometimes are helpful in patients with generalized dystonia too. In patients with generalized dystonia, it is not feasible to treat all manifestations, but it is useful to treat particularly problematic features. For example, patients with generalized dystonia that involves the neck and trunk are at high risk for development of pain and/or acquired myelopathy due to constant and forceful bending of the spine. In these patients, targeting the axial manifestations with botulinum toxin can avert or delay the need for surgery.
The use of electromyography (EMG) guidance for delivery of botulinum toxins varies widely among practitioners. Some consider it unnecessary in most cases, while others consider it essential for all. We prefer a more customized approach that depends on patient needs. EMG guidance is rarely required in patients with blepharospasm or craniofacial dystonia, since the medication is usually delivered subcutaneously and not directly into the muscles. It is also rarely required in cervical dystonia, since careful examination of the abnormal movements together with muscle palpation is sufficient to identify muscles to achieve good results. Exceptions are those with particularly thick necks, very complicated patterns of movement, and those in whom unguided treatments produce unsatisfactory results. On the other hand, patients with distal limb involvement usually require EMG guidance for best results. Here, the many small muscles of the hand or foot are too difficult to identify accurately without EMG.
7.6 Surgery
Multiple prior reviews describe the use of surgical treatments for dystonia in great detail [4,5,66]. We will not repeat these details here, but instead provide some general principles and strategies for their use. For patients who fail medical therapy, surgical approaches should be considered. Selective denervation is a viable option for some patients with cervical dystonia. ITB can be helpful for patients with a combination of spasticity and dystonia, especially children. DBS is the treatment of choice for those with generalized dystonia, and is increasingly applied in those with medically resistant focal or segmental dystonias. While DBS may be available through many medical centers, it should be done where there is special expertise in dystonia, because the outcome depends on operative technique [4].
Article highlights.
Treatment of dystonia begins with proper diagnosis and classification, an appropriate search for underlying etiology, and an assessment of the associated functional impairment.
The therapeutic approach must be tailored to the individual needs of the patient.
Physical therapy and occupational therapy can be useful in many patients.
Botulinum toxin is currently the mainstay of treatment for focal and segmental dystonia.
Oral medications and deep brain stimulation are the mainstays of therapy for generalized dystonia.
This box summarises key points contained in the article.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–72. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 2.Goldman J, Comella C. Treatment Of dystonia. Clin Neuropharmacol. 2003;26(2):102–8. doi: 10.1097/00002826-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 3••.Bhidayasiri R, Tarsy D. Treatment of dystonia. Expert Rev Neurother. 2006;6(6):863–86. doi: 10.1586/14737175.6.6.863. Very thorough review of dystonia treatment options. [DOI] [PubMed] [Google Scholar]
- 4••.Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008;5(2):320–30. doi: 10.1016/j.nurt.2008.01.002. Excellent review of DBS in dystonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks WJJ. Brain surgery for dystonia. In: Stacey MA, editor. Handbook of dystonia. Informa Healtcare Usa, Inc; New York: 2007. pp. 393–406. [Google Scholar]
- 6.Burke RE, Fahn S, Marsden CD. Torsion dystonia: a double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology. 1986;36(2):160–4. doi: 10.1212/wnl.36.2.160. [DOI] [PubMed] [Google Scholar]
