Abstract
PURPOSE
To report optical coherence tomography (OCT) features of patients with autoimmune retinopathy.
DESIGN
Consecutive case series.
METHOD
Eight patients who presented with unexplained loss of central vision, visual field defects, and/or photopsia were diagnosed with autoimmune retinopathy based on clinical features, electroretinogram (ERG) findings, and serum antiretinal antibody analysis. All patients underwent OCT testing of the macula and nerve fiber layer (NFL).
RESULTS
Outer retinal abnormalities and/or decreased macular thickness on OCT were seen in all patients. Macular OCT showed reduced central macular and foveal thicknesses in 6 patients (mean thickness 143 ± 30 μm and 131 ± 29 μm respectively). In all but 1 patient, loss of the photoreceptor layer or disruption of the photoreceptor outer and inner segment junction was noted. Three patients showed only mild to moderate focal NFL loss.
CONCLUSIONS
Retinal atrophy and reduced macular thickness on OCT are predominant features in patients with autoimmune retinopathy. OCT provides objective measures of retinal damage and may offer clues toward understanding the mechanism of visual dysfunction and the diagnosis of autoimmune retinopathy.
Autoimmune- and cancer-associated retinopathy represent an important cause of otherwise unexplained acute or subacute vision loss in adults. These forms of retinal disease result from a presumed immunologic process affecting the retina by autoantibodies directed against retinal antigens.1–3 Acquired immunologically mediated retinal degeneration in the absence of an underlying malignancy is commonly referred to as autoimmune retinopathy, while the term cancer-associated retinopathy is reserved for similar processes but with an associated malignancy at the time of initial evaluation. Autoantibodies against multiple retinal antigens including recoverin, α-enolase, heat-shock proteins, arrestin, transducin, neurofilament protein, carbonic anhydrase II, and TULP1 have been reported in sera of patients with autoimmune- and cancer-associated retinopathy. Patients may describe symptoms such as decreased vision, photopsias, decreased night vision, abnormal color vision, and visual field defects. As fundus evaluation may initially be normal, the diagnosis may be challenging, and ancillary testing including electroretinography (ERG) and serum antibody analysis are helpful in establishing the diagnosis. ERG is typically markedly reduced in amplitude, even in the early stages.1,4 However, in some cases the pathology may be limited to central cone abnormalities that may only be recognized using multifocal ERG testing.2
We believe that optical coherence tomography (OCT) is helpful in the diagnosis and determination of prognosis in patients with autoimmune- and cancer-associated retinopathy. In addition, OCT may offer clues towards understanding the mechanism of visual dysfunction in these patients.
METHODS
We analyzed a consecutive case series of 8 patients with newly diagnosed autoimmune retinopathy. All patients were seen in the department of ophthalmology at the University of Virginia. The diagnosis was based on a detailed ophthalmic examination, automated visual field testing, ERG evaluation, and serum antiretinal antibody detection. Blood samples were collected from all patients and sent to the Ocular Immunology Laboratory (Oregon Health and Science University, Portland, Oregon) for evaluation of antiretinal autoantibodies. Antibody testing was performed using previously described techniques that employed Western blot analysis using proteins extracted from human retinas and immunohistochemistry.5 Following initial screening test, when the serum was suspected to react with known retinal proteins, a separate confirmatory experiment was performed whereby the serum was again incubated with the purified protein on a blot. Many antiretinal autoantibodies have been previously described that may or may not be associated with cancer. The most frequent of those include antibodies against retinal α-enolase (46 kDa), recoverin (23 kDa), and p35 (35 kDa) that predominantly affect photoreceptors, but can also affect bipolar cells and retinal ganglion cells. In this study the antiretinal antibodies tested included antibodies against these 3 retinal antigens as well as antibodies against carbonic anhydrase II (30 kDa), rhodopsin (40 kDa), arrestin (48 kDa), and phosphodiesterase (PDE; 88 kDa). Other less frequently encountered autoantibodies such as antibodies against neurofilament proteins, heat-shock protein 70, TULP1 protein, 40-kDa insoluble protein, transducin-alpha, interphotoreceptor retinoid-binding protein (IRBP), and other retinal proteins of unknown identity were also tested. Additionally, autoantibodies against bipolar cells such as those seen in melanoma-associated retinopathy (MAR) syndrome were tested.
