Abstract
Paclitaxel is commonly used to treat multiple human malignancies, but its mechanism of action is still poorly defined. Human ovarian cancer SKOV3 cells (parental SKOV3) were treated with paclitaxel (1 μM) for 2 days, and the morphologic changes in the cells were monitored for more than 4 months. Parental SKOV3 underwent a markedly morphologic transition from the epithelial to fibroblast-like phenotype following treatment with paclitaxel; the resulting cells were designated as SKOV3-P. The SKOV3-P cells’ proliferative ability was assessed via a 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The molecular characteristics of these cells were assessed via immunocytochemical staining and Western blot analysis. Their invasiveness and tumor formation ability was evaluated via wound-scratch and colony formation assays. The tumorigenicity of SKOV3-P cells was assessed in vivo after subcutaneous injection of tumor cells between injections of parental and paclitaxel-treated cells in nude mice. SKOV3-P cells have decreased the proliferation and invasion ability, decreased colony-forming ability when cultured in Matrigel and lost their tumor formation as compared with parental SKOV3 cells when injected in nude mice. SKOV3-P cells have decreased expression of E-cadherin, cytokeratin, Snail, PI3K, and P-Akt-Ser473, and increased expression of fibronectin, vimentin, Slug, P27, and PTEN. These results demonstrated that paclitaxel can inhibit tumor growth by inducing ovarian cancer epithelial cells toward a benign fibroblast-like phenotype through dysregulation of previously known pathways involved in the regulation of epithelial to mesenchymal transition (EMT), which may represent a novel mechanism for paclitaxel-induced tumor suppression.
Keywords: Ovarian Cancer, Paclitaxel, Epithelial to Mesenchymal Transition, Fibronectin
1. Introduction
Ovarian cancer is one of the most common causes of death among women with gynecologic malignancies [1]. Due to diagnosis in late stage and to the development of dormant drug resistant cancer cells, epithelial ovarian cancers often respond initially to platinum and taxane-based chemotherapy, but a fraction of malignant epithelial cells persist and recur in up to 80% of patients, eventually lead to death of these patients [2, 3]. Paclitaxel is a first-line chemotherapeutic agent that is effective in epithelial ovarian cancer [4] by stabilizing microtubules, inducing cell cycle arrest in the G2-M phase, and activating proapoptotic signaling [5, 6]. SKOV3 ovarian cancer cells exhibit an epithelial morphology and can grow in soft agar with a relatively high colony forming efficiency. In vivo, SKOV3 cells can form moderately differentiated adenocarcinomas and has been regularly used as a model cell line for ovarian cancer xenograft [7]
Cancer initiation and progression are complicated processes that are regulated by a variety of cellular and signaling proteins. Epithelial carcinogenesis requires a co-evolving interaction of cancer cells with the tumor microenvironment, including stromal cells [8]. Epithelial to mesenchymal transition (EMT) is known to play a critical role in embryogenesis [9–12]. Cells undergoing EMT lose their epithelial morphology, undergo cytoskeleton reorganization and acquire a motile phenotype associated with the upregulation and downregulation of several molecules, including tight and adherens junction proteins and mesenchymal markers [13, 14]. Recently, EMT has been found to facilitate the metastasis of human cancer[15, 16]. EMT in cancer cells has been shown to promote metastasis during tumor invasion and dissemination and has also been implicated to resist therapy and disease relapse in general [14, 17–20].
We report here a distinct EMT form of SKOV3 induced by paclitaxel, designated as SKOV3-P, which has acquired a benign fibroblast-like phenotype. Our studies of the biologic and molecular marker properties of these mesenchymal cells revealed an unexpected characteristic that epithelial cancer cells in response to paclitaxel that may be further exploited for therapeutic use.
2. Materials and Methods
2.1. Cell culture
SKOV3 ovarian cancer cells were obtained from the American Tissue Culture Collection and maintained in Eagle’s minimum essential medium (EMEM; Lonza Walkersville, Inc.) containing 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 [21]. The SKOV3 was kayotyped to ensure free of contamination of mouse fibroblasts.
