Abstract
Although the etiologies of sudden cardiac death (SCD) are diverse, genetic mutations associated with cardiomyopathic and channelopathic diseases are major causes, and clinically available genetic tests offer the potential to identify at-risk family members, contribute to risk stratification, and guide therapeutic intervention. Recently, the first large-scale systematic studies exploring the background genetic “noise” rate of these tests have been conducted and offer guidance in interpreting positive genetic test results.
Genetic basis of SCD-predisposing disease
Although the pathological underpinnings of SCD are diverse, approximately two-thirds can be explained following a conventional medical autopsy; the most common are heritable cardiomyopathies such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC).1 Approximately one-third of SCDs cannot be explained by conventional medical autopsies; however, postmortem genetic testing has identified cardiac channelopathies, such as long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT), as common etiologies. The molecular underpinnings of these diseases have been the focus of intense research. At the basic science end of the research spectrum, these studies have broadened our knowledge of cellular electrophysiology and pathological cardiocyte remodeling. Clinically, genetic research offers the possibility of identifying individuals who are predisposed to heart disease, yet clinically negative, and may directly influence therapy, clinical management, and disease prognosis of certain SCD-associated diseases.
As with any clinical test, understanding the spectrum of background noise within a given genetic test is critical to interpreting test results. Specifically, systematic evaluation of a genetic test’s “signal-to-noise” ratio is critical to determining whether an identified variant of undetermined significance might be the biomarker responsible for disease or whether it is a rare genetic variant with no relevance to the disease in question. Although two decades of research have been dedicated to the identification and characterization of disease-susceptibility mutations, only recently has background genetic variation in SCD-predisposing diseases been evaluated systematically.
Long QT syndrome
Congenital LQTS, with a prevalence as high as 1 in 2,500 persons, comprises a group of cardiac channelopathies characterized by delayed cardiac repolarization and increased risk for syncope, seizures, and SCD. Accurate diagnosis of LQTS is a challenge because disease-afflicted individuals may or may not manifest QT prolongation on a resting 12-lead surface electrocardiogram. More than 1,000 distinct mutations have been identified