Abstract
Objective
To evaluate the effect of adaptive iterative dose reduction (AIDR) on image noise and image quality as compared with standard filtered back projection (FBP) in 320-detector row CT coronary angiography (CTCA).
Methods
50 patients (14 females, mean age 68±9 years) who underwent CTCA (100 kV or 120 kV, 400–580 mA) within a single heartbeat were enrolled. Studies were reconstructed with FBP and subsequently AIDR. Image noise, vessel contrast and contrast-to-noise ratio (CNR) in the coronary arteries were evaluated. Overall image quality for coronary arteries was assessed using a five-point scale (1, non-diagnostic; 5, excellent).
Results
All the examinations were performed in a single heartbeat. Image noise in the aorta was significantly lower in data sets reconstructed with AIDR than in those reconstructed with FBP (21.4±3.1 HU vs 36.9±4.5 HU; p<0.001). No significant differences were observed between FBP and AIDR for the mean vessel contrast (HU) in the proximal coronary arteries. Consequently, CNRs in the proximal coronary arteries were higher in the AIDR group than in the FBP group (p<0.001). The mean image quality score was improved by AIDR (3.75±0.38 vs 4.24±0.38; p<0.001).
Conclusion
The use of AIDR reduces image noise and improves image quality in 320-detector row CTCA.
CT coronary angiography (CTCA) is a robust non-invasive imaging modality with high spatial and temporal resolution that enables accurate diagnosis or exclusion of coronary artery disease [1-4]. However, CTCA usually exposes the patient to a substantial amount of radiation (9.4–21.4 mSv) [5-7]. Therefore, several scanning techniques, such as ECG-based tube current modulation, prospective ECG triggering and reduced tube voltage scanning, have been developed to reduce the patient's radiation exposure [6-8]. Reductions of the tube current also lead to lower radiation exposure, as the tube current correlates to dose in a linear fashion. However, lower radiation leads to an increase in CT image noise because the current reconstruction method, filtered back projection (FBP), is unable to consistently generate diagnostic-quality images with reduced tube currents [9].
Recently, the adaptive iterative dose reduction technique has been developed as a new reconstruction algorithm to improve image noise [10-12], and has already been shown to reduce the radiation dose in clinical practice [13-16]. Adaptive iterative dose reduction (AIDR) developed for CT by Toshiba Medical Systems Corporation is a modified iterative reconstruction technique in which the original high-noise image undergoes a number of reconstructions that reduce image noise until the resultant image displays the desired noise level. This technique is expected to reduce the radiation dose for a similar noise level to FBP.
To our knowledge, no study has evaluated the quality of CT images using AIDR. The purpose of this study was to evaluate the effect of AIDR regarding image noise and image quality in comparison with FBP, using the same raw data set for both FBP and AIDR, in 320-detector row CTCA.
Materials and methods
Patients
This study was performed according to the principles of the Declaration of Helsinki, and approved by our institutional review board. Informed consent was obtained from all patients before the CT examination. 50 patients (36 males, 14 females; mean age, 68.2±9.4 years; range, 40–88 years) referred to CTCA for clinical indications were enrolled in this study. Patients who had previous allergic reaction to iodinated contrast material, elevated serum creatinine level (>1.5 mg dl−1) or who were potentially pregnant were excluded.
CT scanning
CT scanning was performed using a 320-detector-row scanner (Aquilion ONE™; Toshiba Medical Systems Corporation, Tokyo, Japan). Patients with a pre-scan heart rate of 65 beats per minute (bpm) or higher were given 20–60 mg of metoprolol (Selokeen; AstraZeneca, Zoetermeer, the Netherlands) orally 1 h before scanning.
