Abstract
Objective
This study evaluated the efficacy and safety of flecainide in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) on top of conventional drug therapy.
Background
CPVT is an inherited arrhythmia syndrome caused by gene mutations that destabilize cardiac ryanodine receptor Ca2+ release channels. Sudden death is incompletely prevented by conventional drug therapy with β-blockers +/− Ca2+ channel blockers. The anti-arrhythmic agent flecainide directly targets the molecular defect in CPVT by inhibiting premature Ca2+ release and triggered beats in vitro.
Methods
We collected data from every consecutive genotype-positive CPVT patient started on flecainide at eight international centers before November 13, 2009. The primary outcome measure was the reduction of ventricular arrhythmias during exercise testing.
Results
Thirty-three patients received flecainide because of exercise-induced ventricular arrhythmias despite conventional, for different reasons not always optimal, therapy (median age of 25 years, range 7 to 68; 70% female). Exercise tests comparing flecainide with conventional therapy alone were available in 29 patients. Twenty-two (76%) patients had either a partial (n=8) or complete (n=14) suppression of exercise-induced ventricular arrhythmias by flecainide (p<0.001). No patient experienced worsening of exercise-induced ventricular arrhythmias. Median daily flecainide dose in responders was 150 mg (range 100 to 300 mg). During a median follow-up of 20 months (range 12 to 40) one patient experienced ICD shocks for polymorphic ventricular arrhythmias, which was associated with low flecainide levels. In one patient, flecainide successfully suppressed exerciseinduced ventricular arrhythmias for 29 years.
Conclusions
Flecainide reduced exercise-induced ventricular arrhythmias patients with CPVT uncontrolled by conventional drug therapy.
Keywords: antiarrhythmia agents, catecholaminergic polymorphic ventricular tachycardia, ventricular arrhythmia
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a malignant inherited arrhythmia syndrome, characterized by physical or emotional stress-induced bidirectional or polymorphic ventricular tachycardia (VT) in structurally normal hearts, with a high fatal event rate in untreated patients (1–3). Approximately 60% of CPVT patients have mutations in genes encoding the cardiac ryanodine receptor Ca2+ release channel (RYR2) or cardiac calsequestrin (CASQ2) (4–6), and these cause spontaneous RyR2 channel openings (7,8). The ensuing rise in cytosolic Ca2+ triggers delayed afterdepolarizations, ventricular premature beats (VPBs), and VT, especially under conditions of β-adrenergic stimulation (9,10).
Hence, β-blockers are considered first-line therapy, but unfortunately they are not completely effective in preventing life-threatening arrhythmias (1–3,11–16). An implantable cardioverter-defibrillator (ICD) is often used in patients with ventricular arrhythmias on β-blocker. However, ICDs are not fully protective, and can be proarrhythmic in CPVT patients, because both appropriate and inappropriate ICD shocks can trigger catecholamine release, subsequently resulting in multiple shocks (“arrhythmic storm”), and death (17,18). Thus, additional therapy is desired for CPVT. Small case series show that left cardiac sympathetic denervation is effective in patients who are insufficiently protected by β-blocker therapy and/or receiving too many ICD shocks (19–22).
Recently, we discovered that the antiarrhythmic agent flecainide directly blocks RyR2 channels, prevents RyR2-mediated premature Ca2+ release, and suppresses triggered beats in myocytes isolated from mouse hearts lacking calsequestrin, an animal model of CPVT (23). This effect is not mediated by Na+ channel block, the conventional mode of action thought to underlie flecainide activity, but rather can be attributed to open state block of RyR2 channels; that is, flecainide directly targets the molecular defect responsible for the arrhythmogenic Ca2+ waves that trigger CPVT in vivo (24). In preliminary work, flecainide also appeared to be effective in two highly symptomatic CPVT patients (23).
Here we collate the data from every CPVT patient started on flecainide at eight international centers and report on the efficacy and safety of flecainide treatment in CPVT.
Methods
Participants and study design
To better understand the efficacy and safety of flecainide in CPVT, we reviewed the chart of every consecutive CPVT patient in whom flecainide was started at eight tertiary referral centers in the Netherlands, Canada, France, Israel, Japan, and the United States before November 13, 2009. All patients had a clinical diagnosis of CPVT (based on exercise-induced bidirectional or polymorphic VT in the absence of structural cardiac disease) and a putative pathogenic mutation in the gene encoding RYR2 or CASQ2. Decisions on flecainide starting dose and dosing increases were made by the treating physician as part of specialized clinical care. Data collection and analysis was done retrospectively by chart review and was approved by the review boards at each participating institution.
Primary and secondary outcome measures
Couplets or VT during exercise are significantly associated with future arrhythmic events in CPVT (2). Since all patients were monitored by repeat exercise testing as part of routine clinical care, we used the reduction of ventricular arrhythmias during exercise testing as the primary outcome measure. The effect of flecainide was quantified by comparing the ventricular arrhythmia score (see below) of the last exercise test on conventional therapy with the ventricular arrhythmia score of the first exercise test after a minimum of five days on the stable flecainide dose. Only patients on an unchanged or lower β-blocker dose during flecainide treatment were included in the primary analysis. Depending on the site, exercise testing was performed using treadmill (standard or modified Bruce protocols), or bicycle ergometer.
