Abstract
Injuries to muscle in the elite athlete are common and may be responsible for prolonged periods of loss of competitive activity. The implications for the athlete and his/her coach and team may be catastrophic if the injury occurs at a critical time in the athlete's diary. Imaging now plays a crucial role in diagnosis, prognostication and management of athletes with muscle injuries. This article discusses the methods available to clinicians and radiologists that are used to assess skeletal muscle injury. The spectrum of muscle injuries sustained in the elite athlete population is both discussed and illustrated.
Injury in sport is an inevitable consequence of the loads placed on the human body during extreme activity. Although certain injuries are common to all sports, others are said to be sports specific, e.g. “rock climber's finger” and “little leaguer's elbow”. By their very nature, sport-specific injuries are rare compared with those sustained across all sports, in which injuries to muscle are among the most prevalent. Analysis of the injury patterns sustained at the Olympic Games in Beijing, China, revealed that, after ankle sprains, the most common injury was thigh muscle strains [1]. Muscle injuries account for up to one-third of all sports-related injuries in elite sport and the implications for the athlete, coach and team/club may be beyond the scope of this article, ranging from the physical to the psychological to the financial [2-4]. Injuries to the muscle–tendon–bone unit are responsible for over 50% of the total of all injuries in elite soccer players [5]. The hamstring muscle unit is implicated as the main culprit in most published series including studies involving Union of European Football Associations Champions League footballers [6] and elite rugby players, in whom there is an average loss of playing time of 17 days as a result of muscle injury [7]. Muscle injuries result from either direct trauma, usually in the form of blunt impact and less frequently penetrating injury, or indirect trauma following excessive eccentric force along the muscle–tendon–bone axis. Imaging of sports-related injury is now pivotal to guiding management and aiding with prognosis. This article will discuss the biomechanics of muscle injury and the imaging spectrum of sports-related muscle injuries and their potential complications.
Imaging techniques
Cross-sectional imaging of muscle and muscle injury in elite sport is usually undertaken by MRI and/or ultrasound. Myositis ossificans aside, plain radiography, CT and nuclear medicine techniques are not routinely used in imaging muscle injury.
MRI provides excellent lesion detection and localisation. The images are anatomical and clearly understood by healthcare professionals and patients alike. Depending on the magnet used, even the largest of athletes can be imaged without difficulty. However, MRI is a scarce resource, expensive, time-consuming, uncomfortable and acquires static images.
Various muscle injury MRI protocols are in use across Europe. Common to all of them should be at least one fat-suppressed fluid-sensitive sequence such as short tau inversion–recovery or fat-saturated proton density-weighted or T2 weighted imaging (Figure 1). Diffusion imaging and other advanced MRI techniques have been mooted as potentially useful in assessing muscle injury but have yet to reach routine status (Figure 2) [8]. At least one T1 weighted image should be acquired to assess for blood products and more importantly for muscle atrophy (Figure 3).
Figure 1.
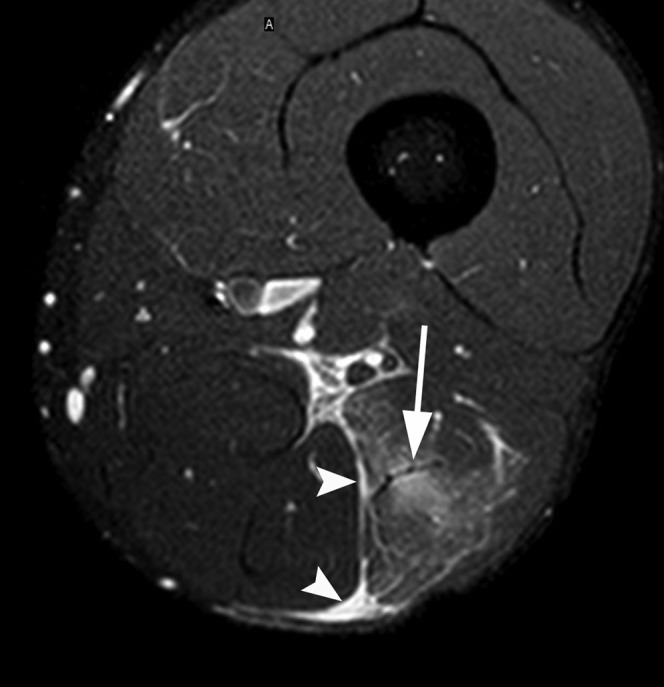
A 28-year-old male professional footballer with grade I distal hamstring strain. Axial fat-saturated proton density-weighted image through the distal thigh demonstrating oedema within the long head of the biceps femoris muscle centred on the musculotendinous junction (arrow). Note the perifascial fluid surrounding the muscle at the injury site (arrowheads).
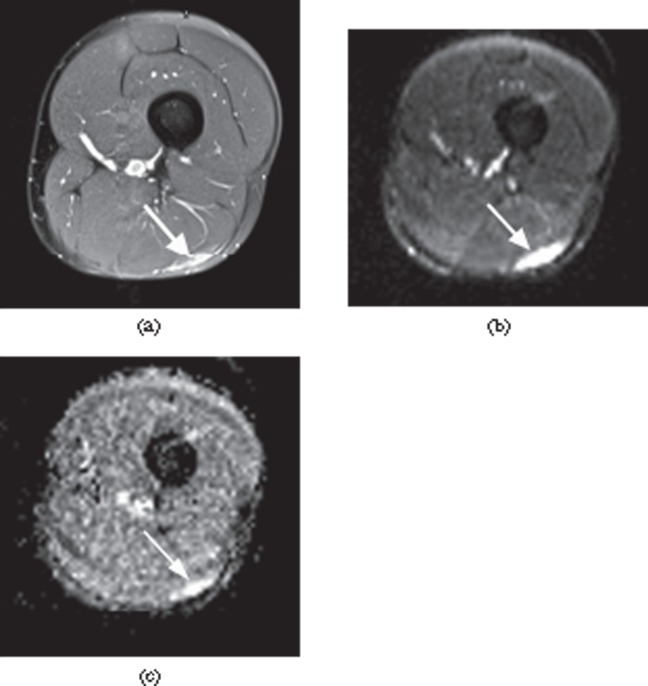
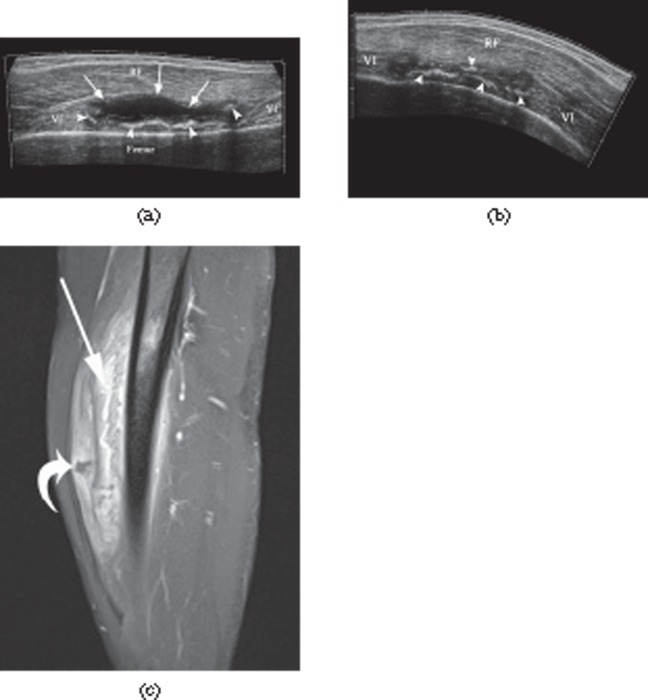
Figure 2.
A 26-year-old male professional footballer with grade I distal hamstring strain. (a) Axial fat-saturated proton density-weighted image demonstrating high signal at the myofascial surface of the long head of biceps muscle (arrow). (b) Axial B050 diffusion-weighted image and (c) apparent diffusion coefficient mapped axial image obtained at the same level showing high signal within the periphery of the muscle (arrow).
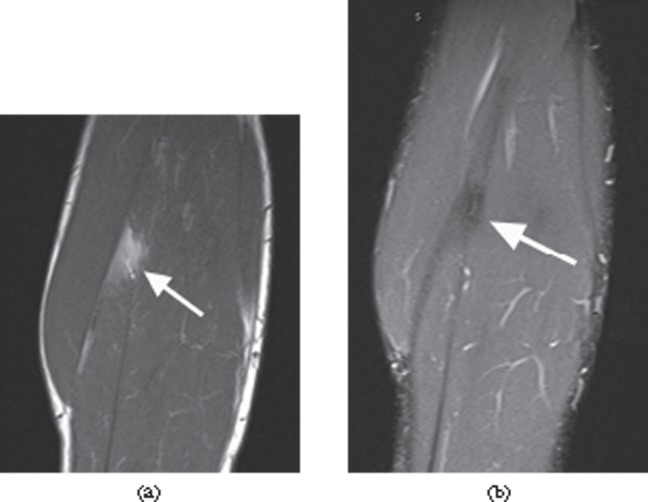
Figure 3.