The volume of contrast material was adapted to the patient's body weight; all the patients received 0.7 ml kg−1 of non-ionic contrast material (Iomeprol, Iomeron 350 mg ml−1 I; Eisai, Tokyo, Japan) injected at a fixed duration of 10 s, followed by 20 ml of 0.9% saline solution injected at the same flow rate as the contrast material [17]. Using a dual-shot injector, the contrast material and saline solution were injected through a 20-gauge intravenous injection catheter inserted into the antecubital vein. The scan delay was set with the use of automatic bolus-tracking technology (Real Prep technique; Toshiba Medical Systems Corporation). As soon as the single density level in the ascending aorta was enhanced by 150 HU over the baseline, the patient was instructed to take a deep breath and hold it 6 s after triggering, the contrast-enhanced CT scan was performed. Tube voltage and tube current were adapted to individual body mass index (BMI) according to the protocol shown in Table 1. Other scanning parameters were a collimation of 320×0.5 mm, a rotation time of 0.35 s and z-coverage value of 140–160 mm in which the entire heart was scanned in a single rotation.
Table 1. Body mass index (BMI)-adapted scanning protocol.
| BMI (kg m−2) | Voltage (kV) | Current (mA) |
| <20 | 100 | 400 |
| 20–22.4 | 100 | 450 |
| 22.5–24.9 | 100 | 500 |
| 25–27.4 | 120 | 550 |
| >27.5 | 120 | 580 |
All the examinations were performed with dose modulation for visualisation of the myocardial or valve motion throughout the cardiac cycle. The window of full tube current was limited to 65–85% of the time elapsing between two consecutive R waves in an electrocardiogram (R–R interval). Outside the window of full radiation, tube current was reduced by 80%. For evaluation of the coronary arteries, data were reconstructed at 75% of the R–R interval with a slice thickness of 0.5 mm and a reconstruction interval of 0.25 mm, using a medium soft-tissue kernel (FC13). If motion artefacts were still present in this phase, images were reconstructed at each 2% interval around the 5% intervals with the fewest motion artefacts at the mid-level of the heart. The raw data were reconstructed with a standard FBP and subsequently AIDR. The reconstructed image data were transferred to a computer workstation (Zio Station System 610; Ziosoft, Tokyo, Japan) for post-processing.
The effective radiation dose of CTCA was calculated as the dose-length product (DLP) multiplied by a conversion coefficient for the chest (k=0.028 mSv mGy cm−1) [18-21].
Quantitative analysis
The following measurements were performed by one reader, who had 7 years of experience in cardiovascular radiology. Calculations of the contrast-to-noise ratios (CNR) in the proximal right coronary artery (RCA) and left main coronary artery (LMA) were performed as previously described [22,23], and comprised the following steps. First, attenuation was measured in a region of interest (ROI) in the proximal RCA and the LMA. ROIs were drawn to be as large as possible; calcifications, plaques and stenoses were carefully avoided. Vessel contrast was calculated as the difference in mean attenuation between the contrast-enhanced vessel lumen and the adjacent perivascular tissue. Second, image noise was determined as the standard deviation (SD) of the attenuation value in an ROI placed in the ascending aorta. Third, the CNR was calculated as the ratio of vessel contrast over noise.
Qualitative analysis
Coronary arteries were classified according to the guidelines of the American Heart Association (15 segments) [24]. Coronary artery analysis was performed in all vessels with at least a 1.5 mm vessel diameter. Overall image quality was assessed by two experienced radiologists, one with 2 years and one with 7 years of experience in cardiac radiology. Both radiologists were blinded to the clinical information and reconstruction method. In case of a disagreement in the data analysis between the two observers, a final decision was obtained by consensus. Overall image quality was assessed on a five-point rating scale for each coronary artery segment: 5, excellent (absence of motion artefacts or noise-related blurring, and excellent vessel opacification); 4, good (minor motion artefacts or noise-related blurring, and good vessel opacification); 3, acceptable (some motion artefacts or noise-related blurring, and fair vessel opacification); 2, suboptimal (marked motion artefact or noise-related blurring, and poor vessel opacification); 1, non-diagnostic. Images with a score of 3 or higher were considered diagnostic.
Statistical analyses
SPSS® software v. 17.0 (IBM SPSS, Armonk, NY) was used for statistical testing. Differences in image quality parameters (vessel contrast, image noise, CNR and image quality) between the two scanning protocol groups were compared using the Mann–Whitney U-test. Pearson correlation analysis was performed to compare BMI with image noise. All data were expressed as mean±SD, and differences were considered to be statistically significant at p<0.05.