2.2. Establishment of a stable cell model and observation of cell morphology
When SKOV3 cells reached 90% confluency, they were washed with phosphate-buffered saline (PBS) and were then continuously exposed to 1 μM paclitaxel for 2 days when 70% – 80% of the cells were killed before replacement with medium with no paclitaxel. The surviving cells were continuously cultured for more than 4 months.
2.3. Western blot analysis
Total proteins were extracted from parental SKOV3 cells and from paclitaxel-induced spindle cells using a lysis buffer previously described [22]. Then, 30 μg aliquots of each lysate were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore Corp.). Afterward, the membranes were blocked with 5% nonfat milk in 1×Tris buffered saline with 0.1% Tween-20 (TBST) for 1 hour at room temperature, washed with 1× TBST three times, and incubated with the appropriate primary antibody overnight at 4°C. After being washed an additional three times with 1×TBST, the cells were incubated with the appropriate secondary antibody in 1% nonfat milk in 1×TBST for 1 hour at room temperature on a rocking platform. Expression of proteins including fibronectin, vimentin, β-catenin, cytokeratin (AE1/AE3) Slug, Snail and E-cadherin were measured using mixed ECL Plus reagents (RPN2132OL/AK, GE Life Sciences Co.) and developed using an X-OMAT 2000 film processor (Kodak). β-actin was used as a protein-loading control. The antibodies used are described in Table 2. The secondary antibodies against mouse immunoglobulin (IgG; RPN4201) and rabbit IgG (RPN4301) conjugated with horseradish peroxidase were obtained from Amersham Bioscience.
Table 2.
Antibodies information used in our paper
| Antibodies name | Company (Cat number) | Dilution | Anti-rabbit or mouse |
|---|---|---|---|
| β-actin | Sigma Aldrich (A5441) | 1:15,000 (WB) | M |
| phosphoinositide 3-kinase (PI3K) | Santa Cruz Biotechnology (sc-8010) | 1:500(WB) | R |
| phosphorylated AKT (Ser473) | Cell Signaling Technology (no. 9271) | 1:500(WB) | R |
| Sail | Abcam Inc. (ab17732) | 1:1000(WB) | R |
| Slug | Cell Signaling Technology (no. 9585) | 1:1000(WB) | R |
| E-cadherin | BD Biosciences (no. 610181) | 1:2500(WB) 1:100(IF) | M |
| Twist | Cell Signaling Technology (no. 4119) | 1:1000(WB) | R |
| P27 | abcam Inc. (ab32034) | 1:1000(WB) | R |
| PTEN | Santa Cruz Biotechnology (sc7974) | 1:200(WB) | M |
| Ki-67 | Biocare Medical (no. CP249B) | 1:100(ICC) | R |
| β-catenin | BD Pharmingen (no. 610153) | 1:1000(WB) | M |
| β-catenin | Cell Signaling Technology (no. 2849) | 1:10(ICC) 1:10(IF) | M |
| Cytokeratin | Imgenex (IMG-80126) | 1:1000(WB) | M |
| Fibronectin | BD Pharmingen (no. 61007) | 1:5000(WB) 1:200(IF) 1:500(ICC) | M |
| Vimentin | abcam Inc. (ab8069) | 1ug/ml(WB) 1:500(IF) | M |
R: rabbit
M: mouse
2.4. Immunofluorescence assays
Treated cells were harvested using trypsin and seeded on slides the day before immunofluorescence assays. The cells were washed twice with 1×PBS. Then the slides were dried at room temperature for 5–10 minutes, fixed with cold acetone for 10 minutes, and washed twice for 5 minutes with PBS. The cells were then blocked with normal horse serum in PBS for 30 minutes at room temperature and incubated with the primary antibodies (Table 2) at the appropriate dilution overnight at 4°C. The next day, the slides were washed with PBS, incubated with the appropriate secondary antibodies (Texas red-conjugated anti-rabbit and anti-mouse) at a dilution of 1:100 in the dark for 1 hour, and then washed an additional time with 1×PBS. The cells were then stained with 4′,6-diamidino-2-phenylindole and photographed with a TE2000-U fluorescent microscope (Nikon).