A 33-year-old male professional footballer with a history of previous calf muscle strain. (a) Sagittal T1 weighted image through the calf demonstrating focal fatty infiltration of the soleus muscle (arrow), which fully suppresses on the fat-suppressed short tau inversion–recovery sagittal image at the same level (b). Without the T1 image, the low-signal area within the muscle could be misinterpreted as an intramuscular scar.
Ultrasound examination of acute muscle injury is becoming increasingly popular in elite sport and is frequently performed by radiologists and sports clinicians in the acute and hyperacute injury setting. Ultrasound has several important potential advantages over MRI such as superior spatial resolution, cost, convenience, portability and dynamic evaluation of the injury. The advent of extended field of view (FOV) imaging allows the user to produce images that most clinicians and patients can clearly understand (Figure 4) [9]. However, diagnostic ultrasound of muscle injury is limited by operator dependency, limited FOV acquisition and reduced injury conspicuity compared with MRI. The operator should be skilled in the technique and have detailed knowledge of compartmental muscle anatomy and experience of assessing normal and abnormal muscle tissue in the static and dynamic state. Linear high-frequency probes are most commonly used (9–17 MHz) and provide excellent near-field resolution but may be limited by their lack of tissue penetration capability. In larger athletes, the deep muscles of the thigh may provide a particular challenge to the operator and in this group it should be noted that ultrasound can miss deep lesions [10]. The use of a curvilinear low-frequency (3–7.5 MHz) probe may be required but at a cost of lower spatial resolution, and small muscle injuries may be missed. Colour or power Doppler imaging is occasionally useful in identifying muscle injury by demonstrating increased blood flow at the injury site (Figure 5).
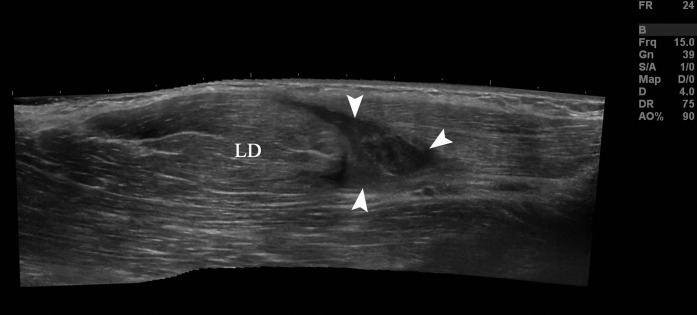
Figure 4.
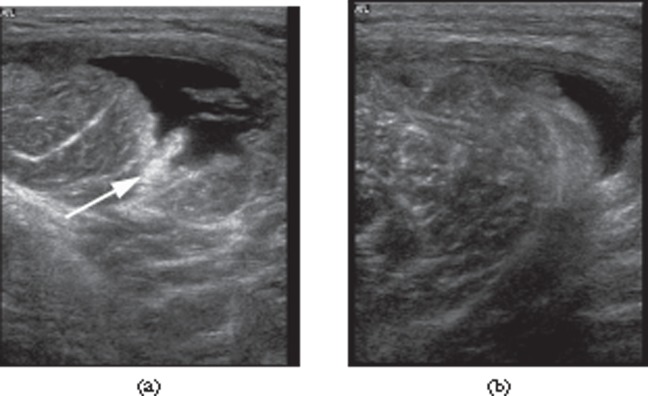
A 22-year-old cricket professional fast bowler with grade II right latissimus dorsi strain. Extended field of view sonogram demonstrating a tear within the latissimus dorsi muscle (LD) surrounded by haematoma (arrowheads).
Figure 5.
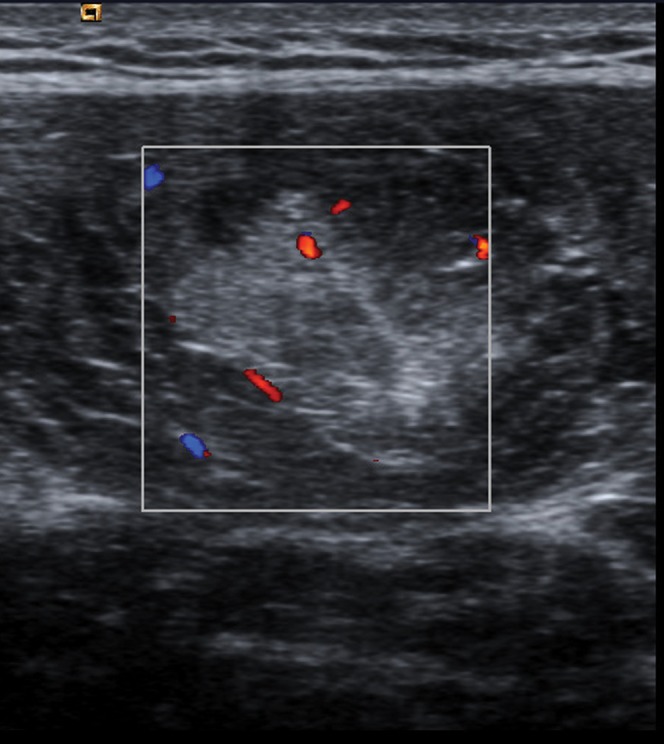
A 27-year-old female elite sprinter with grade I thigh strain. Axial sonogram of the rectus femoris muscle with colour Doppler demonstrating increased blood flow within the muscle at the injury site.
Biomechanics and cross-sectional imaging of muscle injury
Although an injury may occur at any point along the axis of the muscle–tendon–bone unit, this article will focus on injuries sustained primarily to the muscle, musculotendinous junction and myofascial surface. These injuries can be divided into two aetiological groups: direct injury, which is most commonly blunt trauma in the form of a direct blow to the lower limb, and indirect injury, in which excessive eccentric load on the muscle results in tearing of the junction between muscle fibres at the musculotendinous junction or myofascial interface, this being the weakest point in the muscle–tendon unit [11].
Direct muscle injury
Penetrating injury and subsequent muscle laceration is rarely encountered during modern sporting activity. Blunt trauma, however, is extremely common and is most frequently encountered in the lower limb in collision sports such as soccer and rugby. When a massive blunt force (usually the kneecap of the opposing player) is directly applied to the muscle of the recipient limb (usually the anterior mid-thigh), the dissipation of that force will determine the subsequent injury pattern. If the recipient is lucky enough that the main vector of the force is directed to the side of the bone, it is unlikely that any significant muscle injury will ensue. If, however, the force vector is directed towards the bone (usually the femur), the energy dissipated from the superficial to the deep muscle will be large, but the energy dissipated from the deep muscle to the bone will be massive and the muscle will tear at this point. The severity of the injury ranges from a small muscle contusion (Figure 6) to a large haematoma within the muscle itself (Figure 7), between muscles (intermuscular) or at the muscle–bone interface (Figure 8). Jackson and Feagin [12] defined a three-stage clinical grading system to gauge prognosis:
Figure 6.
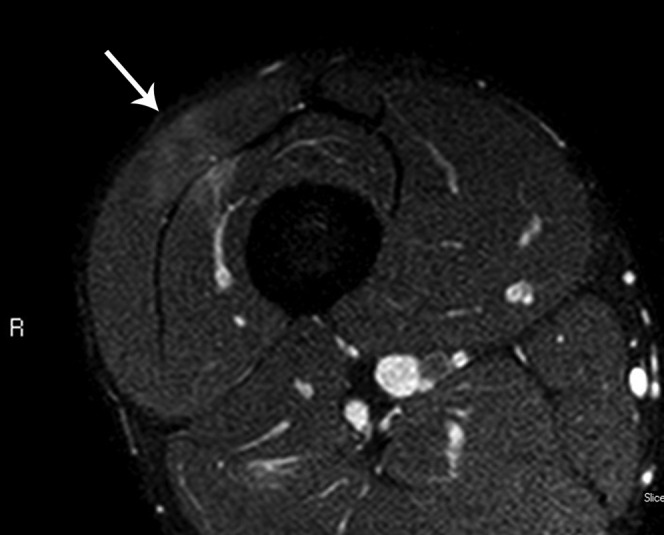
A 25-year-old professional footballer with minor thigh contusion. The arrow indicates the direction of impact of the blow. Note the high signal within the vastus lateralis and vastus intermedius muscles in the line of the force vector. Two-muscle involvement is unusual in eccentric load muscle strains and is more consistent with muscle contusion.
Figure 7.