Secondary outcome measures were the incidence of arrhythmic events (defined as syncope, aborted sudden cardiac death, appropriate ICD shocks, and sudden cardiac death), assessment of well-being and side effects of flecainide, and monitoring of proarrhythmic effects of flecainide, in particular QRS duration during exercise and increase in the ventricular arrhythmia burden (25,26).
Definitions of ventricular arrhythmia
Exercise testing was analyzed and scored using the following predefined parameters (modified from Rosso et al.) (27):
Ventricular arrhythmia score, defined by the worst ventricular arrhythmia (1=no or isolated VPBs, 2= bigeminal VPBs and/or frequent VPBs (>10 per minute), 3=couplet, 4=non-sustained VT (NSVT; ≥3 successive VPBs));
Presence of either of the parameters of the ventricular arrhythmia score, and presence of bidirectional VT (>3 successive VPBs with a beat-to-beat alternating right and left QRS axis);
Sinus rate at the onset of ventricular ectopy, most often an isolated VPB;
Maximum number of VPBs during a 10-seconds period;
Ratio of VPBs/sinus beats during the 10-seconds period with the maximum number of VPBs. Reaching a ventricular arrhythmia score of one was considered complete suppression of ventricular arrhythmias. Other ventricular arrhythmia score improvements were considered partial suppression.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median (range), and categorical variables as number (percentage). Related samples were compared using the paired Wilcoxon signed ranks test for continuous and ordinal variables, and the McNemar test for dichotomous variables. Independent continuous variables were compared by means of the Mann-Whitney U test. A two-tailed p value <0.05 was considered statistically significant. Statistical analysis was carried out with SPSS software package, version 15.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 33 genotype-positive CPVT patients from 21 families were started on flecainide at eight tertiary care centers (Table 1). All patients had persistent physical or emotional stress-induced ventricular arrhythmias documented by exercise testing, Holter recordings, or the ICD interrogation, and/or persistent symptoms of palpitations, syncope, cardiac arrest, or appropriate ICD shocks, while on β-blockers +/− Ca2+ channel blockers. Twenty-three (70%) of the patients were female. The median age at the start of flecainide therapy was 25 (range 7 to 68) years. Thirty-one (94%) patients were treated with β-blockers, and four (12%) of them also received Ca2+ channel blockers (Table 1). In one patient (#13), flecainide was stopped because of side effects before exercise testing could be repeated, in another patient (#27) the β-blocker dose was increased during flecainide treatment, and two patients (#7 and #30) did not receive β-blocker therapy when flecainide was started (Table 1). In the remaining 29 patients, exercise tests on combination therapy of flecainide with conventional drugs at unchanged or lower doses were available for analysis. In 17 (59%) patients, baseline exercise testing was performed less than 48 hours before flecainide initiation.
Table 1.
Baseline characteristics and flecainide therapy parameters
| Patient | Sex | Mutation* | Age at first symptom, years |
Proband or relative |
Presenting symptom |
Age at diagnosis, years |
Cardiac arrest |
ICD | Age at baseline, years |
Drug therapy at baseline, mg (mg per kg body weight) |
Indication for starting flecainide treatment |
Daily starting / stable flecainide dose, mg (mg per kg body weight)† |
Follow- up, months |
Response to flecainide treatment |
Side effects of flecainide |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1‡ | Female | A4091T | 5 | Proband | Seizure | 6 | Yes | Yes | 13 | Nadolol 160 (2.4) Verapamil 180 (2.7)§ |