- 7.Greene P, Shale H, Fahn S. Analysis of open-label trials in torsion dystonia using high dosages of anticholinergics and other drugs. Mov Disord. 1988;3(1):46–60. doi: 10.1002/mds.870030107. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S. High dosage anticholinergic therapy in dystonia. Neurology. 1983;33(10):1255–61. doi: 10.1212/wnl.33.10.1255. [DOI] [PubMed] [Google Scholar]
- 9.Tsao C. Low-dose trihexyphenidyl in the treatment of dystonia. Pediatr Neurol. 1988;4:381. doi: 10.1016/0887-8994(88)90089-6. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AE, Lang AE, Saint-Cyr JA, et al. Cognitive processes in idiopathic dystonia treated with high-dose anticholinergic therapy: implications for treatment strategies. Clin Neuropharmacol. 1991;14(1):62–77. doi: 10.1097/00002826-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Burke RE, Fahn S. Pharmacokinetics of trihexyphenidyl after short-term and long-term administration to dystonic patients. Ann Neurol. 1985;18:35–40. doi: 10.1002/ana.410180107. [DOI] [PubMed] [Google Scholar]
- 12.Greene P. Baclofen in the treatment of dystonia. Clin Neuropharmacol. 1992;15:276–88. doi: 10.1097/00002826-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Greene PE, Fahn V. Baclofen in the treatment of idiopathic dystonia in children. Mov Disord. 1992;7(1):48–52. doi: 10.1002/mds.870070109. [DOI] [PubMed] [Google Scholar]
- 14.Sandyk R. Blepharospasm- successful treatment with baclofen and sodium valproate. A case report. S Afr Med J. 1983;64:955–6. [PubMed] [Google Scholar]
- 15.Sandyk R. Treatment of writer’s cramp with sodium valproate and baclofen. A case report. S Afr Med J. 1983;63:702–3. [PubMed] [Google Scholar]
- 16.Brennan M, Ruff P, Sandyk R. Efficacy of a combination of sodium valproate and baclofen in meige’s disease (idiopathic orofacial dystonia) Br Med J (Clin Res Ed) 1982 Sep 25;285(6345):853. doi: 10.1136/bmj.285.6345.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anca MH, Zaccai TF, Badarna S, et al. Natural history of oppenheim’s dystonia (Dyt1) In Israel. J Child Neurol. 2003;18:325–30. doi: 10.1177/08830738030180050701. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic J. Medical therapy and botulinum toxin in dystonia. Adv Neurol. 1998;78:169–83. [PubMed] [Google Scholar]
- 19.Hughes AJ, Lees AJ, Marsden CD. Paroxysmal dystonic head tremor. Mov Disord. 1991;6(1):85–6. doi: 10.1002/mds.870060118. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13(4):402–11. doi: 10.1002/ana.410130406. [DOI] [PubMed] [Google Scholar]
- 21.Davis TL, Charles PD, Burns RS. Clonazepam-sensitive intermittent dystonic tremor. South Med J. 1995;88(10):1069–71. doi: 10.1097/00007611-199510000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher NA, Thompson PD, Scadding JW, et al. Successful treatment of childhood onset symptomatic dystonia with levodopa. J Neurol Neurosurg Psychiatry. 1993;56(8):865–7. doi: 10.1136/jnnp.56.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett RE, Yahr MD, Duvoisin RC, et al. Torsion dystonia and spasmodic torticollis- results of treatment with L-dopa. Neurology. 1970;20(11):107–13. doi: 10.1212/wnl.20.11_part_2.107. [DOI] [PubMed] [Google Scholar]
- 24.Coleman M. Preliminary remarks on L-dopa therapy of dystonia. Neurology. 1970;20(11):114–21. doi: 10.1212/wnl.20.11_part_2.114. [DOI] [PubMed] [Google Scholar]
- 25.Mandell S. The treatment of dystonia with L-dopa and haloperidol. Neurology. 1970;20(11):103–6. doi: 10.1212/wnl.20.11_part_2.103. [DOI] [PubMed] [Google Scholar]
- 26.Greene P, Shale H, Fahn S. Experience with high dosages of anticholinergic and other drugs in the treatment of torsion dystonia. Adv Neurol. 1988;50:547–56. [PubMed] [Google Scholar]
- 27.Lang AE. Dopamine agonists and antagonists in treatment of idiopathic dystonia. Adv Neurol. 1988;50:561–70. [PubMed] [Google Scholar]
- 28•.Jinnah HA, Hess EJ. Experimental therapeutics for dystonia. Neurotherapeutics. 2008;5:198–209. doi: 10.1016/j.nurt.2008.01.001. Discusses a multifaceted approach to drug discovery in dystonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nygaard TG, Marsden CD, Fahn S. Dopa-responsive dystonia: long-term treatment response and prognosis. Neurology. 1991;41:174–81. doi: 10.1212/wnl.41.2_part_1.174. [DOI] [PubMed] [Google Scholar]