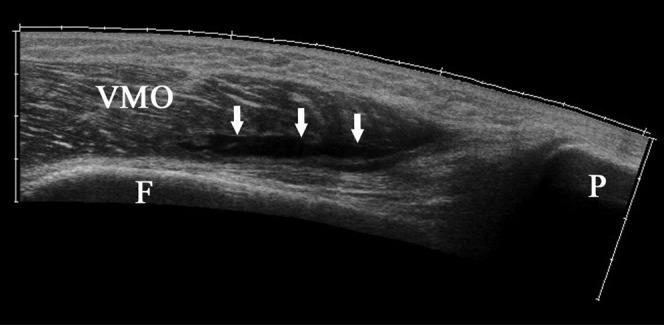
A 31-year-old male amateur footballer with intramuscular haematoma. Extended field of view sonogram demonstrating a laceration (arrows) within the posterior aspect of the vastus medialis obliquus (VMO) muscle anterior to the femoral cortex (F).
Figure 8.
A 32-year-old male professional footballer with a deep surface thigh haematoma. (a) Coronal and (b) axial short tau inversion–recovery MRI of the anterior thigh demonstrating a large haematoma deep to the vastus intermedius muscle (arrowheads) adjacent to the femoral cortex (F). Note the laceration into the muscle (arrow) and the layering of blood products on the axial image (curved arrow). The player was imaged 2 weeks after the original injury and had completed two full games in the interval between the injury and the MRI scan.
Mild contusion: range of motion less than one-third normal. Average loss of activity is 6 days.
Moderate contusion: range of motion one-third to two-thirds of normal. Average loss of activity is 56 days.
Severe contusion: greater than two-thirds loss of range of motion. Average loss of activity is more than 60 days.
Interestingly, the size of an intramuscular haematoma on imaging does not always correlate with loss of function and the clinical–MRI grading system used for indirect muscle tears (see below) cannot be applied to blunt trauma. Some elite athletes are able to function at the highest level of sport despite massive macroscopic injury on imaging (Figure 8).
Imaging of muscle contusion and haematoma
Ultrasound
On ultrasound, a contusion is seen as an ill-defined area of hyperechogenicity within the muscle that crosses fascial boundaries (Figure 9). In the hyperacute situation, the injured muscle initially appears swollen and may be isoechoic with adjacent unaffected muscle [13,14]. If the impact force is great enough, there will be significant rupture of muscle fibres and bleeding into a potential space, resulting in haematoma formation (Figure 7). In the first 24–48 h, the haematoma will appear as an irregularly outlined muscle laceration separated by hypoechoic fluid with marked increased reflectivity in the surrounding muscle (Figure 10). During this period, the haematoma may solidify and become hyperechoic to the surrounding muscle. After 48–72 h, the haematoma develops into a clearly defined hypoechoic fluid collection with an echogenic margin. This echogenic margin gradually enlarges and “fills in” the haematoma in a centripetal fashion (Figure 10) [14]. If the haematoma is causing intense pain and/or exerts local mass effect on adjacent neurovascular structures, or is placing the limb at risk of compartment syndrome, then evacuation of the clot may be necessary. This is usually performed under ultrasound guidance 10–14 days after the initial injury [15].
Figure 9.
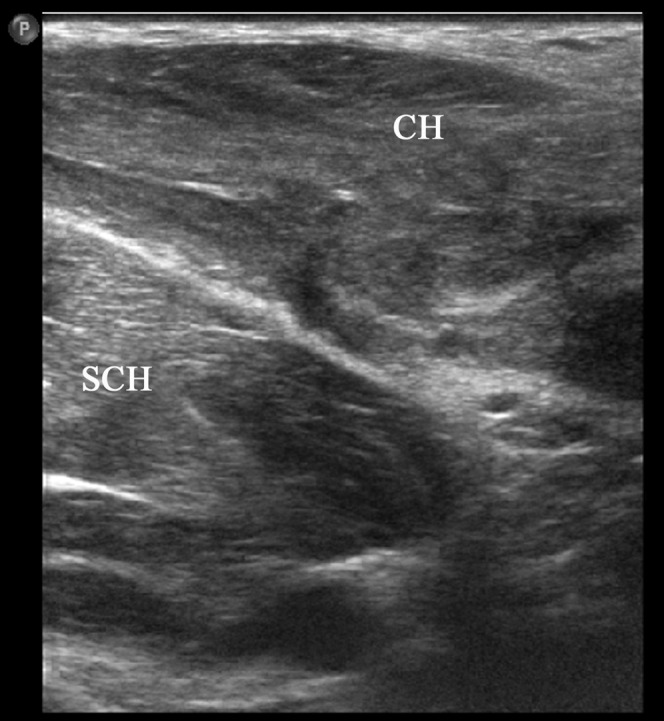
A 27-year-old male elite boxer presenting with pectoralis muscle contusion following punch injury to the chest. Note the generalised reflectivity within the clavicular (CH) and sternocostal (SCH) heads of the pectoralis major muscle.
Figure 10.
A 26-year-old male professional footballer with thigh haematoma. (a) Axial sonogram of the anterolateral thigh 2 days following a direct blow to the lateral side. Note the echogenic torn muscle tissue (arrow). (b) Axial sonogram taken 2 weeks later showing filling in of the haematoma.
MRI
In the initial phase, the contusion causes oedema and interstitial haemorrhage leading to muscle swelling and characteristic feather-like high signal within the muscle on fat-suppressed fluid-sensitive sequences (Figure 6). The presence of blood products may result in areas of faint high signal on T1 weighted images in the subacute early phase (Figure 11). Although these same imaging findings may be seen in grade I eccentric loading muscle strain, MRI usually shows oedema affecting different muscles along the line of the force vector and crossing fascial planes, a finding which is uncommon in indirect muscle strain injury. Moreover, the two conditions are usually easily distinguished clinically.
Figure 11.
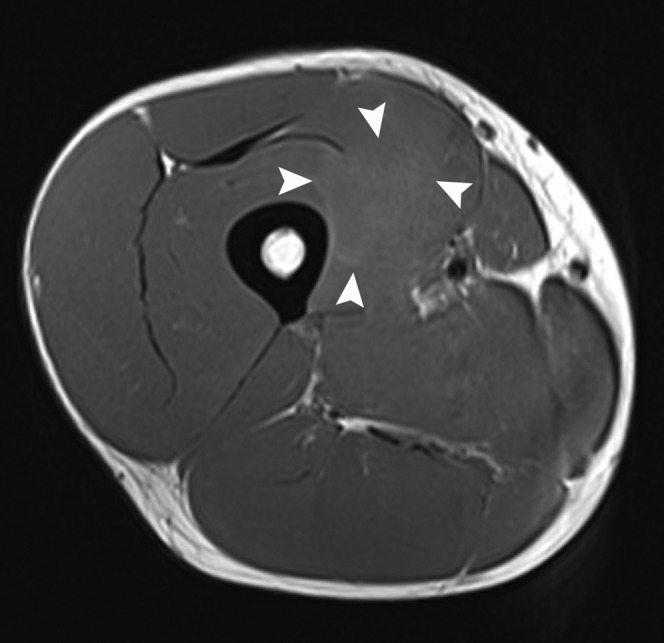
A 32-year-old male professional footballer with a thigh deep surface haematoma. Axial T1 weighted MRI of the anterior thigh of the same player taken at the same position and time as the axial short tau inversion–recovery image in Figure 8. Note the faint high-signal margins of the haematoma consistent with subacute early phase blood products (arrowheads).
As the haematoma develops, the morphology of the injury follows the same pattern as that described for ultrasound. Initially, there is an irregular muscle laceration followed, after 48 h, by a more clearly defined fluid collection within the muscle. The muscle surrounding the haematoma often remains diffusely high signal on the fluid-sensitive sequences (Figure 8). The signal characteristics of the haematoma will change in time depending on the nature of the predominant blood product within it (Table 1). There are two further stages of the haematoma healing process: fibrosis and/or calcification. Fibrosis of the margins of the haematoma will contract the lesion over time. Calcification of the haematoma wall leads to the development of myositis ossificans (see below).
Table 1. MRI appearances of evolving muscle haematoma.
| Stage | Blood product | T1 signal intensity | T2 signal intensity |
| Hyperacute (<4 h) | Intracellular oxyhaemoglobin | Intermediate | Bright |
| Acute (4–6 h) | Extracellular oxyhaemoglobin | Intermediate | Dark |
| Early subacute (6–72 h) | Intracellular methaemoglobin | Bright | Dark |
| Late subacute (72 h–4 weeks) | Extracellular methaemoglobin | Bright | Bright |
| Chronic (>4 weeks) | Haemosiderin | Dark | Dark |
Indirect muscle injury
During eccentric muscle contraction, the muscle is at risk of disruption as the force of active contraction is added to the passive stretching force being applied to the muscle–tendon unit [11]. Sudden onset pain usually localised to a single muscle occurring during a period of eccentric muscle contraction, most commonly a sprint, is the typical presentation of a muscle strain. Pre-disposing risk factors may be athlete related or muscle specific. Athlete-related risk factors include age, male sex, improper warm-up and fatigue. Muscle-specific risk factors include previous injury within the same muscle, and muscles that have a high proportion of fast twitch (type II) fibres, cross multiple joints, have complex anatomy or are regularly subject to eccentric loading [11]. Hence, the biceps femoris, rectus femoris and medial gastrocnemius are the most commonly strained muscles in elite sport [16,17].