2.5. Immunocytochemical staining assays
Immunocytochemical staining for β-catenin, fibronectin, and Ki-67 using an avidin-biotin-peroxidase complex was performed as previously described [23]. The dilutions of these antibodies are listed in Table 2. When parental SKOV3 and SKOV3-P cells were grown on glass coverslips and reached 70% confluency, slides were washed with PBS and fixed with pure cold acetone for 10 minutes on ice. After treatment with 0.3% hydrogen peroxide for 10 minutes and 1.5% normal goat serum to block endogenous peroxidase activity and nonspecific protein binding, respectively, the cells were incubated overnight with the primary antibodies at 4°C in a humidified chamber. The following morning, cells were incubated with biotinylated goat anti-mouse IgG for 30 minutes, counterstained with hematoxylin, and examined and photographed under a BV40 microscope (Olympus)
2.6. In vitro cell proliferation assays
Serial dilutions of cells in culture medium were prepared, and 100 μl of the dilutions (containing 1×104, 5×103, and 1×103 per 100 μl) was added into triplicate wells of a 96 well microtiter tissue culture plate. Cells were incubated for 12 hours, and 10 μl of 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) reagent was added to each well. Three control wells that were incubated with only medium to allow for absorbance reading. Two days later, 100 μl of reagent with detergent (MTT; Sigma-Aldrich) was added to all the wells when a purple precipitate was clearly visible in the cells. Two hours later, the absorbance in each well was measured at 570 nm using a plate reader (uQuant, BIO-TEK instruments, INC). The values from triplicate readings were averaged, and the average value for the control wells was subtracted.
2.7. Wound-scratch assays
Parental SKOV3 and SKOV3-P cells (1 × 105) were plated in six-well plates for 24–48 hours (until they reached 90% confluency). Confluent cells were uniformly scratched using sterile pipette tips. Then, the medium was replaced with fresh EMEM without fetal bovine serum. The cells were photographed with microscope (Olympus) and counted in several pre-marked areas at 0, 48, and 96 hours.
2.8. Colony formation in vitro and tumor formation in nude mice and PKH25 staining of tumor cells
BD Matrigel matrix (BD Biosciences) was thawed overnight on ice at 4°C. Then, 5,000 parental SKOV3 and 5,000 SKOV3-P and cells were cultured in a mixture of Matrigel and EMEM at a 1:1 ratio and seeded at a total volume of 300 μl on a 24-well plate on ice. This cell mixture with Matrigel was solidified at 37°C for 10 minutes, and then 0.5 ml of EMEM was added. The medium was replenished every 48 hours, and the clones were counted 7 days after seeding.
To determine the ability of the cells to form tumors, we administered bilateral injections of SKOV3-P cells and parental SKOV3 cells s.c. into 6- to 8-week-old female athymic nude mice (National Cancer Institute). Before injection, 2 nude mice were injected with 1×106 SKOV3-P cells stained with PKH26 (Sigma-Aldrich). After staining with PKH26 for 5 minutes, the staining was stopped when mixed with fetal bovine serum, and the cells were washed with complete EMEM three times. Each subcutaneous injection consisted of 1×104, 1×105, and 1×106 SKOV3-P or 1×103, 1×104, and 1×105 parental SKOV3 cells. At day 18, two mice injected with 1×106 PKH26-stained SKOV3-P can be palpated small nodules in the injection sites and the bumps were removed for frozen section examination. The other mice were kept in a specific pathogen-free environment and checked for tumor development every 2 days for 2 months. The mice were euthanized by CO2 inhalation. The tumors were excised, fixed in 10% formalin overnight, and subjected to routine histologic examination following hematoxylin and eosin staining. All mouse experiments were performed in accordance with guidelines approved by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
2.9. Statistical analysis
For analysis of the data from the in vitro experiments, Statistical comparisons of the data from in vitro experiments were performed using the non-paired Student t-test. Differences between groups were considered statistically significant at a P value of less than 0.05. Data are reported as mean ± standard deviation.