OCT of the macula and nerve fiber layer (NFL) was performed on all of these patients using a fourth-generation Zeiss Stratus OCT (Carl Zeiss Ophthalmic Systems, Dublin, California, USA), which was the most recent OCT version commercially available at the time of initiation of the study. All 6 high-definition radial line scans of the macular OCT imaging as well as all 3 scans of the NFL OCT for each patient were reviewed and analyzed for reliability of thickness measurements. Only reliable scans, according to previously reported guidelines,6 were included. Quantitative data are presented as mean ± standard deviation.
Patients were offered treatment with oral prednisone and subsequently intravenous immunoglobulin (IVIG) 400 mg/kg for 5 consecutive days every month for 3 months. Treatment was repeated only in case of documented improvement of antiretinal antibody titers and visual function (as indicated by visual acuity, visual fields, and ERG) measured 2 to 4 weeks after initial treatment.
RESULTS
Seven women and 1 man, with a mean age of 59 ± 15 years, were included in the study (Table). In all patients a complete ophthalmic, medical, and family history failed to suggest hereditary forms of retinal degeneration or inflammatory retinal disease. The clinical, electrophysiological, laboratory, and OCT findings are summarized in the Table. Best-corrected visual acuity at presentation ranged from 20/20 to 1/200 E (defined as the ability to see the 20/200 “E” Snellen optotype at a distance of 1 foot). All 8 patients tested positive for serum antiretinal autoantibodies. Two patients had a history of malignancy: ovarian cancer in 1 patient and non-Hodgkin lymphoma as well as breast cancer in the other. The malignancies had been diagnosed years prior to presentation. Three patients had associated systemic autoimmune diseases (rheumatoid arthritis, Graves disease, systemic lupus erythematosus, and antiphospholipid antibody syndrome).
TABLE.
Characteristics of Patients With Autoimmune Retinopathy
| Patient # (Age, Sex) | Presenting Symptoms | BCVA at Presentation | Fundus Findings | Serum Antiretinal Antibodies | ERG | Macular OCT Findings/Central Macular Thickness | Nerve Fiber Layer Thickness | Associated Autoimmune Disease/Malignancy |
|---|---|---|---|---|---|---|---|---|
| 1 (85, M) | Progressive loss of vision from 20/60 and 20/25 over 1 year, poor peripheral vision, photopsia | HM OD 20/70 OS |
Mild optic nerve head pallor, attenuated vessels, pigment clumping in macula | 30 kDa (carbonic anhydrase), 46 kDa (α-enolase) 52 and 67 kDa |
Extinguished rod and cone responses | Loss of photoreceptor layer; bilateral epiretinal membrane; 273 μm OD 220 μm OS |
Unremarkable | RA |
| 2 (51, F) | Progressive loss of vision over 2 years, photopsia | 20/200 OU | Old PRP & focal laser, regressed PDR, attenuated vessels | 46 kDa (α-enolase) | Extinguished rod and cone responses | Loss of photoreceptor layer; 92 μm OD 101 μm OS |
Moderate thinning inferiorly OD | None |
| 3 (54, F) | Progressive loss of vision over 1 year | 20/20-OU | Unremarkable | 30 kDa (carbonic anhydrase), 33 kDa | Normal rod, increased implicit time on cone responses | Central macular thinning with no apparent Photoreceptor loss; 176 μm OD 167 μm OS |
Mild thinning temporally OU | Lymphoma, breast cancer, cryoglobulinemia, hepatitis C |