NSVT (on Holter recordings) | 300 (4.5) | 25 | Complete | No |
| 2 | Female | R2401H | 6 | Proband | Syncope | 6 | No | No | 7 | Nadolol 15 (0.9) | NSVT (on Holter recordings) | 96 (5.6) / 120 (7.1) | 22 | No | No |
| 3‡ | Male | CASQ2: 532+1G>A (38) | NA | Relative | None | 3 | No | Yes | 12 | Metoprolol 125 (2.3) Verapamil 120 (2.2)§ |
NSVT (on ICD recordings) + frequent ICD shocks | 100 (1.9) / 150 (2.8) | 28 | Complete | No |
| 4‡ | Female | E4076K | 28 | Relative | Syncope | 31 | No | No | 37 | Metoprolol 100 (1.6) | Couplets + side effects | 100 (1.6) / 150 (2.4) | 23 | Partial | No |
| 5 | Female | S4124G | NA | Relative | None | 31 | No | No | 36 | Bisoprolol 5 (0.08) Verapamil 240 (3.7)§ |
NSVT + side effects | 100 (1.5) / 150 (2.3) | 28 | Partial | No |
| 6 | Female | S4124G | 45 | Proband | Syncope | 50 | No | No | 68 | Bisoprolol 2.5 (0.04) | NSVT + side effects | 75 (1.2) / 150 (2.4) | 13 | Partial | Sinus arrest and dizziness |
| 7 | Female | S4124G | 26 | Relative | Cardiac arrest | 26 | Yes | No | 41 | None|| | NSVT | 150 (2.2) | 22 | Partial | Dizziness |
| 8‡ | Male | S4124G | 8 | Relative | Syncope | 8 | No | No | 10 | Metoprolol 50 (1.9) | Couplets | 50 (1.9) / 100 (3.7) | 22 | Partial | No |
| 9‡ | Male | E4187Q | NA | Proband | None (detected by cardiological examination after SCD son) | 47 | No | No | 53 | Metoprolol 200 (2.4) | NSVT + side effects | 150 (1.7) | 20 | Partial | No |
| 10‡ | Male | E4187Q | NA | Relative | None | 19 | No | Yes | 25 | Metoprolol 200 (2.7) | NSVT | 150 (2.0) | 20 | No | No |
| 11‡ | Female | E4187Q | NA | Relative | None | 14 | No | Yes | 20 | Metoprolol 150 (2.6) | NSVT | 100 (1.8) | 20 | Complete | No |
| 12‡ | Male | E4187Q | NA | Relative | None | 11 | No | Yes | 17 | Metoprolol 100 (1.6) | NSVT | 100 (1.6) / 300 (4.8) | 20 | Partial | No |
| 13 | Female | E1724K | 13 | Relative | Syncope | 13 | No | No | 25 | Metoprolol 25 (0.4) | Couplets | 100 (1.3) # | NA# | NA# | Fatigue, dizziness, chest pain |
| 14 | Female | E1724K | 9 | Proband | Syncope | 15 | No | No | 50 | Sotalol 160 (2.1) | Bigeminy/ frequent VPBs + side effects | 100 (1.3) | 20 | No | No |
| 15‡ | Male | R420W | NA | Relative | None | 38 | No | No | 49 | Metoprolol 100 (1.3) | Couplets | 150 (1.9) / 300 (3.9) | 19 | Complete | No |
| 16‡ | Male | R420W | NA | Relative | None | 12 | No | No | 16 | Metoprolol 100 (1.7) | NSVT | 100 (1.7) | 19 | Complete | No |
| 17 | Female | Y4962C | NA | Relative | None | 41 | No | No | 45 | Atenolol 25 (0.4) | NSVT | 150 (2.5) | 12 | Complete | No |
| 18‡ | Female | M2605V, A4510T, 14757-6_7CT>TA | NA | Proband | None (detected by exercise testing at preparticipation screening) | 40 | No | No | 40 | Metoprolol 100 (1.4) | Couplets | 200 (2.9) | 18 | Partial | No |
| 19 | Female | R420W | 33 | Proband | Syncope | 33 | No | Yes | 36 | Bisoprolol 5 (0.08) | Bigeminy/ frequent VPBs | 100 (1.5) | 17 | Complete | No |
| 20 | Male | R420W | NA | Relative | None | 11 | No | No | 12 | Atenolol 25 (0.7) | Couplets | 100 (2.6) | 23 | Complete | No |
| 21‡ | Female | G3946S | 14 | Proband | Syncope | 15 | No | No | 34 | Nadolol 160 (2.7) | Couplets | 200 (3.3) | 18 | Complete | No |
| 22 | Female | R420Q | 14 | Proband | Syncope | 15 | No | Yes | 20 | Bisoprolol 1.25 (0.03) | Couplets | 200 (4.0) | 17 | No | No |
| 23‡ | Female | R2474G | 1 | Proband | Convulsion without fever | 11 | No | Yes | 18 | Atenolol 100 (2.1) Verapamil 120 (2.6) |
NSVT | 150 (3.2) | 20 | Complete | No |
| 24 | Female | R420W | NA | Relative | None | 20 | Yes | No | 24 | Metoprolol 25 (0.4)** | Bigeminy/ frequent VPBs + side effects | 100 (1.8) | 17 | Complete | No |
| 25 | Female | E1724K | 10 | Proband | Syncope | 31 | No | No | 39 | Carvedilol 2.5 (0.05) | NSVT | 100 (2.2) | 14 | Partial | No |
