TO THE EDITOR
The serotonin transporter (SLC6A4) is a key regulator of serotonergic neurotransmission. Like most genes, SLC6A4 expression is partially regulated by genetic variation. The most heavily studied variant affecting this expression is the polymorphism known as the serotonin transporter linked polymorphic region (5HTTLPR). In those of northern European ancestry, this variation exclusively consists of two alleles; a long (l) variant that consists of 16 repeats and a short (s) variant that consists of 14 repeats of ~22 bp element. In a large number of studies, it has been repeatedly demonstrated that the short variant is associated with 60% of the transcriptional activity as long allele [Lesch et al., 1996]. Unfortunately, the mechanism through which this differential expression is conveyed is unclear but may be mediated via the diverse interactions of a number of transcription factors including CCCTC-binding factor (CTCF) [Ali et al., 2010].
In individuals of other ancestry, in particular the African-Americans, another 5HTTLPR variant exists and is referred to as the extra long (xl) allele which is ~81 bp longer than the l allele [Gelernter et al., 1997]. Although it has been speculated that this longer variant act as “super long” alleles with respect to transcriptional activity, to the best of our knowledge, this activity has not been directly examined, depriving the field of hard data on which to build a better understanding of the regulatory mechanisms through which variation at this locus effects gene expression.
It is also particularly useful to examine regulation of gene activity in an African American population because several race-by-genotype interaction effects have been reported [Widom and Brzustowicz, 2006; Propper et al., 2007], suggesting to some that the regulation of serotonin reuptake may be differentially related to 5-HTTLPR genotype in African American versus European-American individuals [Williams et al., 2003], with opposite effects expected in the two groups. Because we have previously examined the relationship of 5-HTTLPR genotype to mRNA production in a European-American sample [Philibert et al., 2008], it is possible for us to directly compare the direction of association between genotype and gene activity in each using the same methods.
In order to examine transcriptional properties of these alleles, we genotyped DNA from female subjects participating in the Family and Community Health Care Takers Study (FACHS) [Cutrona et al., 2000]. The FACHS isa longitudinal examination of health and health-related behaviors in care taker/offspring dyads in rural African American families whose methods and procedures have been approved by the University of Iowa Institutional Review Board study.
To accomplish our objective, we genotyped DNA from FACHS female subjects for whom we had lymphoblast cell lines to identify individuals homozygous for short or long variant, as well as individuals who were either heterozygous or homozygous for the xl allele. We next measured SLC6A4 gene expression in RNA from lymphoblast cell lines for each of these individuals and analyzed the relationship between genotype and gene expression.
The genotyping of the 5HTTLPR was conducted as previously described [Philibert et al., 2008]. The amount of SLC6A4 transcript as well as the levels of five housekeeping gene transcripts (CALR, UBC, RPL7A, RPS19, and RPS20), were determined in triplicate as previously described using Taqman™ reagents (Applied Biosystems, Foster City, for SLC6A4, we used Hs00169010), and a BioMark Platform (Fluidigm, South San Francisco, CA) as previously described [Shields et al., in submission]. Next, the relative amount of SLC6A4 transcript was then normalized to total RNA levels using the geometric mean of five housekeeping genes and the resulting Ct scores converted to z-scores. Finally, the resulting genotype and gene expression data were analyzed using the regression subroutine contained within JMP version 9 (SAS Institute, Cary, SC).
DNA from a total of 480 female FACHS subjects were genotyped with the overall frequencies of the s, l and xl alleles being 26%, 70%, and 4%, respectively. From the material derived from thesesubjects, we selected 86 cell lines that were homozygous for the long allele, 26 that were homozygous for the short allele, 21 that were heterozygous for the extra-long allele (8 s,xl and 13 l,xl) and one that was homozygous for the xl allele.
Figure 1 gives the relationship between genotype and expression level. Ordinal regression analysis using a simple 0 (ss), 1 (ll) and 2 (at least one xl allele) model demonstrated a significant relationship between genotype and gene expression (P < 0.05) with the order of transcriptional efficiency being xl > l > s. This pattern is consistent with the hypothesis that the xl variant will be associated with increased transcriptional efficiency. It is also noteworthy that the relative transcriptional efficiency of the l and s alleles is in the same direction in this African American sample as was reported earlier by our lab and others for a European American sample [Philibert et al., 2008]. Accordingly, there is support for a simple model in which length of the polymorphic region is linearly associated with transcriptional activity, but no support for the hypothesis that regulation of the highly conserved serotonin transmitter system is differentially regulated by African Americans and European Americans. Conversely, because the xl allele is present, there is potential for the 5HTTLPR to provide an expanded range of potential mRNA responses and environmental interactions in gene–environment interaction studies in African American cohorts.
FIG. 1.
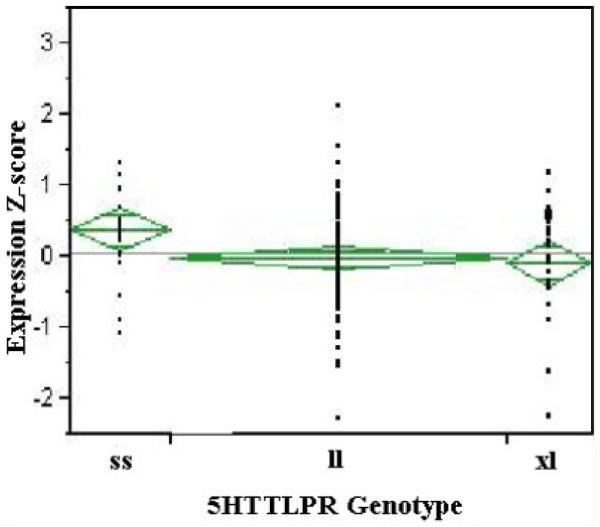
The relationship between 5HTTLPR genotype and SLC6A4 transcript levels in lymphoblast RNA prepared from blood contributed by female FACHS subjects. Higher z-scores denote lower levels of expression with each unit of z-score being equal to approximately 1.6 Ct counts. Group sizes: ss (n = 26), ll (n = 86), or at least one xl allele (n = 24). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb]
Footnotes
The authors have no conflicts of interest with respect to this communication.
REFERENCES
- Ali FR, Vasiliou SA, Haddley K, Paredes UM, Roberts JC, Miyajima F, Klenova E, Bubb VJ, Quinn JP. Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by CTCF. J Neurochem. 2010;112(1):296–306. doi: 10.1111/j.1471-4159.2009.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Hessling RM, Brown PA, Murry V. Direct and moderating effects of community context on the psychological well-being of African American women. J Pers Soc Psychol. 2000;79(6):1088–1101. doi: 10.1037//0022-3514.79.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet Part B. 2008;147B(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C, Willoughby M, Halpern CT, Carbone MA, Cox M. Parenting quality, DRD4, and the prediction of externalizing and internalizing behaviors in early childhood. Develop Psychobiol. 2007;49(6):619–632. doi: 10.1002/dev.20249. [DOI] [PubMed] [Google Scholar]
- Shields B, Vijayendran M, Plume JT, Monick MM, Gerrard M, Brody GH, Beach SR, Philibert RA. Smoking, Genetic, Epigenetic, and G x Methylation Interaction Effects in the Regulation of Interleukin 6 Receptor Expression J Tob Nicotine Res. in preparation. in submission. [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” Childhood abuse and neglect, maoa genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60(7):684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function[ast] Neuropsychopharmacology. 2003;28(3):533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]