| 4 (70, F) | Rapid decline in acuity from 20/30 over 4 months | 20/200 OU | Focal RPE atrophy OD, normal OS Central window defects on FA OU |
145 kDa (IRBP) | Not available | Loss of photoreceptor layer; 128 μm OD 129 μm OS |
Unremarkable | SLE, antiphospholipid antibody syndrome |
| 5 (69, F) | Rapid decline in acuity from 20/40 over 3 months | 20/200 OD 20/70 OS |
Unremarkable | 46 kDa (α-enolase) | Normal rod, significantly reduced cone responses | Loss of photoreceptor layer; 173 μm OD 168 μm OS |
Unremarkable | None |
| 6 (49, F) | Central visual loss over 6 months | 6/200 E OU | Unremarkable | 35–36 kDa and 44 kDa | Delayed and significantly reduced cone and rod responses | Central macular thinning with abnormal foveal depression; mild disruption of photoreceptor OS/IS junction; 151 μm OD 146 μm OS |
Unremarkable | None |
| 7 (78, F) | Decreased vision over 6–8 months, photopsia | 20/70 OU | Attenuated vessel, scattered RPE changes OU | 50 kDa, 62 kDa, and 67 kDa | Extinguished rod and cone responses | Loss of photoreceptor layer; 149 μm OD 147 μm OS |
Moderate thinning inferiorly OD; moderate thinning superiorly & inferiorly OS | Ovarian cancer |
| 8 (40, F) | Decreased acuity over 2 months, severely affected visual field on Humphrey 24-2 and 10-2 testing, photopsia OD only | 20/70 declined to 1/200 E OD 20/20 OS |
Bilateral hypopigmentation of inferior retina | 40 kDa | Normal rod and cone responses on full-field ERG; reduced macular response OD on multifocal ERG | Disruption of photoreceptor OS/IS junction; 277 μm OD 206 μm OS |
Unremarkable | Graves disease Positive ANA |
ANA = antinuclear antibody; BCVA = best-corrected visual acuity; E = 20/200 “E” Snellen optotype; ERG = electroretinogram; F = female; FA = fluorescein angiogram; HM = hand motion; IRBP = interphotoreceptor retinoid-binding protein; M = male; OCT = optical coherence tomography; OS/IS = outer segment/inner segment; PDR = proliferative diabetic retinopathy; PRP = panretinal photocoagulation; RA = rheumatoid arthritis; RPE = retinal pigment epithelium; SLE = systemic lupus erythematosus.
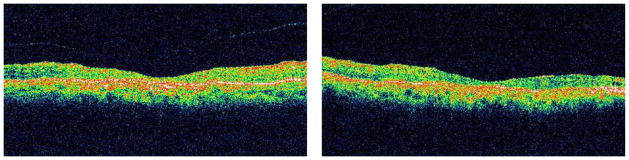
Outer retinal abnormalities and/or decreased macular thickness on OCT were seen in all of the patients. Macular OCT showed reduced central macular and foveal thicknesses in 6 patients (mean thickness 143 ± 30 mm and 131 ± 29 mm respectively). There was no significant difference in central macular thickness measurements between the right and left eyes (P value = .14). In the other 2 patients the mean central macular and foveal thicknesses were not decreased and measured 244 ± 36 and 223 ± 36 mm respectively. In all but 1 patient (Patient 3) loss of the photoreceptor layer (Figure 1) or disruption of the photoreceptor outer and inner segment (OS/IS) junction (Figure 2) was noted on macular OCT. OCT of the NFL was largely unremarkable in all patients, with only mild to moderate focal NFL loss in 3 patients.
FIGURE 1.
Macular optical coherence tomography of the right (Left) and left eye (Right) of a patient with anti-α-enolase-positive autoimmune retinopathy (Patient 2) shows marked retinal thinning and atrophy of the outer retinal layers, including the outer nuclear layer and photoreceptors, in both eyes.
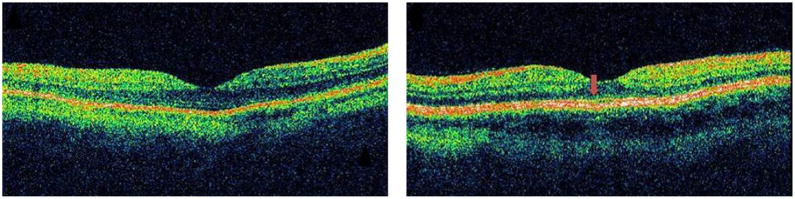
FIGURE 2.