in 13 LQTS-susceptibility genes; however, approximately 70 to 75% of clinically robust LQTS is due to mutations residing in three genes: the KCNQ1-encoded IKs potassium channel (Kv7.1, LQT1), the KCNH2-encoded IKr potassium channel (Kv11.1, LQT2), and the SCN5A-encoded INa sodium channel (Nav1.5, LQT3) (ref. 2). These mutations result in perturbed channel function or trafficking and pathologically disturb the resulting cardiac action potential. All clinically available LQTS genetic tests include these three major disease-susceptibility genes, and a patient with a robust clinical diagnosis of LQTS has a 75% chance that a rare mutation will be found in one of these genes.3
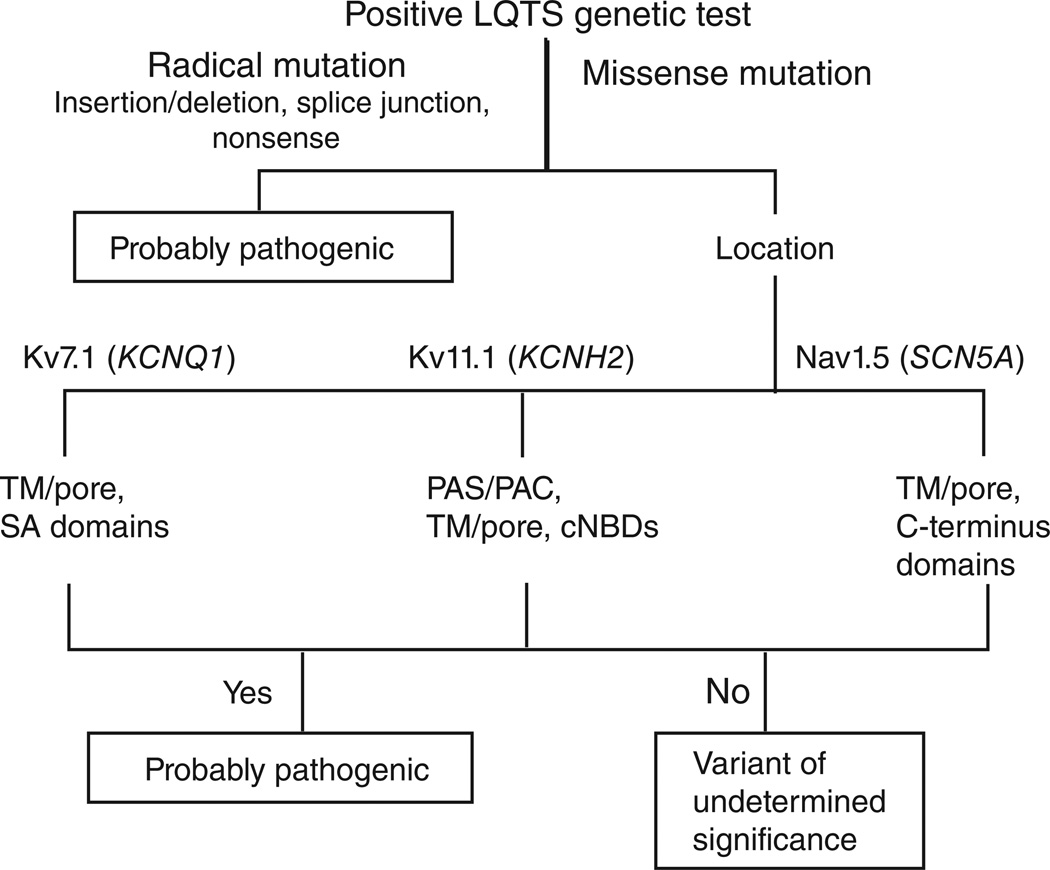
We have recently shown that the background rate of rare, amino acid-or protein-altering genetic variants for KCNQ1, KCNH2, and SCN5A in ostensibly healthy individuals is approximately 5% (ref. 4). Radical mutations that significantly altered the channel protein, such as insertions or deletions, splice-site mutations, or nonsense mutations that result in premature protein truncation, were strongly associated with LQTS causality if identified in any of these three genes. This is reflected in a high estimated predictive value (EPV), namely, >99%, that a genetic test positive for these mutations is disease-associated. Conversely, genetic tests positive for missense mutations, which alter a single amino acid, were also strongly associated with LQTS when localizing to Kv7.1 and Kv11.1.
Specifically, Kv7.1 missense mutations demonstrated an EPV of 71% when found in the N-terminal domain, whereas mutations outside of this domain had an EPV ranging from 94 to 100%. Kv11.1 missense mutations localizing to the N terminus (outside the perarnt-sim–associated C-terminal (PAS/PAC) domain) or the C terminus (outside the cyclic nucleotide-binding cyclic nucleotide-binding domain (cNBD)) were of questionable disease association, demonstrating EPVs of 26% and 56%, respectively. Kv11.1 mutations elsewhere had a 74 to 100% probability of being a LQTS-causative mutation when identified in a patient with a robust LQTS phenotype.
Missense SCN5A mutations were more likely associated with disease causality when localizing to transmembrane/linker domain (EPV 88%) or the C terminus (EPV 75%) of Nav1.5. Importantly, the pathogenicity of a missense mutation localizing to either the domain I–II or the domain II–III cytoplasmic linker loops within Nav1.5 cannot be predicted. Overall, among the missense mutations, those localizing to the pore regions of any of these three channels were associated with the highest likelihood of disease pathogenicity. These findings are summarized in Figure 1.
Figure 1.