| 26‡ | Female | F2215L | 5 | Proband | Cardiac arrest | 10 | Yes | No | 24 | Propranolol 140 (2.8) | NSVT (on Holter recordings) + syncope + palpitations | 100 (2.0) | 13 | No | No |
| 27 | Female | R4157H | 56 | Relative | Palpitations | 57 | No | Yes | 57 | Bisoprolol 5 (0.08) †† | NSVT | 150 (2.3) | 31 | NA†† | No |
| 28 | Female | M3978I | 14 | Relative | Syncope | 15 | No | Yes | 25 | Nadolol 40 (0.7) | Frequent VPBs + syncope | 150 (2.5) | 31 | Complete | Nausea and dizziness |
| 29 | Female | M3978I | 14 | Proband | Syncope | 14 | No | Yes | 26 | Bisoprolol 5 (0.06) ‡‡ | Bigeminy/frequent VPBs | 150 (3.1) | 32 | No | No |
| 30 | Female | M3978I | 13 | Relative | Syncope | 32 | No | No | 45 | None§§ | Bigeminy/frequent VPBs | 150 (2.3) | NA|||| | Partial | Nausea and dizziness |
| 31 | Female | M3978I | 13 | Relative | Syncope | 38 | No | No | 50 | Bisoprolol 5 (0.09) | VPBs + palpitations | 100 (1.8) | NA## | No | Nausea and dizziness |
| 32 | Male | V4771I | 4 | Proband | Syncope with seizure | 18 | No | No | 18 | Sotalol 240 (3.2) | NSVT | 200 (2.7) | 29 years*** | Complete | No |
| 33‡ | Female | R2401H | 9 | Proband | Syncope | 9 | No | Yes | 17 | Nadolol 160 (2.5) | Syncope with VF and arrhythmic storm (recorded on ICD log) | 150 (2.3) | 40 | Complete | No |
| Total | Female: 23 (70%) | RyR2: 32 (97%) | Median: 13 (range, 1–56) | Probands: 15 (45%) | Symptoms: 21 (64%) | Median: 18 (range, 3–57) | Yes: 4 (12%) | Yes: 12 (36%) | Median: 25 (range, 7–68) |
β-blocker: 31 (94%) Ca2+ channel blocker: 4 (12%) |
Severe ventricular arrhythmia: 26 (79%); symptoms: 5 (15%) | Median: 100 (range, 50–300) / 150 (range, 100–300) | Median: 20 (range, 12–40) | Complete: 14/31 (45%); partial: 10/31 (32%) | Yes: 6 (18%) |
RYR2 mutations unless otherwise indicated.
Stable dose was identical to starting dose when only one dose is displayed.
Patients who were treated with a first-line β-blocker at an optimal dose (n=15).
Verapamil was discontinued when flecainide was started.
This patient discontinued β-blocker therapy during three consecutive pregnancies, and thereafter agreed with her treating cardiologist to persistently discontinue β-blocker therapy and avoid exercise.
Flecainide was discontinued within a few days and before exercise testing on flecainide could be performed.
Metoprolol was discontinued and flecainide was started because of intolerable side effects.
This patient was not included in the primary analysis, because the bisoprolol dose was also increased.
This patient discontinued β-blocker therapy on her own initiative after flecainide treatment was started and before an exercise test on combined therapy could be performed. The ventricular arrhythmia score on flecainide monotherapy did not change as compared with the baseline exercise test on β-blocker.
This patient discontinued β-blocker therapy because of side effects.
This patient discontinued flecainide and restarted β-blocker therapy on her own initiative.
This patient discontinued flecainide because of side effects after exercise testing on β-blocker and flecainide was performed.
This patient was excluded from the follow-up calculation.
ICD = implantable cardioverter defibrillator; NSVT = nonsustained ventricular tachycardia; VPB = ventricular premature beat; SCD = sudden cardiac death; NA = not applicable; VF = ventricular fibrillation.
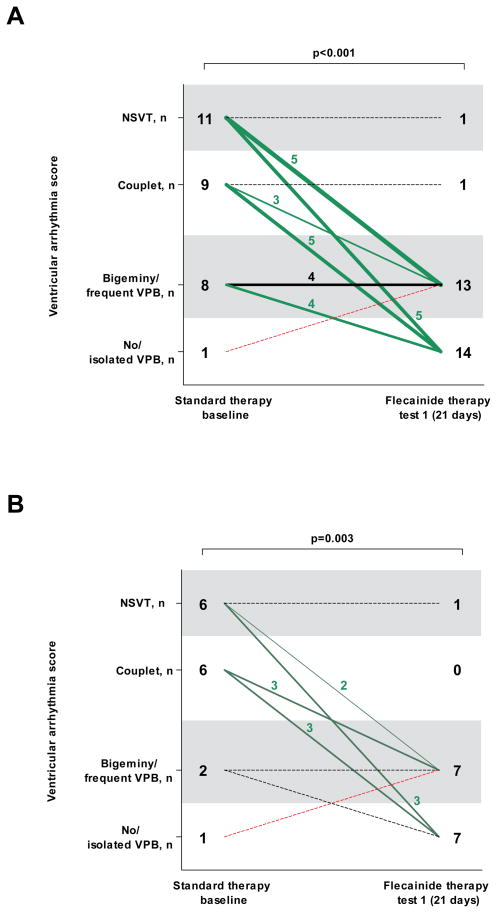
Flecainide therapy reduces exercise-induced ventricular arrhythmias
Flecainide treatment improved the ventricular arrhythmia score in 22 (76%) patients (p<0.001; Figure 1A). Fourteen (48%) patients had complete suppression of ventricular arrhythmias (including seven patients without any VPB), and 8 (28%) had partial suppression. None of the patients experienced significant (ie, couplet or VT) worsening of the exercise-induced ventricular arrhythmia score (Figure 1A).