Macular optical coherence tomography of a patient with autoimmune retinopathy (Patient 8) shows preservation of the outer retina and outer nuclear layer with normal central macular thickness in both eyes. However, disruption of the photoreceptor inner/outer segment junction is evident in the right eye only (Left). Note normal photoreceptor inner/outer segment junction in the left eye (Right, arrow).
All patients received 1 mg/kg oral prednisone for at least 4 weeks with no improvement of their symptoms. In addition, 3 patients (Patients 1, 2, and 8) were treated with IVIG. Only 1 patient (Patient 8) had improvement of field of vision (Figure 3) and ERG with only mild improvement in acuity. The other 2 patients did not experience improvement in visual function, although significant subjective improvement in photopsia was reported.
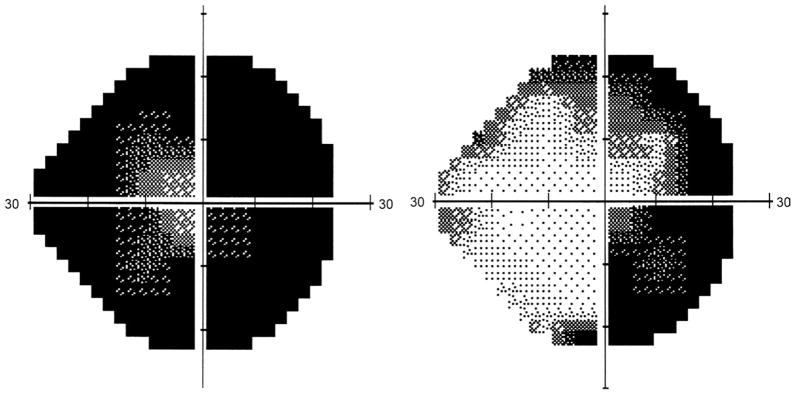
FIGURE 3.
Humphrey automated 24-2 visual field of the right eye of a patient with autoimmune retinopathy (Patient 8) before (Left) and after (Right) treatment with 2 cycles of intravenous immunoglobulin. Note the improvement in visual field after treatment. This was associated with an improvement in visual acuity and multifocal electroretinography and a drop in the antiretinal antibody titer.
DISCUSSION
The diagnosis of autoimmune retinopathy remains extremely challenging. A high index of suspicion is required and extensive investigation is sometimes performed before reaching the correct diagnosis. All our patients presented with unexplained visual loss, with or without photopsias, with no or subtle nonspecific findings on ocular examination. Many of these patients would have probably undergone exhaustive neurologic and neuro-ophthalmologic evaluation had it not been for the OCT findings pointing toward retinal thinning and outer retinal pathology. These OCT findings led to the suspicion of autoimmune retinopathy even prior to electroretinography. Moreover, we believe that the high yield of serologic testing confirming the diagnosis in this consecutive case series was largely attributable to the high index of suspicion generated by the OCT findings. It should also be noted that antiretinal antibodies may be present in the normal population and their presence does not necessarily indicate retinopathy. For example, while anti-recoverin autoantibody is not typically present in the normal population, the frequency of anti-α-enolase autoantibody is approximately 10% in healthy subjects; however, this is not well defined for other antiretinal autoantibodies.7,8 When antiretinal antibodies are present in high titers, retinopathy is more likely. In addition, it was found that autoantibodies against retinal proteins from patients with retinopathy were cytotoxic to retinal cells, in contrast to those from healthy subjects, probably through recognition of additional unique regions on their target retinal antigen.9 Therefore, testing for cytotoxicity is important to determine the pathogenic potential of circulating antiretinal antibodies. In this respect, OCT findings as noted in this study may serve as an additional marker of the pathogenic potential of circulating antiretinal antibodies.