Algorithm for interpreting a positive genetic test for long QT syndrome (LQTS). Radical mutations that significantly alter the coding protein, such as insertions or deletions, alteration of the intron or exon splice sites, and nonsense mutations that introduce a premature protein truncation, are probably LQTS-associated. Missense mutations localizing to KCNQ1-encoded Kv7.1 (within the TM/ linker/pore or the SA domain), KCNH2-encoded Kv11.1 (within the PAS/PAC, TM/linker/pore, or the cNBD domains), and SCN5A-encoded Nav1.5 (within the TM/linker or the C-terminal domains) are associated with LQTS. Mutations localizing outside these specific domains of Kv11.1 and Nav1.5 are of uncertain disease significance. cNBD, cyclic nucleotide-binding domain; PAS/PAC, per-arnt-sim–associated C-terminal; SA, subunit assembly; TM, transmembrane.
Arrhythmogenic right ventricular cardiomyopathy
ARVC, a potentially lethal genetic cardiovascular disorder characterized by myocyte loss and fibro-fatty tissue replacement of the right ventricle, has a prevalence of 1 in 1,000 to 5,000 individuals. The variable clinical course can range from subclinical structural abnormalities to right ventricular tachyarrhythmias to right ventricular or biventricular failure.5 Mutations in several ARVC-susceptibility genes that encode essential components of desmosomal proteins have been identified: PKP2-encoding plakophilin 2, DSP-encoding desmoplakin, DSG2- encoding desmoglein 2, DSC2-encoding desmocollin 2, JUP-encoding junction plakoglobin, and TMEM43-encoding transmembrane protein 43. The clinically available ARVC genetic test encompasses these major genes with a yield of approximately 40 to 60% (ref. 6).
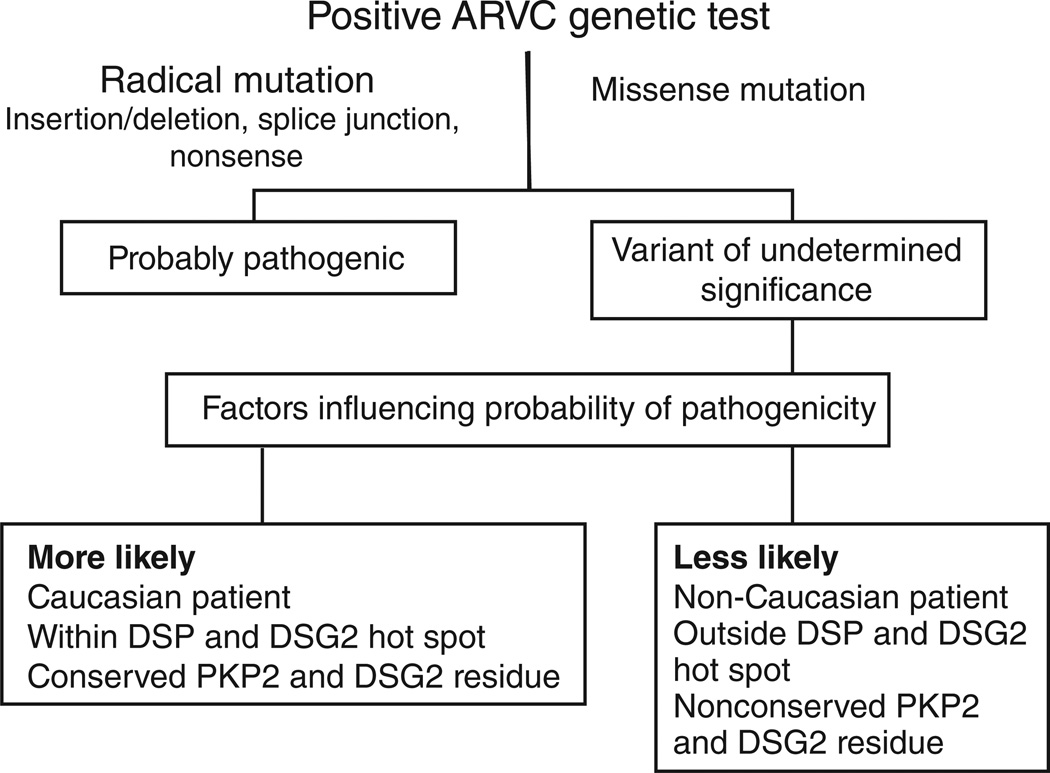
We have recently shown that the overall background “genetic noise” rate for the ARVC gene test panel is approximately 16%, more than three times higher than for LQTS.7 Despite the higher prevalence of background noise, radical mutations were rare in disease-free individuals and were thus associated strongly with disease pathogenicity. Although radical mutations were probably disease-causative if found in any ARVC-associated gene, missense mutations were associated with disease pathogenesis only in specific situations. Akin to LQTS-associated mutations localizing preferentially to the transmembrane pore channels, ARVC-causative mutations localized to critical structure–function domains of the proteins comprising the desmosome. Specifically, missense mutations localizing to the N-terminal regions of DSP and DSG2 were probably pathogenic, whereas mutations elsewhere could not be distinguished