Figure 1. Ventricular arrhythmia score per patient at the baseline exercise test on standard therapy and at the first exercise test on the final (stable) flecainide dose in the entire cohort (n=29; panel A), and in the patients who were treated with a first-line β-blocker at an optimal dose (N=15, panel B).
NSVT indicates nonsustained ventricular tachycardia; VPB, ventricular premature beat.
The number of patients in each ventricular arrhythmia category and change of ventricular arrhythmia category is shown. The line thickness indicates the number of patients, a dotted line represents one patient. The median time interval between the two tests is shown. All exercise tests were on an unchanged β-blocker dose.
Flecainide treatment also significantly improved all other predefined parameters of exercise-induced ventricular arrhythmia (Table 2). For example, patients on flecainide achieved significantly higher heart rates before ventricular ectopy occurred. Independently, flecainide caused a significant reduction in maximum sinus rate during exercise, even though a higher mean workload was achieved. As expected (28), flecainide prolonged the PR interval (149±21 vs. 160±24 ms; p=0.003), and the QRS duration (83±9 vs. 89±11 ms; p=0.005), but did not change the QTc interval (399±26 vs. 405±19 ms; p=0.171) at rest. These parameters remained within the normal range at rest and during peak exercise in all patients, except for a slightly prolonged resting PR interval (220 ms) in one patient (#20).
Table 2.
Exercise test results at the baseline exercise test on standard therapy and at the first exercise test on the final (stable) flecainide dose
| Variable | Standard therapy baseline (n=29) | First exercise test on stable flecainide dose (n=29) | p value |
|---|---|---|---|
| Time after start flecainide, days | - | 21 (5–363) | - |
| Sinus rate at baseline, beats per minute | 57±10 | 59±9 | 0.061 |
| Sinus rate at maximal exercise, beats per minute | 145±23 | 133±18 | 0.002 |
| Maximum workload attained, METS | 11±3 | 12±4 | 0.042 |
| Sinus rate at onset of ventricular arrhythmias, beats per minute | 113±19 | 118±19 | 0.046* |
| Maximum number of VPBs during a 10-seconds period† | 12±5 | 5±5 | <0.001 |
| Ratio of VPBs to sinus beats during the 10-seconds period with the maximum number of VPBs† | 1.2±0.8 | 0.4±0.4 | <0.001 |
| Isolated VPB | 29 (100) | 22 (76) | 0.016 |
| Bigeminal VPBs | 28 (97) | 13 (45) | <0.001 |
| Frequent VPBs (>10 per minute) | 27 (93) | 14 (48) | 0.001 |
| Couplet | 20 (69) | 2 (7) | <0.001 |
| Nonsustained ventricular tachycardia | 11 (38) | 1 (3) | 0.002 |
| Longest ventricular salvo, VPBs† | 5 (3–9) | 4 | - |
| Bidirectional NSVT | 4 (36) | - | - |
Data are mean ± standard deviation, median (range), or n (%).
Only the 22 patients who still had ventricular ectopy at the first exercise test on the stable flecainide dose were included in this analysis.
Data were available for 28 patients (not available in patient #32).
METS = metabolic equivalents; VPB = ventricular premature beat; NSVT = nonsustained ventricular tachycardia.
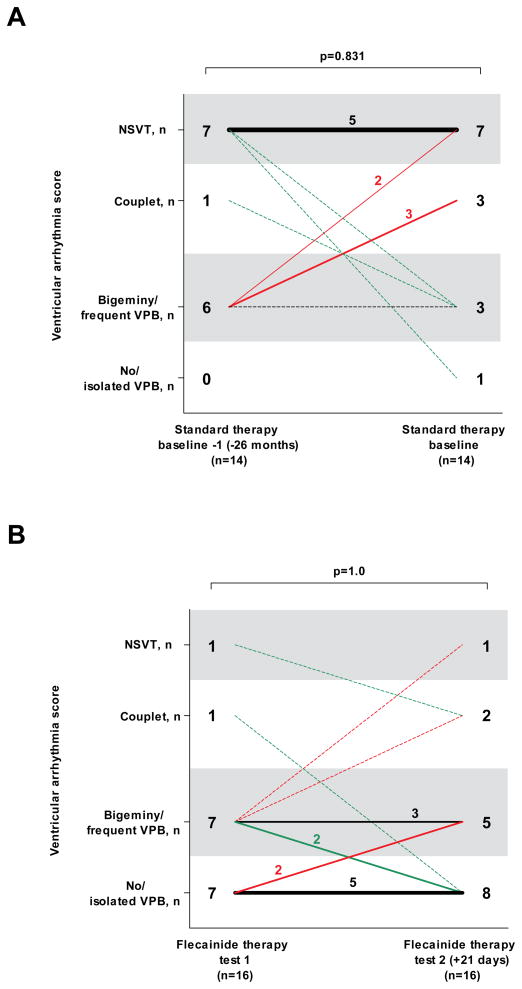
We next assessed the reproducibility of exercise testing as a measure of the ventricular arrhythmia burden in CPVT. While not available for all patients, a subset of patients underwent repeated exercise testing either on the same dose of conventional therapy (n=14), or on the same flecainide dose (n=16). In both cases, the ventricular arrhythmia score of the second exercise test was not statistically different from that of the first exercise test (Figure 2). Similarly, all other predefined parameters of exercise-induced ventricular arrhythmia also did not change significantly (e.g., the maximum number of VPBs during a 10-seconds period was 5±5 at the first exercise test on the stable flecainide dose, and 6±6 at the second exercise test on the same flecainide dose (p=0.556)), suggesting that ventricular arrhythmia scores obtained from exercise testing are reproducible measures of drug efficacy in CPVT and that tachyphylaxis was not present.