3. Results
3.1. Paclitaxel induced SKOV3 cells to form fibroblast-like SKOV3-P cells
Paclitaxel is known to inhibit the cell growth by a G2-M-phase transition [5]. As expected, after SKOv-3 cells were exposure to paclitaxel for 2 days, most of the cells died and cell morphology changed becoming longer and irregular in shape, varied in size, with unclear cell borders. One week after paclitaxel treatment, parental SKOV3 cells became rounded and enlarged morphologically. The morphology of parental SKOV3 is shown in Figure 1A-a. The epithelial and spindle cells co-exist in the same flask following the treatment of paclitaxel. When the cells reached more than 80% confluence, more epithelial cells appeared among the spindle cells. One month after paclitaxel treatment, there are a number of morphologically distinct giant cancer cells with many cell branches extended from them (Figure 1B-a). These large cells continued to generate more branches and developed numerous spindle cells with continued cultures (Figure 1B-b and -d). Spindle cell morphology can be maintained for at least 6 months when paclitaxel-treated SKOV3 cells were cultured in regular EMEM (not shown) although there was no obvious change in the total number of large cells. However, the spindle morphology reverted to an epithelial appearance when cultured cells reached more than 80% confluence, demonstrating that these phenotypes are reversible. Cells with reversible SKOV3-P constituted 5–15% the total population depending on the level of confluence.
Figure 1.
Paclitaxel induces a fibroblast-like phenotype in SKOV3 cells. (A) Different morphologic characteristics of parental SKOV3 cells and SKOV3-P cells. a) Morphology of parental SKOV3 cells. b–f). Morphologic variations of SKOV3-P cells derived from SKOV3 cells. (B) Parental SKOV3 cells were treated with 1μM paclitaxel for 48 hours and then recovered over the period of 4 months in EMEM (×10)
3.2. Expression of EMT-related proteins in SKOV3-P cells
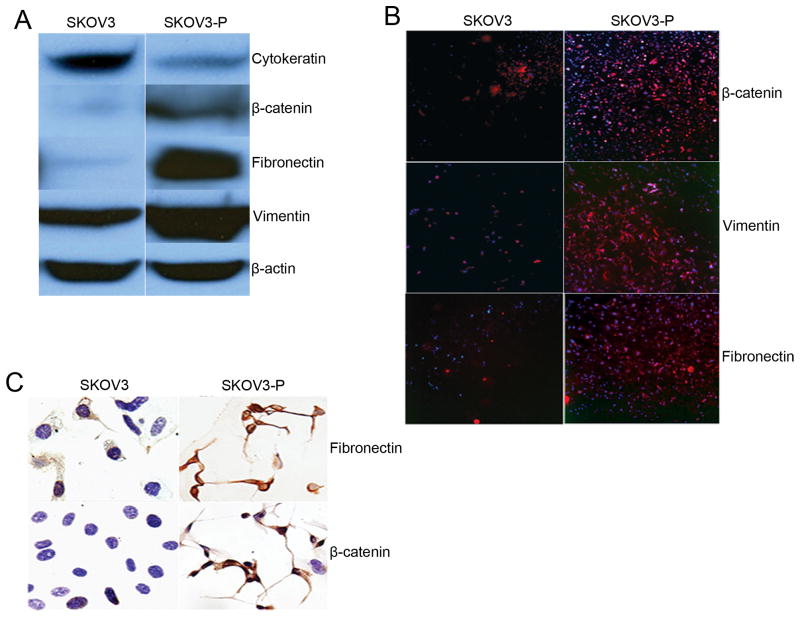
SKOV3-P cells developed an elongated, fibroblast-like morphology with a scattered and unorganized growth pattern in culture, which was consistent with an epithelial to mesenchymal transition of SKOV3 cell. Western blot analysis was performed using lysates of the SKOV3-P and parental SKOV3 cells to confirm the decreased expression of epithelial-related proteins including cytokeratin (AE1/AE3), increased expression of mesenchymal protein including fibronectin, vimentin, and an EMT-regulatory protein β-catenin (Figure 2A). Increased expression of fibronectin, vimentin, and β-catenin in SKOV3-P cells was further confirmed by immunohistochemical and immunofluorescent staining (Figure 2B and 2C).