- 30.Ichinose H, Ohye T, Takahashi E, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutation in the Gtp-cyclohydrolase I gene. Nat Genet. 1994;8:232–42. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 31.Nygaard T, Wilhelmsen K, Risch N, et al. Linkage mapping of dopa-responsive dystonia (Drd) to chromosome 14q. Nat Genet. 1993;5:386–91. doi: 10.1038/ng1293-386. [DOI] [PubMed] [Google Scholar]
- 32.Steinberger D, Blau N, Goriuonov D, et al. Heterozygous mutation in 55-untranslated region of sepiapterin reductase gene (Spr) in a patient with dopa-responsive dystonia. Neurogenetics. 2004;5:187–90. doi: 10.1007/s10048-004-0182-3. [DOI] [PubMed] [Google Scholar]
- 33.Steinberger D, Korinthenber R, Topka H, et al. Dopa-responsive dystonia: mutation analysis of Gch1 and analysis of therapeutic doses of L-dopa. Neurology. 2000;55:1735–37. doi: 10.1212/wnl.55.11.1735. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa Y, Graf WD, Wong H, et al. Dopa-responsive dystonia simulating spastic paraplegia due to tyrosine hydroxylase (Th) gene mutations. Neurology. 2001;56:260–53. doi: 10.1212/wnl.56.2.260. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez-Jimenez F, Garcia-Ruiz P, Molina J. Drug-induced movement disorders. Drug Saf. 1997;16:180–204. doi: 10.2165/00002018-199716030-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zuddas A, Cianchetti C. Efficacy of risperidone in idiopathic segmental dystonia. Lancet. 1996;347:127–8. doi: 10.1016/s0140-6736(96)90257-3. [DOI] [PubMed] [Google Scholar]
- 37.Reeves R, Liberto V. Treatment of essential blepharospasm with quetiapine. Mov Disord. 2003;18(9):1072–3. doi: 10.1002/mds.10485. [DOI] [PubMed] [Google Scholar]
- 38.Karp BI, Goldstein SR, Chen R, et al. An open trial of clozapine for dystonia. Mov Disord. 1999;14(4):652–7. doi: 10.1002/1531-8257(199907)14:4<652::aid-mds1015>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Burbaud P, Guehl D, Lagueny A, et al. A pilot trial of clozapine in the treatment of cervical dystonia. J Neurol. 1998;245(6–7):329–31. doi: 10.1007/s004150050229. [DOI] [PubMed] [Google Scholar]
- 40.Wolf M, Mosnaim A. Improvement of axial dystonia with the administration of clozapine. Int J Clin Pharmacol Ther. 1994;32:282–3. [PubMed] [Google Scholar]
- 41.Thiel A, Dressler D, Kistel C, et al. Clozapine treatment of spasmodic torticollis. Neurology. 1994;44:957–8. doi: 10.1212/wnl.44.5.957. [DOI] [PubMed] [Google Scholar]
- 42.Kenney C, Hunter C, Jankovic J. Long-term tolerabilty of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord. 2006;22(2):193–7. doi: 10.1002/mds.21222. [DOI] [PubMed] [Google Scholar]
- 43.Jankovic J. Treatment of hyperkinetic movement disorders with tetrabenazine: a double-blind crossover study. Ann Neurol. 1982;11:41–7. doi: 10.1002/ana.410110108. [DOI] [PubMed] [Google Scholar]
- 44.Jankovic J, Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48(2):358–62. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 45.Jankovic J, Orman J. Tetrabenazine therapy of dystonia, chorea, tics, and other dyskinesias. Neurology. 1988;38(3):391–4. doi: 10.1212/wnl.38.3.391. [DOI] [PubMed] [Google Scholar]
- 46.Pekkenberg H, Fog R. Spontaneous oral dyskinesia. Results of treatment with tetrabenazine, pimozide, or both. Arch Neurol. 1974;31:352–3. doi: 10.1001/archneur.1974.00490410100014. [DOI] [PubMed] [Google Scholar]
- 47.Reches A, Hassan M, Jackson V, et al. Lithium attenuates dopamine depleting effects of reserpine and tetrabenazine but not of alpha methyl-P-tyrosine. Life Sci. 1983;33:157–60. doi: 10.1016/0024-3205(83)90408-3. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa T, Ushizima I, Ono N. Modifications by litium of behavioral responses to methamphetamine and tetrabenazine. Psychopharmacologia. 1975;42:243–8. doi: 10.1007/BF00421263. [DOI] [PubMed] [Google Scholar]
- 49.Ohara S. Mexiletine in the treatment of spasmodic torticollis. Mov Disord. 1998;13(6):934–40. doi: 10.1002/mds.870130612. [DOI] [PubMed] [Google Scholar]
- 50.Muller J. Riluzole therapy in cervical dystonia. Mov Disord. 2002;17(1):198–200. doi: 10.1002/mds.1200. [DOI] [PubMed] [Google Scholar]