Results
CT scans were successfully performed without complications on all 50 patients. The mean BMI of the study population was 23.9±2.8 (range, 18.0–30.1). 2 patients (4%) were underweight (BMI<18.5), 31 patients (62%) were of normal weight (BMI=18.5–24.9), 16 patients (32%) were overweight (BMI=25–29.9) and 1 patient (2%) was obese (BMI>30). All the examinations were performed within a single heartbeat with ECG gating. The mean heart rate during acquisition of CT scans was 53.1±6.7 bpm (range, 37–65 bpm) and the mean effective radiation dose was 12.5±6.0 mSv.
Quantitative image quality parameters are shown in Table 2. Image noise in the aorta was significantly lower in axial CT images reconstructed with AIDR than in those reconstructed with standard FBP (21.4±3.1 HU vs 36.9±4.5 HU; p<0.001). There were no significant correlations between BMI and image noise in axial CT images reconstructed with AIDR (r=−0.14, p=0.35), or between BMI and those reconstructed with FBP (r=−0.10, p=0.51) (Figure 1). No significant differences were observed between FBP and AIDR for the mean vessel contrast (HU) in the proximal coronary arteries (proximal RCA and LMA). Consequently, CNRs in the proximal coronary arteries were significantly higher for AIDR than for FBP (p<0.001) (Table 2). Using AIDR, image noise reduced by 42.0±3.3% with an increase of CNR by 72.3±11.8% in proximal RCA and by 69.5±13.0% in LMA, when compared with FBP.
Table 2. Quantitative image quality parameters.
| Parameter | FBP | AIDR | p-value |
| Image noise (HU) | 36.9±4.5 | 21.4±3.1 | <0.001 |
| Vessel contrast of the RCA (HU) | 555.7±87.2 | 553.2±88.3 | 0.83 |
| Vessel contrast of the LMA (HU) | 564.7±69.3 | 560.9±74.2 | 0.78 |
| CNR in the RCA | 15.2±2.4 | 26.1±4.0 | <0.001 |
| CNR in the LMA | 15.7±2.1 | 26.5±3.8 | <0.001 |
AIDR, adaptive iterative dose reduction; CNR, contrast-to-noise ratio; FBP, filtered back projection; LMA, left main coronary artery; RCA, right coronary artery.
Data are mean±standard deviation unless otherwise specified.
Figure 1.
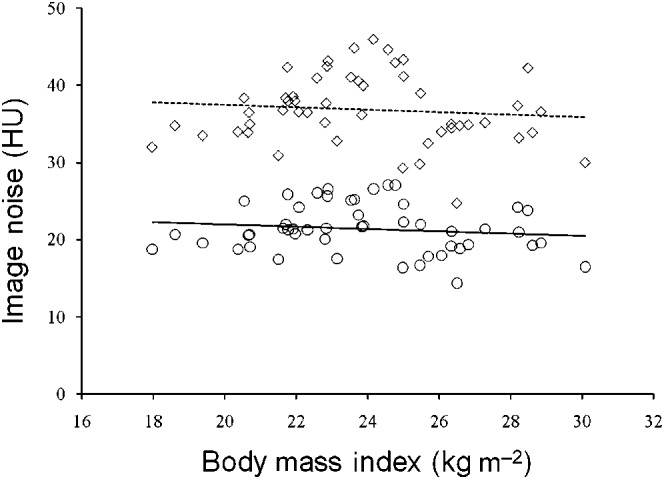
Plots of image noise (HU) vs body mass index (BMI; kg m−2). No significant differences were observed between BMI and image noise in axial CT images reconstructed with filtered back projection (FBP; r=−0.10, p=0.51; dotted line), and between BMI and those reconstructed with adaptive iterative dose reduction (AIDR; r=−0.14, p=0.35; solid line). Diamonds, FBP group; circles, AIDR group.
Results of qualitative assessment of image quality are shown in Table 3. In both reconstruction methods, a total of 630 coronary artery segments with at least a 1.5-mm vessel diameter were available for evaluation. The rate of overall diagnostic image quality in the FBP group was 92.9% of all segments (585/630), while 98.3% of segments (619/630) were diagnostic in the AIDR group. Among 630 segments, 45 (7.1%) were considered non-diagnostic in the FBP group, and 11 (1.7%) in the AIDR group. The mean image quality score was significantly improved by AIDR (3.75±0.38 in the FBP group and 4.24±0.38 in the AIDR group; p<0.001). A representative case is shown in Figure 2.