Several grading systems have been described based on clinical examination and histology [18,19]. The most commonly used system has a three-step clinical grading score as follows:
Grade I (stretch injury): a small tear resulting in <5% loss of function.
Grade II (partial tear): a larger tear with 5–50% loss of function.
Grade III (complete rupture): >50% loss of function.
Imaging of indirect muscle injury
The role of imaging in acute muscle injury has changed from merely confirming a clinical diagnosis to defining the exact location of the injury within the muscle, the size of muscle disruption at the tear site, and the longitudinal length and cross-sectional area of muscle oedema, as these factors have all been used to assist with predicting outcome following muscle strain injury. Pomeranz and Heidt [20] assessed 14 athletes with hamstring injury and suggested that the following MRI findings predict a prolonged “convalescence interval”: >50% cross-sectional area muscle involvement, intramuscular haemorrhage (high on T1 and T2), distal musculotendinous junction tears and ganglion-like fluid collections in muscle (Figure 12).
Figure 12.
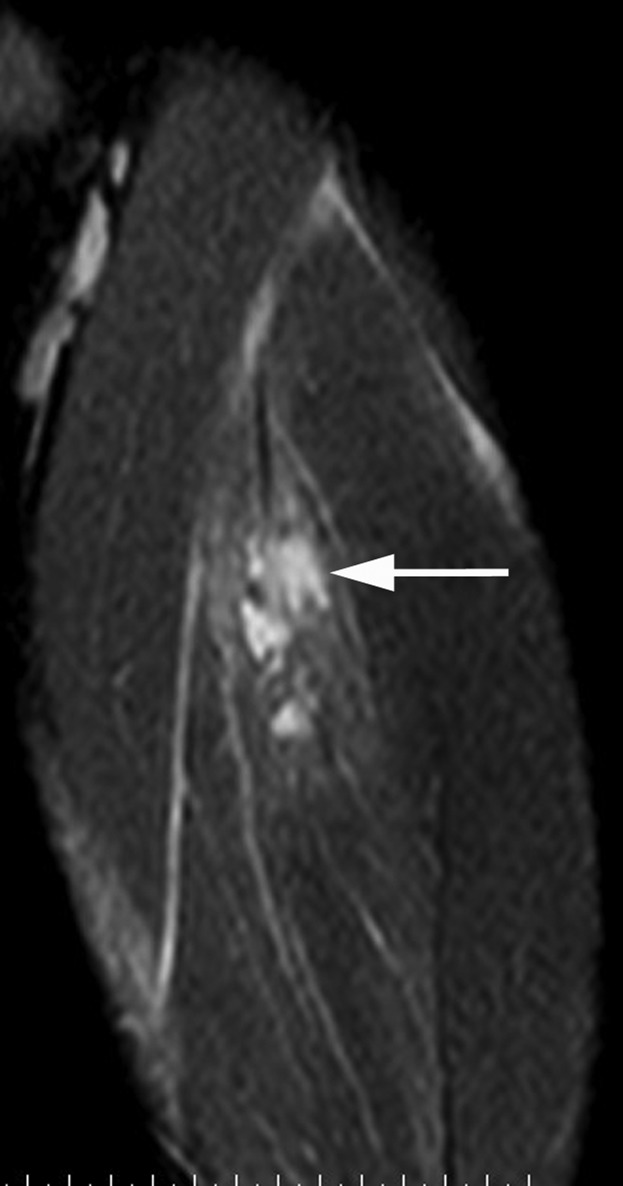
A 26-year-old male professional footballer with grade I thigh strain. Coronal short tau inversion–recovery image of the anterior thigh demonstrating ganglion-type cystic fluid collections within the anterior rectus femoris muscle (arrow).
Slavotinek et al [21] assessed 37 athletes with hamstring injuries and described a linear relationship between rehabilitation time and abnormal percentage cross-sectional area/muscle volume on MRI. In this study, there was no relationship between location within the muscle, i.e. proximal or distal, and prolonged recovery time. The muscle involved, i.e. biceps femoris, semimembranosus or semitendinosis, had no relationship to recovery time.
In 2003, Verral et al [22] assessed 83 athletes with acute hamstring strain using MRI. This study emphasised the importance of a normal MRI as a good predictor of early return to full activity. On average, those athletes with normal MRI were out of full activity for 16 days compared with 27 days in those with an abnormal MRI.
Connell et al [10] performed a longitudinal study of MRI and ultrasound findings in acute clinical muscle strains in 60 Australian rules football players at baseline, 2 weeks and 6 weeks. They described a linear relationship between longitudinal length of the injury at baseline on MRI and prolonged recovery time. In contrast to the Slavotinek study [21], Connell et al [10] concluded that injuries to the biceps femoris were associated with prolonged recovery time compared with injuries to the other muscles of the hamstring muscle complex. Ultrasound was equivalent to MRI in the sensitivity of the detection of acute muscle injury at baseline but tended to underestimate the degree of oedema in the muscle over the 6-week follow-up period. They concluded, therefore, that ultrasound was as useful as MRI in depicting acute hamstring injuries and, because of lower costs, may be the preferred imaging technique but that MRI was more sensitive for follow-up imaging of healing injuries.
Following a muscle strain, the risk of re-injury is high, particularly in the first 8 weeks, and recurrent strains tend to be larger than the original injury [23-25].
Ultrasound
Peetrons and colleagues described the first sonographic three-point grading system for muscle strain injury [26,27].
Grade I strain: Peetrons’ “elongation injury”
Ultrasound of grade I injury may either be normal or show focal or general areas of increased echogenicity at the injury site occupying <5% of the muscle substance (Figure 13). Peetrons included small cavities within the muscle measuring no more than 10 mm as a grade I injury. However, in the authors' experience, the presence of muscle fibre disruption on ultrasound is more indicative of a grade II injury. Occasionally, perifascial fluid may be seen that may be hyperechoic owing to the presence of blood in the extravascular space.
Figure 13.
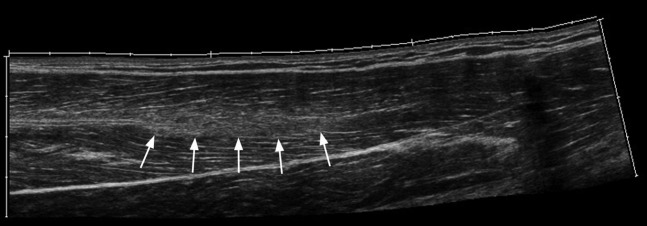
A 28-year-old female elite heptathlete with grade I thigh strain. Longitudinal extended field of view image through the rectus femoris muscle demonstrating increased reflectivity centrally within the muscle obscuring the central tendon (arrows).
Grade II strain: Peetrons’ “partial rupture”
A grade II injury involves >5% but <100% disruption of the cross-sectional area of the muscle. Typically, there is discontinuity of the echogenic perimysial striae either at the musculotendinous (Figure 14) or myofascial junction (Figure 15) [28]. An intramuscular fluid collection may be seen surrounded by a hyperechoic halo. Dynamic scanning during contraction may increase the conspicuity of the tear. Intermuscular and perifascial fluid collections are common in grade II injuries.
Figure 14.
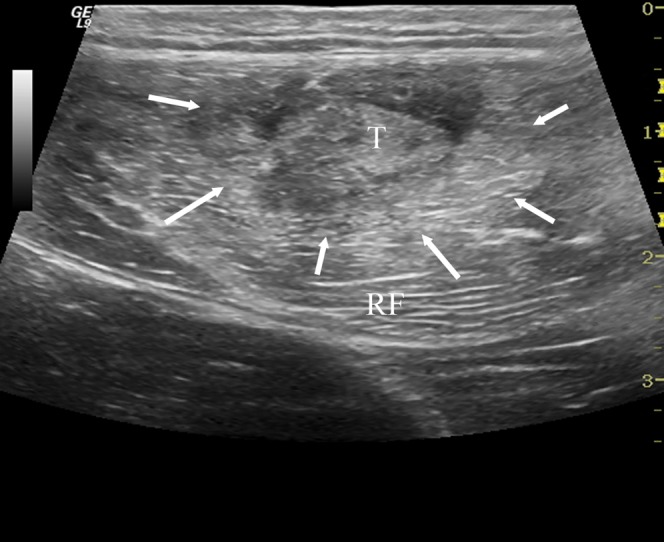
A 30-year-old male professional footballer with grade II thigh strain. Axial ultrasound image demonstrating increased reflectivity within the rectus femoris (RF) muscle (arrows) centred on the musculotendinous junction (T).
Figure 15.