- 51.Couper-Smartt J. Lithium in spasmodic torticollis. Lancet. 1973;2:741–2. doi: 10.1016/s0140-6736(73)92581-6. [DOI] [PubMed] [Google Scholar]
- 52.Koller WC, Biary N. Lithium ineffective in dystonia. Ann Neurol. 1983;13:579–80. doi: 10.1002/ana.410130520. [DOI] [PubMed] [Google Scholar]
- 53.Isgreen WP. Carbamazepine in torsion dystonia. Adv Neurol. 1976;14:411–16. [PubMed] [Google Scholar]
- 54.Biary N, Koller W. Effect of alcohol on dystonia. Neurology. 1985;35:239–43. doi: 10.1212/wnl.35.2.239. [DOI] [PubMed] [Google Scholar]
- 55.Lang AE, Riley DE. Tizanidine in cranial dystonia. Clin Neuropharmacol. 1992;15:142–7. doi: 10.1097/00002826-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Fox SH. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord. 2002;17:145–9. doi: 10.1002/mds.1280. [DOI] [PubMed] [Google Scholar]
- 57•.Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53(1):3–9. doi: 10.1159/000083259. Nice review of botulinum toxin mechanism of action. [DOI] [PubMed] [Google Scholar]
- 58.Simpson DM. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2008;70:1699–706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37(4):616–23. doi: 10.1212/wnl.37.4.616. [DOI] [PubMed] [Google Scholar]
- 60.Blitzer A. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann Otol Rhinol Laryngol. 1989;98(2):93–7. doi: 10.1177/000348948909800202. [DOI] [PubMed] [Google Scholar]
- 61.Troung DD. Double-blind controlled study of botulinum toxin in adductor spasmodic dysphonia. Laryngoscope. 1991;101(6 Pt 1):630–4. doi: 10.1288/00005537-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Hallett M, Benecke R, Blitzer A, Comella CL. Treatment of focal dystonias with botulinum toxin. Toxicon. 2009;54(5):628–33. doi: 10.1016/j.toxicon.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pullman S. Approach to the treatment of limb disorders with botulinum toxin a: experience with 187 patients. Arch Neurol. 1996;53:617–24. doi: 10.1001/archneur.1996.00550070055012. [DOI] [PubMed] [Google Scholar]
- 64.Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213–17. doi: 10.1002/mds.870090216. [DOI] [PubMed] [Google Scholar]
- 65.Jankovic J, Schwarz K. Ressponse and immunoresistance to botulinum toxin injections. Neurology. 1995;45:1743–6. doi: 10.1212/wnl.45.9.1743. [DOI] [PubMed] [Google Scholar]
- 66•.Arce CA. Selective denervation in cervical dystonia. In: Stacey MA, editor. Handbook of dystonia. Informa Healthcare USA, Inc; New York: 2007. pp. 381–92. Thorough review of selective denervation. [Google Scholar]
- 67.Bertrand CM. Selective peripheral denervation for spasmodic torticollis: surgical technique, results, and observations in 260 cases. Surg Neurol. 1993;40:96–103. doi: 10.1016/0090-3019(93)90118-k. [DOI] [PubMed] [Google Scholar]
- 68.Chen X. Selective denervation and resection of cervical muscles in the treatment of spasmodic torticollis: long-term follow-up results in 207 cases. Stereotact Funct Neurosurg. 2000;75(2–3):96–102. doi: 10.1159/000048389. [DOI] [PubMed] [Google Scholar]
- 69.Braun V, Richter HP. Selective peripheral denervation for spasmodic torticollis: 13 year experience with 155 patients. J Neurosurg. 2002;97:207–12. doi: 10.3171/spi.2002.97.2.0207. [DOI] [PubMed] [Google Scholar]
- 70.Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, et al. Selective peripheral deneration for the treatment of intractable spasmodic torticollis: experience with 168 patients at the mayo clinic. J Neurosurg. 2003;98:1247–54. doi: 10.3171/jns.2003.98.6.1247. [DOI] [PubMed] [Google Scholar]
- 71.Munchau A. Prospective study of selective peripheral denervation for botulinum-toxin resistant patients with cervical dystonia. Brain. 2001;124(Pt 4):769–83. doi: 10.1093/brain/124.4.769. [DOI] [PubMed] [Google Scholar]
- 72.Narayan RK, Loubser PG, Jankovic J, et al. Intrathecal baclofen for intractable axial dystonia. Neurology. 1991;41:1141–2. doi: 10.1212/wnl.41.7.1141. [DOI] [PubMed] [Google Scholar]
- 73.Ford B. Use of intrathecal baclofen in the treatment of patients with dystonia. Arch Neurol. 1996;53(12):1241–6. doi: 10.1001/archneur.1996.00550120049016. [DOI] [PubMed] [Google Scholar]