from background genetic noise. Interestingly, missense mutations were found to be associated with disease causality when identified in Caucasians or when localized to amino acid residues that were conserved across species. Specifically, amino acid sites that were highly conserved across species within PKP2 and DSG2 were associated with ARVC disease pathogenicity. These findings are summarized in Figure 2.
Figure 2.
Algorithm for interpreting a positive genetic test for arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC). Radical, non-missense mutations that result in premature protein truncation are likely to be ARVC-associated. Missense mutations are of uncertain disease relevance; however, missense mutations identified in Caucasian patients, mutations localized with hot-spot domains within DSP-encoding desmoplakin and DSG2-encoding desmoglein 2 or more conserved residues of PKP2-encoding plakophilin 2 and DSG2 are more likely to be associated with ARVC pathogenicity.
Hypertrophic cardiomyopathy
HCM, defined as hypertrophy of the heart without a clinically identifiable etiology, is the most common inherited cardiovascular disease, affecting 1 in 500 people. Clinically, HCM has a heterogeneous presentation with varying degrees of hypertrophy, fibrosis, myocyte disarray, left ventricular outflow tract obstruction, ventricular septal morphology, associated symptoms, such as the presence of syncope or chest pain, and sudden-death susceptibility. HCM is a disease marked by variable penetrance and phenotypic expressivity; families hosting the same HCM-associated mutation can demonstrate youthful SCD with minimal septal hypertrophy or profound hypertrophy with normal survival.
Mutations in genes encoding components of the cardiac sarcomere, which is responsible for providing the molecular force of myocyte contraction, have traditionally been associated with HCM pathogenesis. These mutations localize to proteins of the thick myofilament (MYH7-encoded myosin heavy chain, MYL2-encoded regulatory myosin light chain, and MYL3-encoded essential myosin light chain), the intermediate myofilament (MYBPC3-encoded cardiac myosin binding protein C), and the thin myofilament (ACTC-encoded actin, TNNC1-encoded cardiac troponin C, TNNI3-encoded cardiac troponin I, TNNT2-encoded cardiac troponin T, and TPM1-encoded α-tropomyosin). Recently, genes encoding components of the cardiac Z-disk as well as calcium-handling and calcium-regulatory proteins have been associated with HCM in sarcomere-negative individuals.8 Overall, hundreds of mutations have been identified in at least 27 putative HCM-susceptibility genes.