Table 3. Qualitative assessment of image quality.
| Assessment | FBP | AIDR |
| Image quality score | 3.75±0.38 | 4.24±0.38 |
| Total number of coronary artery segments | 630 | 630 |
| Segments with diagnostic image quality (n=630) | 585 (92.9) | 619 (98.3) |
| Non-diagnostic segments (n=630) | 45 (7.1) | 11 (1.7) |
AIDR, adaptive iterative dose reduction; FBP, filtered back projection.
Data are mean±standard deviation, number, and number (percentage).
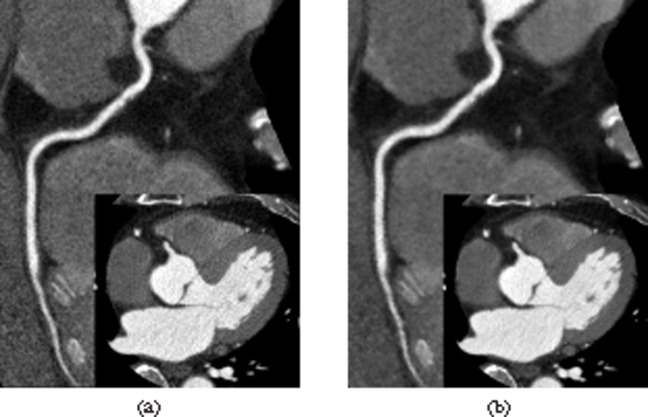
Figure 2.
Comparison of image noise and quality in a 63-year-old man with a body mass index of 28.2 kg m−2. (a, b) Curved-planar reconstruction images of the right coronary artery (RCA) and axial images of the proximal RCA (insets) reconstructed with filtered back projection (a) and adaptive iterative dose reduction (b) algorithms. (a) Image noise of 30.0 HU and vessel contrast in the proximal RCA of 464.6 HU. Image quality was rated as good. (b) Image noise of 16.5 HU and vessel contrast in the proximal RCA of 461.9 HU. Image quality was rated as excellent.
Discussion
This is the first study to evaluate the effect of AIDR on image noise and image quality as compared with standard FBP in 320-detector row CTCA. In this study, the use of AIDR resulted in significant noise reduction with no change of CT attenuation (HU) and vessel contrast, as well as improvement of CNRs and image quality.
Iterative reconstruction techniques have already been used for image reconstruction of single photon emission CT and positron emission tomography because they are insensitive to noise [25-27]. For image reconstruction of CT, FBP has traditionally been used in clinical examination, and iterative reconstruction has not been used because of its huge computational cost. It is reported that the computational cost of iterative reconstruction is about two to three orders of magnitude larger than that of FBP [28]. However, recent advances in computer processing hardware have enabled the clinical use of iterative reconstruction for CT images, and this technique has already been shown to reduce image noise and improve image quality, permitting radiation dose reduction in chest and abdominal CT scans [13-16].
In this study, the use of AIDR reduced image noise by 42%, with an improvement of CNR by approximately 70% when compared with FBP. Qualitative assessment of image quality was also improved by AIDR, probably reflecting this significant decrease in image noise and improved CNR. For other CT systems and manufacturers, there are a few reports on the use of iterative reconstruction techniques for CTCA, adaptive statistical iterative reconstruction (ASIR; GE Healthcare, Waukesha, WI) [29] and iterative reconstruction in image space (IRIS; Siemens Medical Solutions, Forchheim, Germany) [30]. In these reports, image noise was reduced by 17–28%, with no change of CT attenuation or vessel contrast. As the use of AIDR resulted in a high reduction rate of image noise (42%) in our study, this method would also have a potential for radiation dose reduction in CTCA. In the future, we have to evaluate how much radiation dose would be reduced with AIDR to obtain the same noise levels and image qualities as higher dose protocols reconstructed with FBP, and whether the diagnostic accuracy would be maintained when the radiation dose is reduced.