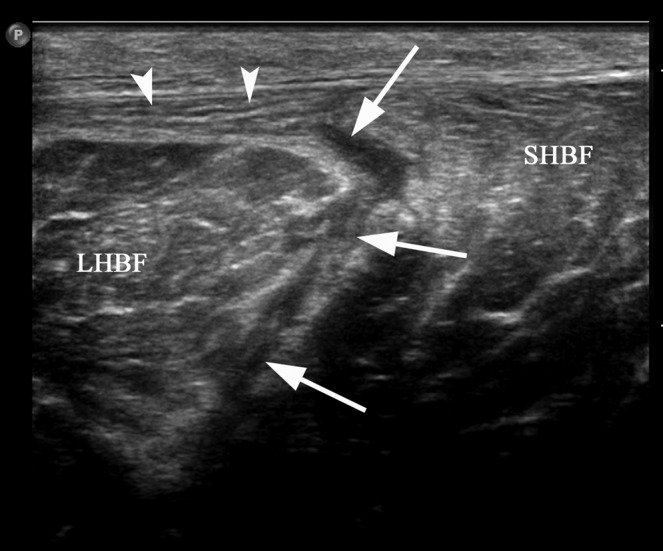
A 24-year-old male professional footballer with grade II hamstring strain. Axial ultrasound image demonstrating fluid (arrows) within the fascial space between the long (LHBF) and short head of biceps (SHBF) muscles and partial stripping of the muscle from the overlying fascia (arrowheads) consistent with a grade II injury.
“Tennis leg”
Partial detachment of medial gastrocnemius at the aponeurosis with soleus muscles is referred to as “tennis leg” [29] (Figure 16). The condition clinically mimics a high Achilles tendon rupture as a palpable and audible “snap”. Deep venous thrombosis is both a differential diagnosis and a complication of this condition and assessment of the calf veins at the time of the scan is mandatory [30].
Figure 16.
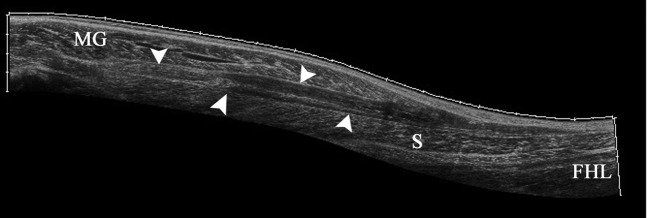
A 36-year-old female netball player with “tennis leg”. Longitudinal extended field of view sonogram of the calf demonstrating fluid (arrowheads) in the fascial space between medial gastrocnemius (MG) and soleus (S) superficial to the flexor hallucis longus (FHL) muscle.
Grade III strain: Peetrons’ “complete rupture”
Ultrasound demonstrates complete discontinuity of the muscle at the musculotendinous junction. The surrounding muscle is hyperechoic and intermuscular, perifascial and subcutaneous fluid collections are common [31] (Figure 17). Surgery is rarely required but has been advocated in avulsion injuries with >2–3 cm of retraction [19].
Figure 17.
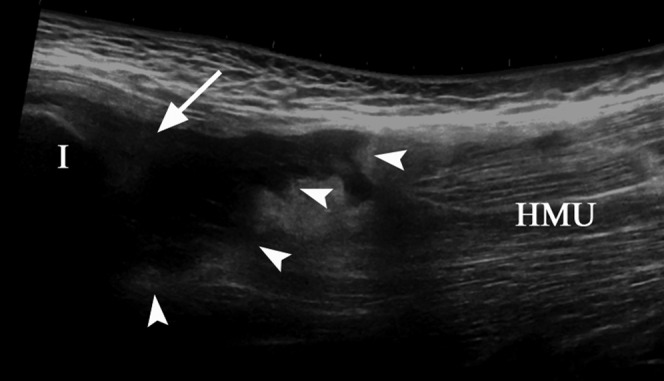
A 26-year-old male professional footballer with grade III hamstring tear. The refracted hamstring muscle unit (HMU) has separated (arrowheads) from the tendons (arrow) attached to the ischial tuberosity (I).
MRI
Grade I strain
Increased signal is seen within the muscle at the injury site on fluid-sensitive fat-suppressed sequences. The high signal is due to oedema and blood radiating from the musculotendinous junction along the muscle fascicles producing a feathery-type pattern within the muscle (Figure 18). There is no significant disruption of muscle architecture (<5% cross-sectional area). As on ultrasound, perifascial fluid may be seen on MRI in grade I injury.
Figure 18.
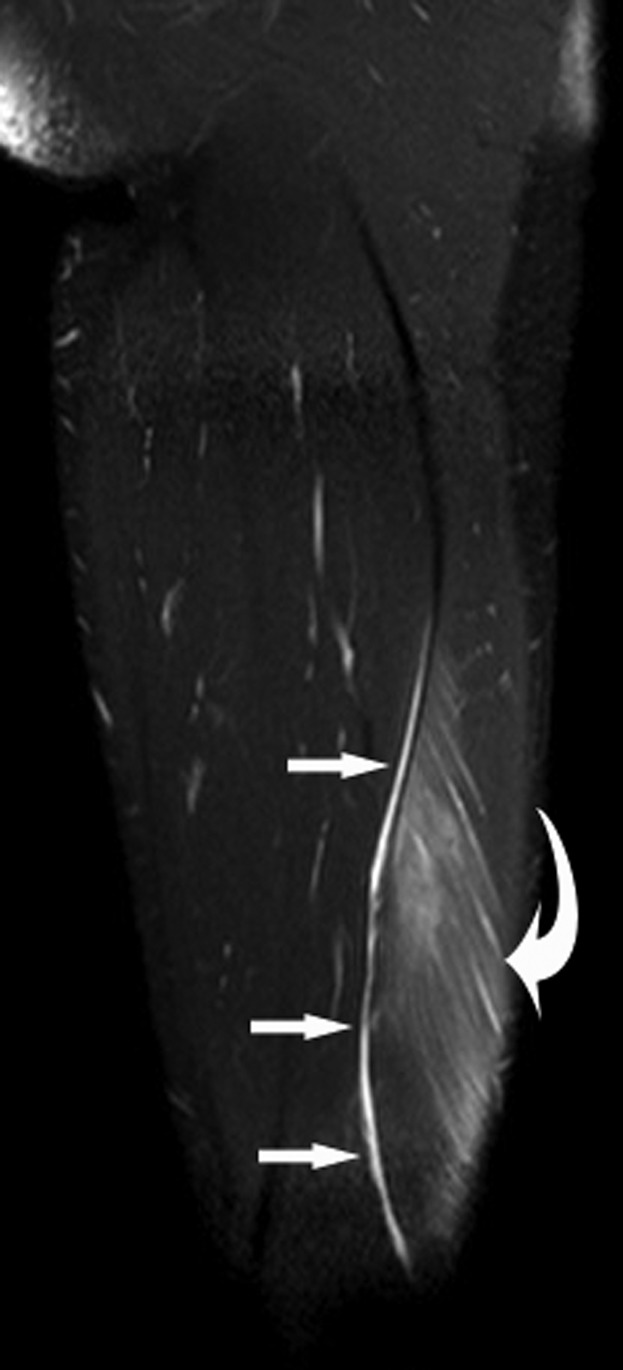
A 28-year-old male professional footballer with grade I distal hamstring strain. Sagittal short tau inversion–recovery MRI of the thigh. Note the feathery high signal along the muscle fibres (curved arrow) and the small slither of fluid in the epifascial space (arrows).
Grade II strain
The key MRI finding in a grade II injury is distortion of normal muscle architecture at the injury site (Figure 19). This results in haematoma formation usually at the musculotendinous junction. The feathery muscle oedema pattern described in grade I injury will also be present. There may be some laxity of the central tendon within the muscle (Figure 20).
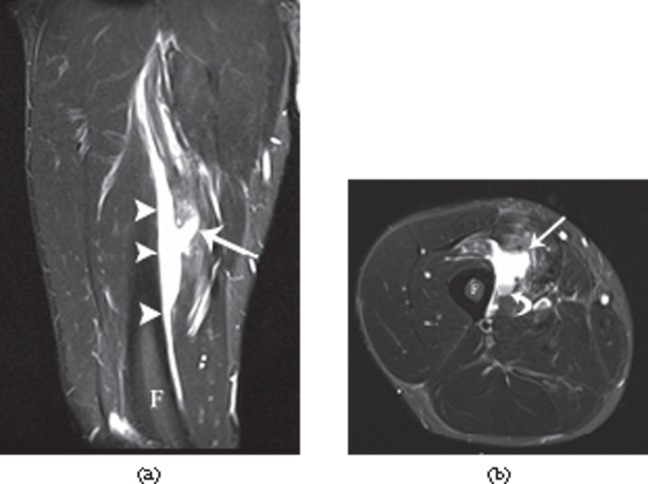
Figure 19.