Figure 2.
The expression of EMT-related proteins. (A) Expression of various proteins in parental SKOV3 and SKOV3-P cells as determined by Western blot analysis. (B) Positive expression images (red) merged with Hoechst 33342 staining. Expression of fibronectin, vimentin, E-cadherin, and β-catenin in parental SKOV3 and SKOV3-P cells assessed using immunofluorescent staining. (C) Immunocytochemical staining of fibronectin and β-catenin.
3.3. Decreased ability of migration and proliferation of SKOV3-P cells
Wound-scratch assays were used to compare the migratory ability of SKOV3-P and parental SKOV3 cells at 0, 48, and 96 hour. At 96 hours, the mean number of parental SKOV3 cells that migrated across the wound was about 3-fold higher than the number of SKOV3-P cells that migrated across the wound (P < 0.001; Figure 3A and 3B). In addition, we compared the proliferative potential of SKOV3-P and parental SKOV3 cells using Ki-67 immunohistochemical staining and a modified MTT assay. Approximately 90% of the parental SKOV3 cells and approximately 30% of the SKOV3-P cells had positive Ki-67 immunohistochemical staining. The MTT assay with various numbers of parental SKOV3 cells and SKOV3-P cells showed a decrease in the growth of SKOV3-P cells compared with the parental SKOV3 cells (P < 0.005; Figure 3C and 3D).
Figure 3.
The ability of migration and proliferation in the SKOV3-P and parental SKOV3 cells. (A) Wound-scratch assays were performed to compare the migratory potential of SKOV3-P and parental SKOV3 cells at 0, 48, and 96 hour. (B) Quantitative results are shown as a wound-healing index, which indicated differences in the numbers of cells at 96-hour. Y-axis indicates the number of migratory cells. (C) Ki-67 immunohistochemical staining verified that SKOV3-P cells had lower proliferative potential than parental SKOV3 cells. (D) Proliferative potential of SKOV3-P and parental SKOV3 cells at 48 hours after plating assessed using a modified MTT assay with various cell numbers. SKOV3-P cells exhibited a decrease in growth compared with parental SKOV3 cells (*P < 0.005).
3.4. Decreased ability of tumor forming of SKOV3-P cells
Seven days after seeding, parental SKOV3 cells had formed more colonies than SKOV3-P cells. The parental SKOV3 colonies were noticeably larger than the SKOV3-P clones. The SKOV3-P cells maintained a relatively static state when grown in Matrigel after seeding (Figure 4A). In vivo, 1×106 SKOV3-P cells were injected subcutaneously, s.c, into six nude mice including two nude mice injected with PKH26-stained SKOV3-P with Matrigel. The 1×106 SKOV3-P cells included most of spindle cells and a small amount of the reversible SKOV3-P with epithelial state. Small 0.2- to 0.3-cm nodules at the injection site could be palpated at day 18 after injection and disappeared 2 months later. Two mice injected with PKH26-stained SKOV3-P were sacrificed at day 18, the nodules were removed and frozen section was performed. Fluorescence microscopy revealed that almost all the cells were stained red with PKH26 (Figure 4B-e), thereby indicating that cells in the nodule were of SKOV3-P origin. Hematoxylin and eosin staining revealed the presence of benign fibroblast-like cells with rare morphologically recognizable tumor cells (Figure 4B-d), while high grade carcinoma cells formed in all mice at 60 days after injection with parental SKOV3 cells (Figure 4B-b and Table 1). These data demonstrated that SKOV3-P induced by paclitaxel had characteristics of benign fibroblasts, including slow proliferative and migration ability and decreased ability of forming a tumor.