In this study, tube voltage and tube current were adapted to individual BMI. As an increase in BMI confers a higher image noise in CTCA, BMI-adapted tube voltage and current protocol has been introduced to achieve similar image noise for all sizes of patients [31]. In the present study, the tube voltage of 100 kV was used for the patients with BMI <25 and tube current was determined according to the patient's BMI from 400 mA to 580 mA, the maximum setting for this scanner. Since the resulting image noise in data sets reconstructed with AIDR as well as FBP was similar in all patients independent of BMI, the results of this study show that our proposed BMI-adapted parameters proved successful in compensating for BMI, and the use of AIDR reduced image noise in a similar manner in all patients.
We acknowledge the following limitations in this study. First, the small number of patients may restrict the informational value of our results. Future studies with larger patient populations are needed to confirm our preliminary experience. Second, the mean body weight of patients examined in this study is smaller than those of average American and European subjects. Therefore, further studies are required to determine whether our results also apply to heavier patients. Third, coronary attenuation and CNRs were selectively evaluated in the proximal RCA and LMA. Distal segments were not evaluated because the small diameters of distal segments do not allow placement of an ROI without including parts of the vessel wall and adjacent tissue, and thereby causing partial volume effects. Finally, we did not assess the diagnostic accuracy of CTCA images by comparing our findings with the reference standard invasive coronary angiography. Further studies must be aimed at assessment of whether CTCA images reconstructed with AIDR would improve the diagnostic accuracy for the diagnosis and exclusion of coronary artery disease compared with those reconstructed with FBP.
In conclusion, use of AIDR reduces image noise and improves image quality for CTCA performed with a 320-detector row CT scanner, when compared with standard FBP. AIDR would have a potential for further radiation dose reduction in CTCA.
References
- 1.Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol 2005;46:552–7 [DOI] [PubMed] [Google Scholar]
- 2.Nikolaou K, Knez A, Rist C, Wintersperger BJ, Leber A, Johnson T, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol 2006;187:111–17 [DOI] [PubMed] [Google Scholar]
- 3.Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J 2005;26:1482–7 [DOI] [PubMed] [Google Scholar]
- 4.Herzog C, Zwerner PL, Doll JR, Nielsen CD, Nguyen SA, Savino G, et al. Significant coronary artery stenosis: comparison on per-patient and per-vessel or per-segment basis at 64-section CT angiography. Radiology 2007;244:112–20 [DOI] [PubMed] [Google Scholar]
- 5.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–7 [DOI] [PubMed] [Google Scholar]
- 6.Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 2006;113:1305–10 [DOI] [PubMed] [Google Scholar]
- 7.Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, et al. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol 2008;18:1809–17 [DOI] [PubMed] [Google Scholar]
- 8.Earls JP, Berman EL, Urban BA, Curry CA, Lane JL, Jennings RS, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology 2008;246:742–53 [DOI] [PubMed] [Google Scholar]
- 9.Kalra MK, Maher MM, Sahani DV, Blake MA, Hahn PF, Avinash GB, et al. Low-dose CT of the abdomen: evaluation of image improvement with use of noise reduction filters—pilot study. Radiology 2003;228:251–6 [DOI] [PubMed] [Google Scholar]
- 10.Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol 2009;193:764–71 [DOI] [PubMed] [Google Scholar]
- 11.Liu YJ, Zhu PP, Chen B, Wang JY, Yuan QX, Huang WX, et al. A new iterative algorithm to reconstruct the refractive index. Phys Med Biol 2007;52:L5–13 [DOI] [PubMed] [Google Scholar]
- 12.Cheng LCY, Fang T, Tyan J. Fast iterative adaptive reconstruction in low-dose CT imaging. Proceedings of the IEEE International Conference on Image Processing. New York, NY: IEEE, 2006. pp. 889–92 [Google Scholar]