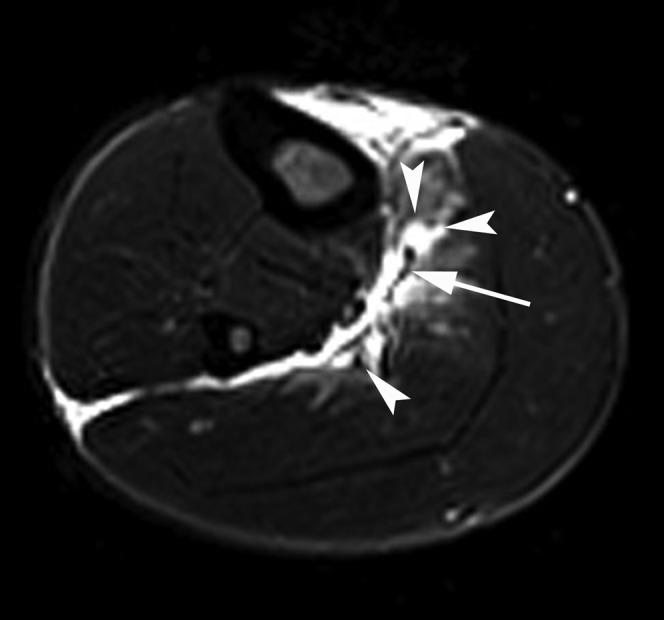
A 29-year-old male professional footballer with grade II calf strain. Axial short tau inversion–recovery MRI of the calf. Note the separation of the muscle (arrowheads) away from the deep soleus tendon (arrow) and the prominent epifascial fluid on the deep, medial and lateral surfaces of the muscle.
Figure 20.
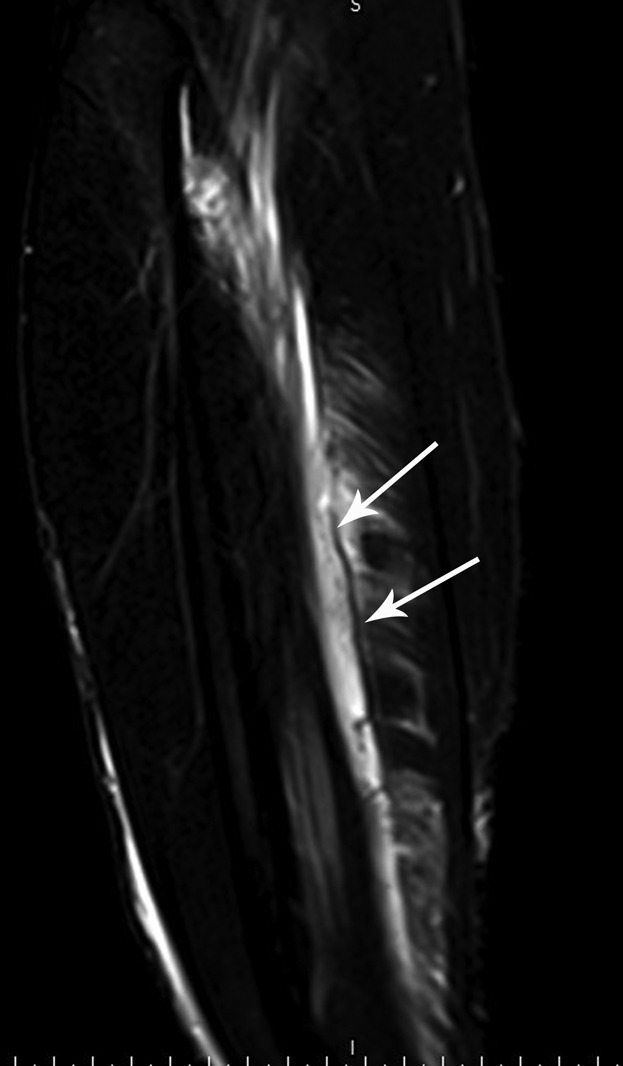
A 29-year-old male professional footballer with grade II calf strain. Coronal fat-saturated proton-density MRI of the calf. There is laxity within the central tendon (arrows) at the epicentre of the muscle injury.
Grade III strain
There is complete disruption of the musculotendinous unit with haematoma filling the space between the two (Figure 21). Grade III injuries are rare compared with grade I and II injuries. A grade IIIB injury is occasionally used to describe avulsion injuries of the musculotendinous unit from the bony attachment. The signal intensity of the haematoma within the muscle will follow the same pattern as that described in Direct muscle injury (above).
Figure 21.
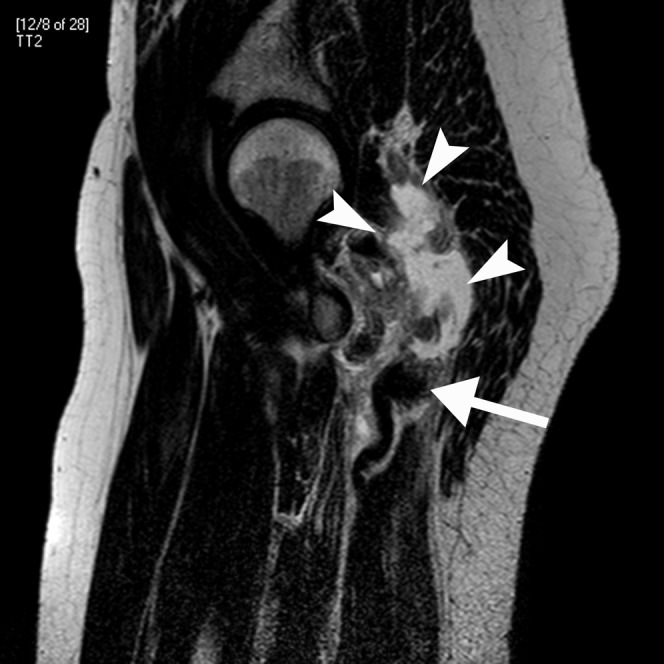
A 38-year-old female footballer with grade III hamstring avulsion. Sagittal T2 weighted MRI. The hamstring tendons have avulsed (arrow) and the tear gap is filled by heterogeneous signal-intensity haematoma (arrowheads).
Delayed onset muscle soreness
Delayed onset muscle soreness (DOMS) is usually observed 12–24 h after unaccustomed strenuous exercise but may also be seen in elite athletes, typically during pre-season when training intensity is increased rapidly or in the early post-rehabilitation phase following injury. The severity of symptoms correlates with the intensity and duration of the activity [32]. The soreness increases to a peak between 24 and 72 h and then subsides by 7 days [33]. MRI may demonstrate increased intramuscular signal on fat-suppressed fluid-sensitive sequences, similar in appearance to grade I muscle strain but typically affecting more than one muscle and possibly more than one compartment [34]. Ultrasound may be normal or demonstrate geographical hyperechogenicity affecting several muscles across different compartments (Figure 22). Imaging alone may not be able to distinguish DOMS from grade I muscle strain and clinical correlation is required. DOMS classically presents at 24–48 h and completely resolves after 4–5 days with conservative treatment, whereas a grade I strain will present acutely and takes 1–2 weeks to resolve.
Figure 22.
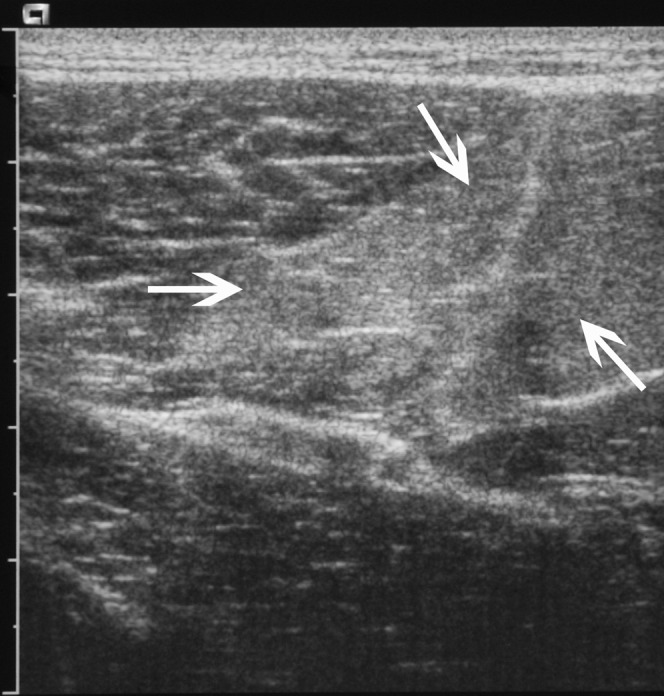
A 26-year-old male rugby player with delayed onset muscle soreness. There is ill-defined increased reflectivity (arrows) within the semitendinosis muscle in the posterior thigh.
Imaging of the complications of muscle injury
Muscle, unlike bone, has limited capacity for regeneration following injury and the majority of the healing is by scar formation [35]. The volume of muscle necrosis and the size of the haematoma will determine the volume of scar formation. Hence, the severity of the injury will be the key determining factor in the subsequent appearances on imaging, irrespective of the mechanism (direct or indirect as described above). The presence of an intramuscular scar alters the normal muscle contraction vector, reducing strength and increasing fatigue [36,37]. The combination of altered muscle biomechanics and increased muscle fatigue results in an increased risk of re-injury [38,39].