Figure 4.
The subcutaneous tumorigenicity in nude mice and the Expression of PI3K/Akt signal markers. (A) Clone formation assays showed that parental SKOV3 cells grown in Matrigel formed large clones 7 days after seeding. However, EMT cells grown in Matrigel maintained a relatively static growth after seeding. (B) Tumor formation ability of SKOV3-P and parental SKOV3 cells. One million of SKOV3-P stained with PKH26 was subcutaneously injected into nude mice with Matrigel. Small 0.2 – 0.3 cm bumps could be palpated at day 18 after injection and disappeared 2 months later. a) Gross picture of tumor arising from s. c. injection of 1000 parental SKOV3 cells. b) HE staining of tumor generated from parental SKOV3 cells (HE×20). c) Gross picture of bump arising from s. c. injection of 106 SKOV3-P cells. d) HE staining showed fibroblast cells from nodule generated from SKOV3-P (HE×20). e) At day 18, small bump with whole capsules in the injection sites were removed and subjected for frozen section analysis. Almost all the cells were red under fluorescence microscopy, demonstrating an origin from injected SKOV3-P cells. (C) Differences in the expression of EMT and PI3K/Akt signal-related markers in parental SKOV3 and SKOV3-P cells. B. Expression of PI3K/Akt signal markers P27 and PTEN, PI3K and P-Akt. β-actin was used as a loading control. (D) Expression of EMT-related markers in parental SKOV3 and SKOV3-P cells. β-actin was used as a loading control.
Table 1.
Tumor formation ability of SKOV3-P and parental SKOV3 cells.
| SKOV3 | Cell number of injection | Number of nude mouse | Day after injection | Average tumor size |
|---|---|---|---|---|
| SKOV3 | 103 | 4 | 60 | 35*30*28mm3 |
| SKOV3 | 104 | 4 | 60 | 80*78*95mm3 |
| SKOV3 | 105 | 4 | 60 | 95*90*85mm3 |
| SKOV3-P | 104 | 4 | 60 | no tumor |
| SKOV3-P | 105 | 4 | 60 | no tumor |
| SKOV3-P | 106 | 4 | 60 | no tumor |
| SKOV3-P (PKH-26) | 106 | 2 | 18 | 0.2–0.3cm bumps |
3.5. Expression of EMT biomarkers and other signaling molecules in SKOV3-P cells
We determined the potential molecular mechanism involved in this process. Because PI3K-Akt signaling is frequently disrupted in human cancers and plays a major role in the balance between cell proliferation and apoptosis, we examined proteins involved in the PI3K-Akt signaling pathway. Western blot analysis revealed that p27 and PTEN expression was higher and PI3K and p-Akt expression was lower in SKOV3-P cells than in parental SKOV3 cells (Figure 4C). In addition, the expression of Slug was higher in SKOV3-P cells than in parental SKOV3 cells, while the expression of Snail was lower in SKOV3-P cells than in parental SKOV3 cells (Figure 4D). The opposing level of expression of Snail and Slug following the treatment of paclitaxel is particularly interesting, as both proteins are well-known transcription factors involved in regulating EMT, although it remains to be tested experimentally that whether such altered ratio of two transcription factors is responsible for the benign phenotype described here. These results demonstrated that paclitaxel inhibits tumor growth through the dysregulation of previously existing pathways known to be involved in regulation of proliferation, apoptosis, and EMT.