- 13.Prakash P, Kalra MK, Digumarthy SR, Hsieh J, Pien H, Singh S, et al. Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr 2010;34:40–5 [DOI] [PubMed] [Google Scholar]
- 14.Gosling O, Loader R, Venables P, Roobottom C, Rowles N, Bellenger N, et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart 2010;96:922–6 [DOI] [PubMed] [Google Scholar]
- 15.Prakash P, Kalra MK, Kambadakone AK, Pien H, Hsieh J, Blake MA, et al. Reducing abdominal CT radiation dose with adaptive statistical iterative reconstruction technique. Invest Radiol 2010;45:202–10 [DOI] [PubMed] [Google Scholar]
- 16.Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol 2010;195:713–19 [DOI] [PubMed] [Google Scholar]
- 17.Tatsugami F, Matsuki M, Inada Y, Kanazawa S, Nakai G, Takeda Y, et al. Feasibility of low-volume injections of contrast material with a body weight-adapted iodine-dose protocol in 320-detector row coronary CT angiography. Acad Radiol 2010;17:207–11 [DOI] [PubMed] [Google Scholar]
- 18.Gosling O, Loader R, Venables P, Rowles N, Morgan-Hughes G, Roobottom C. Cardiac CT: are we underestimating the dose? A radiation dose study utilizing the 2007 ICRP tissue weighting factors and a cardiac specific scan volume. Clin Radiol 2010;65:1013–17 [DOI] [PubMed] [Google Scholar]
- 19.Huda W, Sterzik A, Tipnis S, Schoepf UJ. Organ doses to adult patients for chest CT. Med Phys 2010;37:842–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einstein AJ, Elliston CD, Arai AE, Chen MY, Mather R, Pearson GD, et al. Radiation dose from single-heartbeat coronary CT angiography performed with a 320-detector row volume scanner. Radiology 2010;254:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 2010;194:881–9 [DOI] [PubMed] [Google Scholar]
- 22.Lembcke A, Wiese TH, Schnorr J, Wagner S, Mews J, Kroencke TJ, et al. Image quality of noninvasive coronary angiography using multislice spiral computed tomography and electron-beam computed tomography: intraindividual comparison in an animal model. Invest Radiol 2004;39:357–64 [DOI] [PubMed] [Google Scholar]
- 23.Achenbach S, Giesler T, Ropers D, Ulzheimer S, Anders K, Wenkel E, et al. Comparison of image quality in contrast-enhanced coronary-artery visualization by electron beam tomography and retrospectively electrocardiogram-gated multislice spiral computed tomography. Invest Radiol 2003;38:119–28 [DOI] [PubMed] [Google Scholar]
- 24.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease: report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5–40 [DOI] [PubMed] [Google Scholar]
- 25.Pretorius PH, King MA, Pan TS, de Vries DJ, Glick SJ, Byrne CL. Reducing the influence of the partial volume effect on SPECT activity quantitation with 3D modelling of spatial resolution in iterative reconstruction. Phys Med Biol 1998;43:407–20 [DOI] [PubMed] [Google Scholar]
- 26.Knesaurek K, Machac J, Vallabhajosula S, Buchsbaum MS. A new iterative reconstruction technique for attenuation correction in high-resolution positron emission tomography. Eur J Nucl Med 1996;23:656–61 [DOI] [PubMed] [Google Scholar]
- 27.Riddell C, Carson RE, Carrasquillo JA, Libutti SK, Danforth DN, Whatley M, et al. Noise reduction in oncology FDG PET images by iterative reconstruction: a quantitative assessment. J Nucl Med 2001;42:1316–23 [PubMed] [Google Scholar]
- 28.Wang G, Yu H, De Man B. An outlook on X-ray CT research and development. Med Phys 2008;35:1051–64 [DOI] [PubMed] [Google Scholar]
- 29.Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 2010;195:649–54 [DOI] [PubMed] [Google Scholar]
- 30.Bittencourt MS, Schmidt B, Seltmann M, Muschiol G, Ropers D, Daniel WG, et al. Iterative reconstruction in image space (IRIS) in cardiac computed tomography: initial experience. Int J Cardiovasc Imaging 2011;27:1081–7 [DOI] [PubMed] [Google Scholar]
- 31.Tatsugami F, Husmann L, Herzog BA, Burkhard N, Valenta I, Gaemperli O, et al. Evaluation of a body mass index-adapted protocol for low-dose 64-MDCT coronary angiography with prospective ECG triggering. AJR Am J Roentgenol 2009;192:635–8 [DOI] [PubMed] [Google Scholar]