Intramuscular scar
On ultrasound, an intramuscular scar appears hyperechoic, or heterogeneous hypoechoic, linear or stellate adherent to the musculotendinous junction (Figure 23) [40]. The stellate form usually follows muscle contusion from a direct blow, whereas the linear form typically follows a muscle strain and commonly surrounds the musculotendinous junction.
Figure 23.
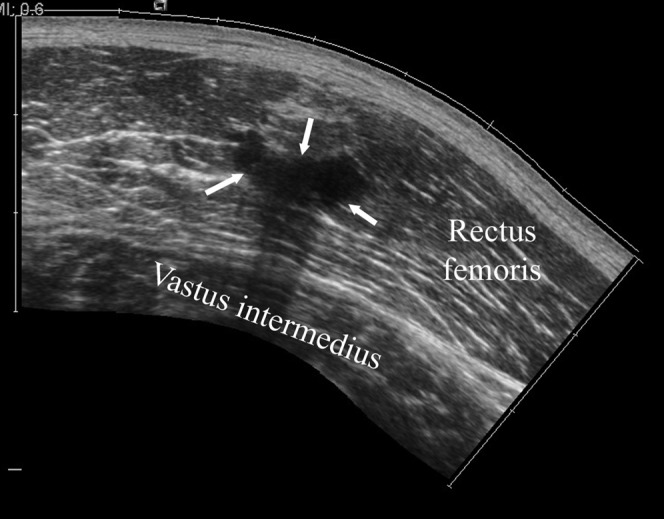
A 29-year-old male professional footballer with intramuscular scar. Longitudinal extended field of view sonogram of the anterior thigh demonstrating a large scar centrally within the rectus femoris muscle (arrows).
On MRI, the scar appears as an area of low signal within the muscle on all sequences adjacent to, or inseparable from, the musculotendinous or myofascial surface (Figure 24). T1 weighted images may reveal local muscle fatty atrophy adjacent to the scar, which may falsely be attributed to a particularly large scar if fat-suppressed imaging is used alone.
Figure 24.
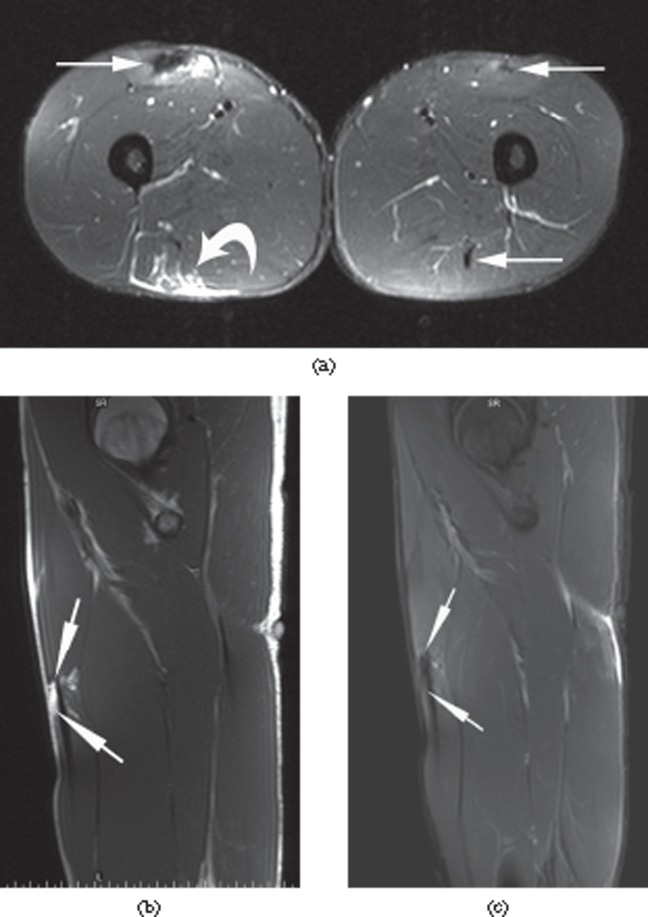
A 27-year-old male professional footballer with acute thigh strain and history of multiple previous thigh strains. (a) Axial short tau inversion–recovery (STIR) MRI demonstrating multiple scars within both thighs (arrows) as well as an acute strain in the right posterior thigh (curved arrow). (b) Sagittal T1 and (c) sagittal STIR imaging through the right thigh demonstrating focal fatty change adjacent to the right rectus femoris scar which fully suppresses on the STIR images (long arrows).
Myositis ossificans
Myositis ossificans may occur following trauma, surgery, burns, neurological insults or immobilisation [41]. However, in the setting of sports-related injuries, direct blunt trauma and muscle haematomas are particularly associated [42,43]. Three distinct phases are recognised clinically: (1) the acute or pseudoinflammatory phase; (2) the subacute or pseudotumoral phase; and (3) the chronic healing phase [44].
Stages 1 and 2 myositis ossificans typically demonstrate non-specific areas of muscle inflammation on both MRI and ultrasound. During the chronic healing phase, osteoid material is laid down at the margin of the haematoma in a lamellar fashion. Peripheral calcification is visible on CT and plain radiographs at approximately 6 weeks and ossification occurs by 6 months (Figure 25) [45]. Sonography detects the early stages of myositis ossificans 2 weeks before radiographic evidence of calcium is evident [46]. Ultrasound initially demonstrates a hypoechoic mass with sheets of peripheral echogenic material (Figure 26) [47,48]. The haematoma element may be aspirated to reduce the size of the lesion (Figure 26). Later, areas of coarse calcification casting acoustic shadows, often parallel to the adjacent diaphysis, may be seen [49]. As mentioned above, the appearances of myositis ossificans on MRI are frequently non-specific and can be easily confused with soft-tissue sarcoma or osteosarcoma. Ultrasound, CT scanning and interval imaging are recommended as percutaneous biopsy may also be misleading.
Figure 25.
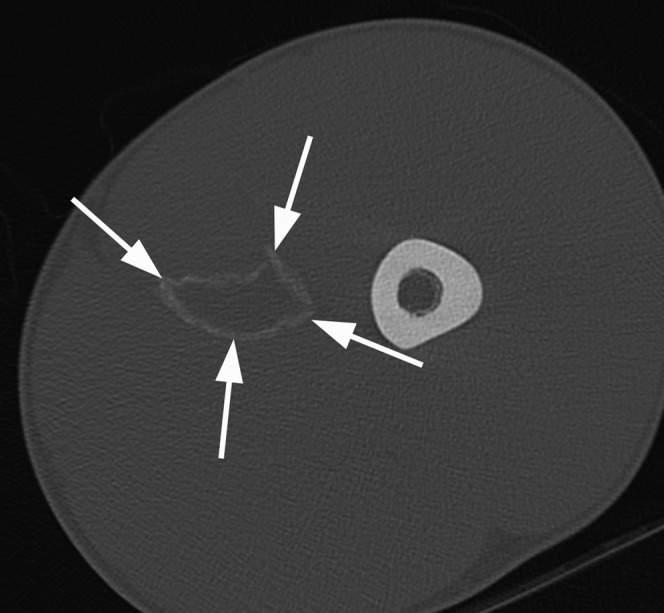
A 17-year-old male professional footballer with myositis ossificans. Axial CT scan of the left thigh demonstrating a rim of mature peripheral calcification (arrows) within the left adductor muscle compartment consistent with myositis ossificans.
Figure 26.
A 25-year-old male rugby player with early myositis ossificans. (a) Longitudinal extended field of view sonogram of the anterior thigh demonstrating a large haematoma (arrows) within the vastus intermedius (VI) deep to rectus femoris (RF). Note the early calcification at the margins of the haematoma (arrowheads). (b) Longitudinal extended field of view sonogram following aspiration of the haematoma so that the gap between the calcification reduces (arrowheads). (c) Sagittal short tau inversion–recovery MRI of the thigh taken 1 week prior to the ultrasound images demonstrates non-specific inflammation within vastus intermedius muscle. Early calcium deposition may be present (curved arrow) and a haematoma is being formed (arrow).
Summary
Muscle injuries are extremely common in elite athletes and are a major cause of loss of competitive playing time. Imaging now plays an ever increasing role in lesion detection, grading and prognosis of muscle injury. MRI is arguably the gold standard imaging technique in muscle injury detection, but ultrasound is being increasingly used in elite sport and, although operator dependent, is readily accessible, cheap and dynamic. Direct and indirect muscle injuries and their complications are readily assessed using either technique.