Outer retinal abnormalities and/or decreased central macular thickness on OCT were seen in all patients with autoimmune retinopathy reported here. Similar macular OCT findings were reported by Mohamed and Harper in a patient with cancer-associated retinopathy secondary to endometrial adenocarcinoma.10 Two of our patients did not have retinal thinning on macular OCT or detectable NFL abnormalities. In 1 of them, the damage was limited to disruption of the photoreceptor OS/IS junction (Patient 8; Figure 2), which corresponded to the depressed central response on multifocal ERG (mfERG) testing. This suggests that careful observation of the OCT line scans is necessary, and extra caution should be invested in examining the various retinal layers, qualitatively, despite normal quantitative analysis. Subtle photoreceptor OS/IS junction abnormalities can be easily overlooked, especially in time-domain OCT images. The other patient (Patient 1) had no retinal thinning despite loss of the photoreceptor layer and a flat ERG. This is likely secondary to the concurrent bilateral epiretinal membranes that the patient had, causing compensatory retinal thickening.
Different mechanisms of cell damage have been suggested for anti-recoverin11,12 and anti-enolase antibodies,13,14 predominantly resulting in apoptosis of retinal cells. Retinal cellular targets reported include the photoreceptors and bipolar cells for anti-recoverin antibodies, and retinal ganglion cells as well as photoreceptors for anti-enolase antibodies.2,11–14 These reported mechanisms of cell death corroborate with our macular OCT findings where all 3 patients (Patients 1, 2, and 5) with anti-enolase antibodies had outer retinal atrophy with loss of the photoreceptor layer. These 3 patients also had loss of central vision consistent with previous findings that anti-enolase autoantibodies seem to target the central retina, as shown by mfERG.2 Although anti-recoverin and anti-enolase antibodies have been most extensively studied and were found to initially affect retinal function, subsequently leading to morphologic damage and retinal degeneration by activating a caspase 3– dependent apoptotic pathway,5,9,11,15 other autoantibodies have also been shown to have similar cytotoxic effects on retinal cells.5,14 –16 Hence, it appears that apoptosis may be a common pathway for retinal autoantibody–induced retinal degeneration. Therefore, the finding of retinal atrophy and thinning on OCT associated with other retinal autoantibodies detected in this study is not surprising.
Another interesting finding was that the NFL was largely normal in most cases despite a relatively chronic course of the retinal disease. The focal NFL defects detected in 3 patients did not correspond to focal visual field defects. Therefore, it appears that the NFL and ganglion cell layers are spared in autoimmune retinopathy. Even among the 3 patients (Patients 1, 2, and 5) with anti-enolase autoantibodies, which is expected to target the ganglion cells, only 1 (Patient 2) had moderate NFL thinning inferiorly only in the right eye. One explanation could be that the changes in the NFL may be below the detection threshold of Stratus OCT. It is also possible that the absence of NFL thinning could be in part attributable to a functional damage by antiretinal antibodies without cell loss leading to normal thickness measurements. However, this remains speculative.
Among the 3 treated patients, the only one that responded to IVIG with improved vision, improved visual field, multifocal ERG, and decreased antiretinal antibody titer was the patient in whom outer retinal damage was limited to disruption of the photoreceptor OS/IS junction on OCT with no reduction in central macular thickness (Patient 8; Figure 3). This may also be of significance knowing that the photoreceptor outer segments are able to regenerate and reorganize.17,18 The process of retinal damage by antiretinal autoantibodies may be potentially reversible if detected early when the retinal damage is limited to the outer segments. However, when the photoreceptor nuclear layer becomes atrophic, as was the case in most of our patients, then the process may be beyond reversibility. These macular OCT findings imply a significant prognostic role for OCT in autoimmune- and cancer-associated retinopathy. They may also help in selecting cases with the potential for visual improvement prior to subjecting patients to the risks and costs of treatment such as steroids, IVIG, or immunosuppression.19,20
Although several drawbacks may be associated with this study primarily relating to the relatively small number of patients, the use of time-domain rather than spectral-domain OCT, and the retrospective nature of the analysis, these findings demonstrate that OCT is useful in the evaluation of patients with suspected autoimmune- and cancer-associated retinopathy. Establishing the diagnosis of autoimmune- and cancer-associated retinopathy is usually difficult and evidence of retinal thinning and/or photoreceptor damage on OCT should alert the physician to the possibility of autoimmune- or cancer-associated retinopathy in the proper clinical setting. All 8 consecutive cases reported here had their OCT evaluation on the day of presentation prior to ERG or serum analysis. They were all tested for antiretinal antibodies because of the high index of suspicion primarily based on OCT findings, and they all tested positive. Thus, OCT is a valuable tool in assessing patients with unexplained visual dysfunction, as is the case with the majority of patients with autoimmune- and cancer-associated retinopathy. Furthermore, OCT findings can be helpful in understanding the mechanism of visual loss in autoimmune retinal damage. Future studies using spectral-domain OCT imaging may provide even better insight into the anatomic correlation in such patients by providing more detailed visualization of the various retinal layers, particularly at the level of the outer retina.