- 74.Albright AL, Barry MJ, Shafron DH, et al. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652–7. doi: 10.1017/s0012162201001190. [DOI] [PubMed] [Google Scholar]
- 75.Albright AL. Intrathecal baclofen for treatment of dystonia. In: Krauss JK, Jankovic J, Grossman R, editors. Surgery for parkinson’s disease and movement disorders. Lippincott Williams &Williams; Philadelphia: 2001. pp. 316–322. [Google Scholar]
- 76.Cooper IS. 20-year followup study of the neurosurgical treatment of dysotnia musculorum deformans. Adv Neurol. 1976;14:423–52. [PubMed] [Google Scholar]
- 77.Yianni J. Globus pallidus internus deep brain stimulation for dystonic conditions: a prospective audit. Mov Disord. 2003;18(4):436–42. doi: 10.1002/mds.10380. [DOI] [PubMed] [Google Scholar]
- 78.Eltahawy HA. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54:613–19. doi: 10.1227/01.neu.0000108643.94730.21. [DOI] [PubMed] [Google Scholar]
- 79.Vidailhet M, Vercueil L, Houeto JL. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–9. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 80.Vidailhet M. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–67. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 81.Kupsch A, Benecke R, Muller J. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 82.Vesper J. Deep brain stimulation of the globus pallidus internus (Gpi) for torsion dystonia: a report of two cases. Acta Neurochir Suppl. 2001;79:83–8. doi: 10.1007/978-3-7091-6105-0_19. [DOI] [PubMed] [Google Scholar]
- 83.Tronnier V, Fogel W. Pallidal stimulation for generalized dystonia: report of 3 cases. J Neurosurg. 2000;92:453–6. doi: 10.3171/jns.2000.92.3.0453. [DOI] [PubMed] [Google Scholar]
- 84.Vercueil L, Pollak P, Fraix V. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001;248:695–700. doi: 10.1007/s004150170116. [DOI] [PubMed] [Google Scholar]
- 85.Katayama M. Chronic stimulation of the globus pallidus internusfor control of primary generalized dystonia. Acta Neurochir Suppl. 2003;87:125–8. doi: 10.1007/978-3-7091-6081-7_26. [DOI] [PubMed] [Google Scholar]
- 86.Zorzi G, Marras C, Nardocci N. Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord. 2005;20:1194–200. doi: 10.1002/mds.20510. [DOI] [PubMed] [Google Scholar]
- 87.Kumar R. Globus pallidus deep brain stimulation for generalized dystonia: clinical and pet investigation. Neurology. 1999;53:871–4. doi: 10.1212/wnl.53.4.871. [DOI] [PubMed] [Google Scholar]
- 88.Kiss ZH. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–86. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- 89.Hung SW, Hamani C, Lozano AM. Long term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–9. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- 90.Ostrem JL. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007;22(13):1885–91. doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- 91.Houser M, Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord. 2005;20(9):1203–5. doi: 10.1002/mds.20522. [DOI] [PubMed] [Google Scholar]
- 92.Foote KD, Sanchez JC, Okun MS. Staged deep brain stimulation for refractory craniofacial dystonia with blepharospasm: case report and physiology. Neurosurgery. 2005;56(2):E415. doi: 10.1227/01.neu.0000147978.67424.42. discussion E415. [DOI] [PubMed] [Google Scholar]
- 93.Damier P, Thobois S, Witjas T. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch Gen Psychiatry. 2007;64:170–6. doi: 10.1001/archpsyc.64.2.170. [DOI] [PubMed] [Google Scholar]
- 94.Candia V. Constraint-induced movement therapy for focal hand dystonia in musicians. Lancet. 1999;353(9146):42. doi: 10.1016/S0140-6736(05)74865-0. [DOI] [PubMed] [Google Scholar]
- 95.Priori A. Limb immobilization for the treatment of focal occupational dystonia. Neurology. 2001;57(3):405–9. doi: 10.1212/wnl.57.3.405. [DOI] [PubMed] [Google Scholar]
- 96.Jankovic J. Can peripheral trauma induce dystonia and other movement disorders? Yes! Mov Disord. 2001;16(1):7–12. doi: 10.1002/1531-8257(200101)16:1<7::aid-mds1005>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]