References
- 1.Junge A, Engebretsen L, Mountjoy ML, Alonso JM, Renström PA, Aubry MJ, et al. Sports injuries during the Summer Olympic Games 2008. Am J Sports Med 2009;37:2165–72 [DOI] [PubMed] [Google Scholar]
- 2.Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997–2000. Br J Sports Med 2002;36:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yard EE, Schroeder MJ, Fields SK, Collins CL, Comstock RD. The epidemiology of United States high school soccer injuries, 2005–2007. Am J Sports Med 2009;37:1798–805 [DOI] [PubMed] [Google Scholar]
- 4.Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med 2004;32:5S–16S [DOI] [PubMed] [Google Scholar]
- 5.Hawkins RD, Hulse MA, Wilkinson C, Hodson A, Gibson M. The association football medical research programme: an audit of injuries in professional football. Br J Sports Med 2001;35:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walden M, Hagglund M, Ekstrand J. UEFA Champions League Study: a prospective study of injuries in professional football during the 2001–2002 season. Br J Sports Med 2005;39:542–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks JHM, Fuller CW, Kemp SPT, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med 2006;34:1297–306 [DOI] [PubMed] [Google Scholar]
- 8.Zaraiskaya T, Kumbhare D, Noseworthy MD. Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging 2006;24:402–8 [DOI] [PubMed] [Google Scholar]
- 9.Lin EC, Middleton WD, Teefey SA. Extended field of view sonography in musculoskeletal imaging. J Ultrasound Med 1999;18:147–52 [DOI] [PubMed] [Google Scholar]
- 10.Connell DA, Schneider-Kolsky ME, Hoving JL, Malara F, Buchbinder R, Koulouris G, et al. Longitudinal study comparing sonographic and MRI assessments if healing hamstring injuries. AJR Am J Roentgenol 2004;183:975–84 [DOI] [PubMed] [Google Scholar]
- 11.Garrett WE., Jr Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc 1990;22:436–43 [PubMed] [Google Scholar]
- 12.Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury with treatment and prognosis. J Bone Joint Surg (Am) 1973;55:95–105 [PubMed] [Google Scholar]
- 13.Aspelin P, Ekberg O, Thorsson O, Wilhelmsson M, Westlin N. Ultrasound examination of soft tissue injury of the lower limb in athletes. Am J Sports Med 1992;20:601–3 [DOI] [PubMed] [Google Scholar]
- 14.Lehto M, Alanen A. Healing of a muscle trauma: correlation of sonographic and histological findings in an experimental study in rats. J Ultrasound Med 1987;6:425–9 [DOI] [PubMed] [Google Scholar]
- 15.Fornage BD. Soft tissue masses: the underutilization of sonography. Semin Musculoskelet Radiol 1999;3:115–34 [DOI] [PubMed] [Google Scholar]
- 16.De Smet AA, Best TM. MR imaging of the distribution and location of acute hamstring injuries in athletes. AJR Am J Roentgenol 2000;174:393–9 [DOI] [PubMed] [Google Scholar]
- 17.Walden M, Hagglund M, Ekstrand J. UEFA Champions League Study: a prospective study of injuries in professional football during the 2001–2002 season. Br J Sports Med 2005;39:542–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan AJ. Quadriceps strain, rupture and Charlie horse. Med Sci Sports Exerc 1969;1:106–11 [Google Scholar]
- 19.O'Donoghue D. Principles in the management of specific injuries. O'Donoghue D. Treatments of injuries to athletes. 4th edn Philadelphia, PA: Saunders; 1984. pp. 39–91 [Google Scholar]
- 20.Pomeranz SJ, Heidt RS. MR imaging of the prognostication of hamstring injury. Radiology 1993;189:897–900 [DOI] [PubMed] [Google Scholar]
- 21.Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR Am J Roentgenol 2002;179:1621–8 [DOI] [PubMed] [Google Scholar]
- 22.Verrall GM, Slavotinek JP, Barnes PG, Fon GT. Diagnostic and prognostic value of clinical findings in 83 athletes with posterior thigh injury. Comparison of clinical findings with magnetic resonance imaging documentation of hamstring muscle strain. Am J Sports Med 2003;31:969–73 [DOI] [PubMed] [Google Scholar]
- 23.Orchard JW. Intrinsic and extrinsic risk factors for muscle strain injuries in Australian football. Am J Sports Med 2001;29:300–3 [DOI] [PubMed] [Google Scholar]
- 24.Orchard JW, Best TM. The management of muscle strain injuries: an early return versus the risk of recurrence. Clin J Sports Med 2002;12:3–5 [DOI] [PubMed] [Google Scholar]
- 25.Koulouris G, Connell DA, Brukner P, Scneider-Kolsky M. Magnetic resonance imaging parameters for assessing risk of recurrent hamstring injuries in elite athletes. Am J Sports Med 2007;35:1500–6 [DOI] [PubMed] [Google Scholar]
- 26.Peetrons P. Echographie musculaire. Feuillets Radiolo 1990;30:217–21 [Google Scholar]
- 27.Peetrons P. Ultrasound of muscle. Eur Radiol 2002;12:35–43 [DOI] [PubMed] [Google Scholar]
- 28.Fornage BD. The case for ultrasound of muscles and tendons. Semin Musculoskelet Radiol 2000;4:375–90 [DOI] [PubMed] [Google Scholar]
- 29.Bianchi S, Martolini C, Abdelwahab IF, Derchi LE, Damiani S. Sonographic evaluation of tears of the gastrocnemius medial head ("tennis leg"). J Ultrasound Med 1998;17:157–62 [DOI] [PubMed] [Google Scholar]
- 30.Delgado GJ, Chung CB, Lektrakul N, Azocar P, Botte MJ, Coria D, et al. Tennis leg: clinical US study of 141 patients and anatomic investigation of four cadavers with MR imaging and US. Radiology 2002;224:112–19 [DOI] [PubMed] [Google Scholar]
- 31.Fornage BD, Touche H, Segal P, Rifkin MD. Ultrasonography in the evaluation of muscular trauma. J Ultrasound Med 1983;2:549–54 [DOI] [PubMed] [Google Scholar]
- 32.Abraham WM. Factors in delayed muscle soreness. Med Sci Sports 1977;9:11–20 [PubMed] [Google Scholar]
- 33.Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 1984;16:529–38 [PubMed] [Google Scholar]
- 34.Nurenberg P, Giddings CJ, Stray-Gundersen, Fleckenstein JL, Gonyea WJ, Pechok RM. MR imaging-guided muscle biopsy for correlation of increased signal intensity with ultrastructural change and delayed-onset muscle soreness after exercise. Radiology 1992;184:865–69 [DOI] [PubMed] [Google Scholar]
- 35.Hurme T, Kalimo H, Lehto M, Jarvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Med Sci Sports Exerc 1991;23:801–10 [PubMed] [Google Scholar]
- 36.Taylor DC, Dalton JD, Seaber AV, Garrett WE. Experimental muscle strain injury. Early functional and structural deficits and the increased risk of reinjury. Am J Sports Med 1993;21:190–4 [DOI] [PubMed] [Google Scholar]
- 37.Speer KP, Lohnes J, Garrett WE. Radiographic imaging of muscle strain injury. Am J Sports Med 1993;21:89–95 [DOI] [PubMed] [Google Scholar]
- 38.Crosier JL. Factors associated with recurrent hamstring injuries. Sports Med 2004;34:681–95 [DOI] [PubMed] [Google Scholar]
- 39.Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Spriggins AJ. Clinical risk factors for hamstring muscle strain: a prospective study with correlation of injury by magnetic resonance imaging. Br J Sports Med 2001;35:435–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JC, Healy JC. Sonography of lower limb muscle injury. AJR Am J Roentgenol 2004;182:341–51 [DOI] [PubMed] [Google Scholar]
- 41.Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathological correlation. AJR Am J Roentgenol 1991;157:1243–8 [DOI] [PubMed] [Google Scholar]
- 42.Huss CD, Puhl JJ. Myositis ossificans of the upper arm. Am J Sports Med 1980;8:419–24 [DOI] [PubMed] [Google Scholar]
- 43.Kransdorf MJ, Murphey MD, Extraskeletal osseous and cartilaginous tumors. In: Imaging of soft-tissue tumors. Philadelphia, PA: WB Saunders; 1997. pp. 317–49 [Google Scholar]
- 44.Bouchardy L, Garcia J. Magnetic resonance imaging in the diagnosis of myositis ossificans circumscripta. J Radiol 1994;75:101–10 [PubMed] [Google Scholar]
- 45.Shirkhoda A, Armin AR, Bis KG, Makris J, Irwin RB, Shetty AN. MR imaging of myositis ossificans: variable patterns at different stages. J Magn Reson Imaging 1995;5:287–92 [DOI] [PubMed] [Google Scholar]
- 46.Peetrons P. Ultrasound of muscle. Eur Radiol 2002;12:35–43 [DOI] [PubMed] [Google Scholar]
- 47.Fornage BD, Eftekhari F. Sonographic diagnosis of myositis ossificans. J Ultrasound Med 1989;8:463–6 [DOI] [PubMed] [Google Scholar]
- 48.Bodley R, Jamous A, Short D. Ultrasound in the early diagnosis of heterotopic ossification in patients with spinal injuries. Paraplegia 1993;31:500–6 [DOI] [PubMed] [Google Scholar]
- 49.Peck RJ, Metreweli C. Early myositis ossificans: a new echographic sign. Clin Radiol 1988;39:586–8 [DOI] [PubMed] [Google Scholar]