Although the genetic causes of HCM are as varied as the clinical phenotype, the majority of mutations are found in sarcomeric genes. This is reflected in the clinically available genetic tests, which all scan for mutations in the canonical HCM-susceptibility genes and variably include the minor or rare genetic subtypes of HCM. The yield of the HCM genetic test varies based on the cohort analyzed, ranging from 25 to 65%, with mutations in MYH7 and MYBPC3 representing the majority of mutation-positive cases.9
To date, no large studies have been done to systematically describe the background genetic noise rate inherent in the HCM genetic test. If the genetic test is confined solely to the five most common HCM subtypes, then we predict a background noise rate akin to that of the LQTS major gene panel. However, if the HCM genetic test panel includes all the minor genes, then it is likely that the noise could rival or even exceed that of the ARVC gene panel. Furthermore, given that the majority of ambiguity in LQTS and ARVC genetic testing lies in the interpretation of rare, functionally uncharacterized missense mutations, it is likely that missense mutations will constitute the majority of false-positive test findings. Comprehensive interrogation of ostensibly healthy individuals through the equivalent of the clinical HCM genetic test, and statistical comparison of these findings with a cohort of clinically robust HCM cases, will provide the first steps in clarifying this ambiguity.
Future directions
Just as completion of the Human Genome Project significantly advanced our understanding of the genetic causes of human disease, completion of the 1000 Genomes Project will rapidly expand our understanding of the genetic variation that makes individuals unique. This comprehensive database of the genomes of more than 1,000 racially and ethnically diverse people, which is currently being finalized, offers a wealth of information to determine the spectrum of healthy variation inherent in genetic tests. Furthermore, currently available techniques for sequencing of all mRNA-encoding DNA—so-called whole-exome sequencing—offers the opportunity for investigators to reasonably sequence the coding regions of every gene. These technological advances and the evolving compendium of human genetic variation must be scrutinized with great caution because we have already observed bona fide LQTS-causative mutations within the early releases of the 1000 Genomes Project. Clinical decisions can, and should, be informed by methodical, reproducible basic and translational scientific investigation; however, the entire clinical picture of the patient must be kept in sharp focus. Just as a negative genetic test should not dissuade a diagnosis in the face of a strong clinical suspicion, identification of a genetic variant in an otherwise unremarkable individual should not solely determine a diagnosis.
ACKNOWLEDGEMENTS
Research in the genetic causes of youthful SCD is supported by National Institutes of Health grants R01-HD42569 and P01-HL94291, a Fondation Leducq Award for the “Alliance for Calmodulin Kinase Signaling in Heart Disease,” and the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.
Footnotes
CONFLICT OF INTEREST
M.J.A. is a consultant for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Transgenomic. M.J.A. and Mayo Clinic Health Solutions also receive royalties from Transgenomic with respect to their FAMILION-LQTS and FAMILION-catecholaminergic polymorphic ventricular tachycardia genetic tests. A.P.L. declares no conflict of interest.
References
- 1.Tester DJ, Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Curr. Opin. Cardiol. 2006;21:166–172. doi: 10.1097/01.hco.0000221576.33501.83. [DOI] [PubMed] [Google Scholar]
- 2.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Effect of clinical phenotype on yield of long QT syndrome genetic testing. J. Am. Coll. Cardiol. 2006;47:764–768. doi: 10.1016/j.jacc.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 4.Kapa S, et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120:1752–1760. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiene G, Corrado D, Basso C. Arrhythmogenic right ventricular cardiomyopathy/dysplasia. Orphanet J. Rare Dis. 2007;2:45. doi: 10.1186/1750-1172-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen-Chowdhry S, Syrris P, McKenna WJ. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2007;50:1813–1821. doi: 10.1016/j.jacc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Kapplinger JD, et al. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J.Am. Coll. Cardiol. 2011;57:2317–2327. doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landstrom AP, Ackerman MJ. Mutation type is not clinically useful in predicting prognosis in hypertrophic cardiomyopathy. Circulation. 2010;122:2441–2450. doi: 10.1161/CIRCULATIONAHA.110.954446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Driest SL, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Yield of genetic testing in hypertrophic cardiomyopathy. Mayo Clin. Proc. 2005;80:739–744. doi: 10.1016/S0025-6196(11)61527-9. [DOI] [PubMed] [Google Scholar]