Figure 2. Ventricular arrhythmia score per patient at the baseline exercise test and at the previous exercise test on the same standard therapy dose (panel A), and at the first and second exercise test on the final (stable) flecainide dose (panel B).
The number of patients in each ventricular arrhythmia category and change of ventricular arrhythmia category is shown. The line thickness indicates the number of patients, a dotted line represents one patient. The median time interval between the two tests is shown. The standard therapy exercise tests were on the same β-blocker +/− Ca2+ channel blocker doses. All exercise tests on flecainide were on the same, stable flecainide dose in combination with an unchanged or lower β-blocker dose. The sinus rate at maximal exercise at the first and second exercise test on flecainide was not significantly different (140±19 vs. 144±20; p=0.245). However, the two patients with a ventricular arrhythmia score of 4 and 3 at the second exercise test did reach a significantly higher maximum sinus rate as compared with the first exercise test (increase of 32 and 19 beats per minute, respectively).
We found that 14 of the 29 patients included in the primary analysis received drug therapy that could be considered suboptimal (i.e., an unusual β-blocker for CPVT (bisoprolol, carvedilol, or sotalol) or a relatively low β-blocker dose (atenolol, metoprolol, or nadolol <1 mg per kg body weight daily) (2). These patients had either side effects on other β-blockers and/or a higher β-blocker dose, or nadolol was not available in their country. To assess whether flecainide was also effective in CPVT patients on optimal conventional therapy, we next analyzed the 15 patients who were treated with a first-line β-blocker at an optimal dose (Table 1). Flecainide significantly improved the ventricular arrhythmia score (p=0.003; Figure 1B), and all other predefined arrhythmia parameters in this subgroup to a similar extent as in the primary analysis.
The ventricular arrhythmia score in the two patients (#7 and #30) who did not receive β-blocker therapy when flecainide was started improved from NSVT to couplet, and from NSVT to bigeminal VPBs and frequent VPBs, respectively.
Flecainide dose in CPVT
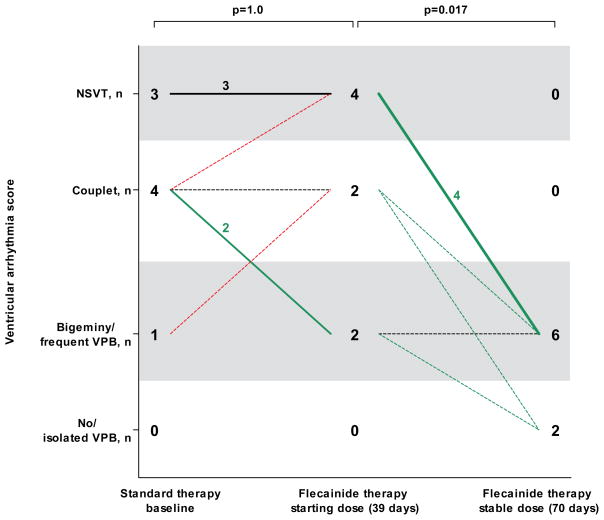
To estimate the optimal dosing of flecainide in CPVT, we analyzed the relationship between starting dose and VT suppression during the first exercise test on flecainide. Patients without suppression of exercise-induced ventricular arrhythmias on the starting flecainide dose received a significantly lower dose (113±39 mg; n=13; p=0.038) compared to patients with either partial (142±38 mg, n=6) or complete ventricular arrhythmia suppression (150±60 mg, n=12). Eight (24%) patients received an increased flecainide dose after the initial exercise test (Table 1). The dose increased from an average daily dose of 96±28 mg to 178±78 mg (range 100 to 300 mg), which resulted in a significant improvement in exercise-induced ventricular arrhythmia score (Figure 3).
Figure 3. Dose-dependence of flecainide in eight CPVT patients who had an increase inflecainide dose.
VPB indicates ventricular premature beat; NSVT, nonsustained ventricular tachycardia.
The number of patients in each ventricular arrhythmia category and change of ventricular arrhythmia category (X-axis) at the last exercise test on the starting flecainide dose (96±28 mg; range 50 to 150 mg) and at the first exercise test on the final (stable) flecainide dose (178±78 mg; range 100 to 300 mg) is shown. The line thickness indicates the number of patients, a dotted line represents one patient. The median time interval from the start of flecainide therapy is displayed. All exercise tests were on an unchanged β-blocker dose.