Acknowledgments
The authors did not receive any funding for this work. Involved in conception and design (A.A., N.G.G.), analysis and interpretation (A.A., N.G.G.), writing the article (A.A.), critical revision of the article (A.A., N.G.G., G.A.), data collection (A.A., S.S.A.), and provision of patients or resources (G.A., N.G.G.). This study was approved by the University of Virginia Institutional Review Board and was compliant with HIPAA regulations.
Biography

Azin Abazari, MD, is currently a clinical assistant professor at the department of ophthalmology at Stony Brook University, Stony Brook, New York. She completed her ophthalmology residency at the University of Virginia and her fellowship in cornea and refractive surgery at Wills Eye Institute.
Footnotes
All authors have completed and submitted the icmje form for disclosure of potential conflicts of Interest and none were reported.
References
- 1.Ohguro H, Yokoi Y, Ohguro I, et al. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137(6):1117–1119. doi: 10.1016/j.ajo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005;139(5):780–794. doi: 10.1016/j.ajo.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 3.Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998;126(2):230–237. doi: 10.1016/s0002-9394(98)00149-4. [DOI] [PubMed] [Google Scholar]
- 4.Ohguro H, Ogawa K, Maeda T, Maeda A, Maruyama I. Cancer-associated retinopathy induced by both anti-recoverin and anti-hsc70 antibodies in vivo. Invest Ophthalmol Vis Sci. 1999;40(13):3160–3167. [PubMed] [Google Scholar]
- 5.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghazi NG, Kirk T, Allam S, Yan G. Quantification of error in optical coherence tomography central macular thickness measurement in wet age-related macular degeneration. Am J Ophthalmol. 2009;148(1):90–96. doi: 10.1016/j.ajo.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996;78(2):120–129. doi: 10.1006/clin.1996.0021. [DOI] [PubMed] [Google Scholar]
- 8.Gitlits VM, Toh BH, Sentry JW. Disease association, origin, and clinical relevance of autoantibodies to the glycolytic enzyme enolase. J Investig Med. 2001;49(2):138–145. doi: 10.2310/6650.2001.34040. [DOI] [PubMed] [Google Scholar]
- 9.Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998;11(6):671–677. doi: 10.1006/jaut.1998.0239. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed Q, Harper CA. Acute optical coherence tomographic findings in cancer-associated retinopathy. Arch Ophthalmol. 2007;125(8):1132–1133. doi: 10.1001/archopht.125.8.1132. [DOI] [PubMed] [Google Scholar]
- 11.Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998;11(5):523–533. doi: 10.1006/jaut.1998.0221. [DOI] [PubMed] [Google Scholar]
- 12.Maeda T, Maeda A, Maruyama I, et al. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001;42(3):705–712. [PubMed] [Google Scholar]
- 13.Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of auto-immune-mediated retinopathy. J Clin Immunol. 2007;27(2):181–192. doi: 10.1007/s10875-006-9065-8. [DOI] [PubMed] [Google Scholar]
- 14.Ren G, Adamus G. Cellular targets of anti-alpha-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun. 2004;23(2):161–167. doi: 10.1016/j.jaut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by autoantibodies of cancer associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38(2):283–291. [PubMed] [Google Scholar]
- 16.Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2(2):63–69. doi: 10.1016/s1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 17.Young RW. The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol. 1971;49(2):303–318. doi: 10.1083/jcb.49.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15(9):700–725. [PubMed] [Google Scholar]
- 19.Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117(4):471–477. doi: 10.1001/archopht.117.4.471. [DOI] [PubMed] [Google Scholar]
- 20.Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–397. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]