4. Discussion
In this study, we showed that SKOV3 cells can differentiate into a fibroblast-like phenotype following treatment of paclitaxel. The resulting SKOV3-P cells had functionally benign behaviors, including slow proliferation and low rates of invasion and little tumor ability. Several studies have demonstrated that EMT plays an important role in generating cancer stem cells and facilitating the transformation, initiation, progression, and metastasis of human cancers [12, 24–25]. On the other hand, it has also been well documented that EMT can contribute to tissue fibrosis in adults by generating fibroblasts [26]. Zeisberg and colleagues reported that endothelial-mesenchymal transition is a form of EMT that occurs during the embryonic development of the heart [27]. In addition, adult fibroblasts are considered to be derived directly from embryonic mesenchymal cells and to increase in number solely as a result of the proliferation of resident fibroblasts [27]. However, data present here, demonstrated that, rather than selecting aggressive and malignant clones through cancer stem-like cells or generating benign fibroblasts from benign epithelial or endothelial cells, certain therapeutic agents such as paclitaxel can induce the epithelial cancer cells to benign fibroblast-like cells. Thus, EMT can overcome the malignant trait of an established cancer through generation of a benign fibroblast-like phenotype.
Our results that EMT induced by paclitaxel involves the dysregulation of several distinct pathways involved in regulation of proliferation, apoptosis, and conventional EMT. We showed high expression of β-catenin and E-cadherin in SKOV3-P as compared with the parental SKOV3 cells. β-catenin is component of Wnt/Wg signaling pathway which can induce the expression of the cell-substrate adhesion molecule fibronectin, a molecule involved in generating fibroblast-like phenotype [28, 29]. As a component of the cadherin adhesion complex, β-catenin is required for linkage of cadherins to the cytoskeleton, thereby conferring adhesiveness to the cells [30, 31]. The loss of E-cadherin or the presence of mutants in carcinoma cells was previously shown to increase invasive potential [32, 33]. Our study showed that expression of E-cadherin is decreased while the β-catenin expression was increased, consistent with other reports that activation of β-catenin pathway is involved in generating an EMT phenotype [28, 34–35]. We further demonstrated that expression of P27 and PTEN was increased and the expression of PI3K and p-Akt was decreased in SKOV3-P cells than in parental SKOV3 cells, which are correlated with the increased expression of β-catenin and Slug. The activation of PI3K/Akt pathway is central to tumorigenesis because it promotes cell survival and inhibits apoptosis via phosphorylation of pro-apoptotic proteins, and mediates cell proliferation by regulating the expression cyclin-dependent kinase inhibitors. Activation of PI3K/Akt signaling occurs in a variety of carcinomas [36, 37]. In addition to affecting cell proliferation and apoptosis, activated Akt upregulates Snail and leads to EMT, it regulates the activity of glycogen synthase kinase 3β (GSK-3β) and its non-phosphorylated form that is responsible for degrading both β-catenin and Snail. Akt phosphorylates GSK-3β and inhibits its expression thus leading to an increase in the signaling of both β-catenin and Slug, ultimately promoting EMT [37–39]. We showed that paclitaxel decreased the activity of PI3K/Akt, in particular, P-Akt-ser473, a key phosphorylation site involved in activation of Akt. Thus, decreased activity in PI3K/AKt pathway is consistent with the benign and fibroblast-like phenotype following the treatment of paclitaxel.
In summary, our data demonstrate that paclitaxel can induce epithelial cancer cells to undergo EMT directly to generate a non-tumorigenic fibroblast-like phenotype. Such mechanisms may be potentially exploited in developing more effective therapeutic strategies for ovarian cancer.
Acknowledgments
This research was supported by an R01 grant (R01CA131183-01A2) and the M.D. Anderson Specialized Programs of Research Excellence (SPORE) in Ovarian Cancer (IP50CA83638) from the National Institutes of Health and grants from the Ovarian Cancer Research Fund. This work was also supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Author contributions: J.L designed research; L.J and Z.S performed research; Y.Y. and X.L. analyzed data. I.M. performed research, and R.C.B and J.L. aided in analysis of the data and preparation of the manuscript.