Clinical follow-up
Three patients (#13, #30 and #31) discontinued flecainide with less than six months of follow-up due to side effects. One patient (#6) required a pacemaker because flecainide exacerbated pre-existing sinus node dysfunction. Flecainide was resumed after pacemaker implantation and this patient was included in the study. In two patients (#7 and #28) the stable flecainide dose was decreased because of dizziness. All other patients tolerated flecainide well without severe side effects. The β-blocker dose was reduced in five patients (#4, #5, #6, #9, and #12) who had a partial suppression of ventricular arrhythmias on flecainide and suffered from side effects of β-blocker therapy (in particular fatigue) before flecainide was started. One patient (#29) refused to take beta-blockers during follow-up, with no worsening of exercise-induced ventricular arrhythmias on flecainide monotherapy.
Thus, 30 out of 33 (91%) patients continued to receive flecainide and were included in the further analysis on the incidence of arrhythmic events. During a median follow-up of 20 months (range 12 to 40 (excluding patient 32#)), VT recurred in only one patient (#1) who experienced several appropriate ICD shocks for polymorphic VT after eight months of flecainide treatment. Her serum flecainide levels were low (0.34 mcg/mL) at the time of the event compared to levels obtained previously (0.75 to 0.82 mcg/mL), suggesting non compliance. She was hospitalized for 48 hours, nadolol and flecainide were resumed at their previous doses, and no further ventricular arrhythmias occurred during a further follow-up of 17 months. The other 29 patients have remained free of arrhythmic events during follow-up. The longest follow-up of 29 years was achieved in patient #32 who presented with exercise-induced VT in 1981. After unsuccessful trials of multiple antiarrhythmic drugs (including mexilitine, amiodarone, propranolol, sotalol, and Ca2+ channel blockers), flecainide (200 mg daily) was added to sotalol (160 mg daily), which resulted in complete suppression of ventricular arrhythmia during exercise testing. Subsequent genotyping revealed a mutation in RYR2. In 2008, an exercise test 48 hours after stopping flecainide and sotalol showed NSVT. After restarting the combined therapy, a subsequent exercise test only showed isolated VPBs, but no VT. In patient #33 flecainide 150 mg daily was started in 2007 because of two syncopes with ventricular fibrillation on the ICD interrogation despite nadolol 240 mg daily. Exercise testing showed complete suppression of ventricular arrhythmias and she has been free from arrhythmic events on flecainide for 40 months.
Discussion
Main findings
Our study demonstrates that flecainide reduces or prevents exercise-induced ventricular arrhythmias in the majority of CPVT patients on conventional drug therapy. These findings are important, because several studies have demonstrated a significant failure rate of current drug therapy (1,3,11–16), including potentially fatal arrhythmic events in 11% of CPVT patients over an 8-year period (2). Based on our clinical experience reported here, flecainide on top of β-blocker therapy should be considered for CPVT patients that otherwise have few alternative therapeutic options. The optimal dose appears to be between 150 to 200 mg daily, with a range from 100 to 300 mg. Daily doses less than 100 mg were associated with a lack of therapeutic response.
Rationale for use of flecainide
CPVT is caused by mutations in the genes encoding RyR2 and CASQ2 (4,5), two proteins that control Ca2+ release from the sarcoplasmic reticulum (SR). As a result of the mutations, Ca2+ is released prematurely and excessively into the cytosol under conditions of catecholaminergic stimulation, generating repetitive spontaneous Ca2+ waves (9,29). The rise of intracellular Ca2+ in turn activates the electrogenic Na+/Ca2+ exchanger (NCX), which produces a transient inward current (ITi). ITi generates delayed afterdepolarizations, which can lead to triggered activity, and the initiation of ventricular arrhythmias (30). Flecainide directly targets the molecular defect in CPVT by inhibiting RyR2 channels and preventing arrhythmogenic Ca2+ waves.(23,24) Flecainide’s Na+ channel blockade further reduces the rate of triggered beats (23,24). This dual action could explain why flecainide is so effective in severe CPVT and provides a rationale for combination therapy with β-blockers. RyR2-mediated SR Ca2+ release importantly regulates the beating rate of sinoatrial nodal cells (31), especially in response to catecholamines (32), and flecainide reduces the rate of spontaneous SR Ca2+ release in myocytes (24). This mechanism may explain why maximum hearts rates were significantly lower in flecainide-treated patients even though workloads were higher compared with baseline exercise testing (Table 2). The reduction in sinus rate during exercise may further contribute to flecainide’s efficacy in CPVT.