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. Journal of Clinical Oncology. 2010;28:3545–3548. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- 3.Bratasz A, Weir NM, Parinandi NL, Zweier JL, Sridhar R, Ignarro LJ, Kuppusamy P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3914–3919. doi: 10.1073/pnas.0511250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Yang XJ, Kundu SD, Pins M, Javonovic B, Meyer R, Kim SJ, Greenberg NM, Kuzel T, Meagher R. Blockade of transforming growth factor-β signaling in tumor-reactive CD8+ T cells activates the antitumor immune response cycle. Molecular cancer therapeutics. 2006;5:1733. doi: 10.1158/1535-7163.MCT-06-0109. [DOI] [PubMed] [Google Scholar]
- 5.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer research. 2002;62:1087. [PubMed] [Google Scholar]
- 6.Ahn HJ, Kim YS, Kim JU, Han SM, Shin JW, Yang HO. Mechanism of taxol-induced apoptosis in human SKOV3 ovarian carcinoma cells. Journal of cellular biochemistry. 2004;91:1043–1052. doi: 10.1002/jcb.20006. [DOI] [PubMed] [Google Scholar]
- 7.Engel JB, Keller G, Schally AV, Halmos G, Hammann B, Nagy A. Effective inhibition of experimental human ovarian cancers with a targeted cytotoxic bombesin analogue AN-215. Clinical Cancer Research. 2005;11:2408–2415. doi: 10.1158/1078-0432.CCR-04-1670. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nature cell biology. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. The Journal of cell biology. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Current opinion in cell biology. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Guarino M. Epithelial-mesenchymal transition and tumour invasion. The International Journal of Biochemistry & Cell Biology. 2007;39:2153–2160. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and β-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 16.Thompson EW, Newgreen DF. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer research. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- 17.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Current opinion in cell biology. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clinical Cancer Research. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 19.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proceedings of the National Academy of Sciences. 2007;104:10069. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clinical Cancer Research. 2010;16:3875–3886. doi: 10.1158/1078-0432.CCR-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G, Xiao X, Rosen DG, Cheng X, Wu X, Chang B, Liu G, Xue F, Mercado-Uribe I, Chiao P. The biphasic role of NF-κB in progression and chemoresistance of ovarian cancer. Clinical Cancer Research. 2011;17:2181. doi: 10.1158/1078-0432.CCR-10-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC. A genetically defined model for human ovarian cancer. Cancer research. 2004;64:1655. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 24.Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell & bioscience. 2011;1:1–7. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. Journal of mammary gland biology and neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 27.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 28.Gradl D, Kühl M, Wedlich D. The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Molecular and cellular biology. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends in Genetics. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 30.Takeichi M. Morphogenetic roles of classic cadherins. Current opinion in cell biology. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 31.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes, Chromosomes and Cancer. 2002;34:255–268. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 32.Shimamura K, Hirano S, McMahon AP, Takeichi M. Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development. 1994;120:2225–2234. doi: 10.1242/dev.120.8.2225. [DOI] [PubMed] [Google Scholar]
- 33.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Molecular cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 34.Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proceedings of the National Academy of Sciences. 1998;95:4374. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solanas G, Porta-de-la-Riva M, Agustí C, Casagolda D, Sánchez-Aguilera F, Larriba MJ, Pons F, Peiró S, Escrivà M, Muñoz A. E-cadherin controls β-catenin and NF-κB transcriptional activity in mesenchymal gene expression. Journal of cell science. 2008;121:2224–2234. doi: 10.1242/jcs.021667. [DOI] [PubMed] [Google Scholar]
- 36.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4 doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 37.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 38.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. Journal of Biological Chemistry. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 39.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nature genetics. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]