Clinical implications
Given the high fatality rate of untreated CPVT patients (1,2), adequate treatment is mandatory and is potentially life-saving. β-blockers are considered first-line therapy. In the largest published series of patients with CPVT, the risk for cardiac arrest (defined as sudden cardiac death, aborted cardiac arrest, and appropriate ICD shocks) despite β-blocker therapy during a mean follow-up period of 8 years was 11% (2). Others have reported very diverse fatal or near-fatal event rates despite β-blocker therapy (1,3,11–16), although the highest event rates may be explained by the predominance of (symptomatic) probands and underdosing of β-blockers. An ICD was recommended for CPVT patients who are survivors of cardiac arrest, or when syncope or sustained VT persists despite maximal tolerable β-blockade (33). Yet, ICDs have potentially harmful effect in CPVT patients (17,18). Moreover, many CPVT patients are children, in whom ICD implantation can lead to significant complications (34). Thus, to avoid ICD implantation and prevent ICD shocks in patients with ICDs, controlling ventricular arrhythmias is of great clinical importance. Alternative therapies are needed for CPVT patients.
Left cardiac sympathetic denervation is an effective alternative when symptoms persist despite β-blockade, but requires surgery, is not universally available, and has only been tested in small cohorts (19–22). The use of Ca2+ channel blockers in addition to β-blockade has been reported to decrease ventricular ectopy in CPVT patients with continuous symptoms and/or exercise-induced ventricular arrhythmias (12,27,35), but is not effective in all patients (27,35,36). From the original six patients treated with verapamil and β-blockers after failure of β-blockers alone, reported by Rosso in 2007 (27), three had clinically significant ventricular arrhythmias during 37±6 months of follow-up (36). Other pharmacologic agents, including Na+ channel blockers, amiodarone, and magnesium, lack of efficacy in CPVT patients (1,12).
In this analysis of all consecutive patients started on flecainide at eight international centers, adding flecainide to standard therapy was effective in further reducing exercise-induced VT and preventing arrhythmic events CPVT patients. To suppress CPVT, adequate dosing of flecainide seems critical. An increased dose may be effective when the initial dose of flecainide fails to suppress VT. Based on these results, flecainide could be added to β-blocker therapy when symptoms or either spontaneous or exercise-induced ventricular arrhythmias persist despite β-blocker.
In our young patient population with no structural heart disease the proarrhythmic effect of flecainide as documented in patients with ischemia and impaired left ventricular function (37) may not be applicable. Consistent with this hypothesis, flecainide did not cause arrhythmic events during a median follow-up of 20 months, which is longer than the mean follow-up of ten months in the Cardiac Arrhythmia Suppression Trial (CAST). The only arrhythmic event was associated with low flecainide serum levels, suggesting that the event was due to the underdosing and not toxicity.
Study limitations
This study reports on our experience of using flecainide in a clinical setting. The number of patients is relatively small, because CPVT is a rare condition and only patients without other treatment alternatives were started on flecainide. However, it is the largest evaluation of a new therapeutic strategy in CPVT patients refractory to current drug therapy, with a median of 20 months follow-up. One patient has received flecainide for 29 years with continuous VT suppression on unchanged doses, and another severely symptomatic patient has been free from arrhythmic events on flecainide for 40 months. Nevertheless, long-term follow-up in more patients would further support the clinical utility of flecainide in CPVT.
Another potential limitation is that we only quantified the effect of flecainide on exercise-induced ventricular arrhythmias, which may not accurately predict fatal arrhythmic events. However, exercise testing is clinically used to guide therapy in CPVT. In a previous study including 70 CPVT patients, exercise-induced couplets or more successive VPBs were significantly associated with future arrhythmic events (sensitivity 0.62; specificity 0.67) (2).
Furthermore, we cannot exclude potential bias introduced by the variability of exercise test results on unchanged treatment, as illustrated in Figure 2. Finally, in 14 patients conventional therapy may be considered suboptimal, as they received an unusual β-blocker for CPVT or a low β-blocker dose for reasons that were previously outlined. However, flecainide was equally effective in the subgroup of CPVT patients who were treated with a first choice β-blocker at an adequate dose (Figure 1B).
Conclusion
In conclusion, our results suggest that flecainide is a safe and effective therapy to reduce ventricular arrhythmias in the majority of CPVT patients who have exercise-induced ventricular arrhythmias despite conventional therapy.
Acknowledgments
Funding Sources
This work was supported by ZorgOnderzoek Nederland Medische Wetenschappen (ZonMW, grant 120610013 to C.W. and A.A.M.W.), by the US National Institutes of Health (grants HL88635, HL71670 to B.C.K, and HL076264 to P.J.K.), by a grant from the Heart and Stroke Foundation of Ontario (grant NA3397 to A.D.K.), by a grant from the French national government named Programme Hospitalier de Recherche Clinique (grant AOR04070, P040411 to A.L.), by a Research Grant for Cardiovascular Diseases (21C-8) from the Ministry of Health, Labour and Welfare, Japan (W.S.), by the American Heart Association Established Investigator Award (grant 0840071N to B.C.K.), and by the Fondation Leducq Trans-Atlantic Network of Excellence, Preventing Sudden Death (grant 05-CVD-01 to A.A.M.W.).
Acknowledgements
None.
Abbreviations
- CASQ2
cardiac calsequestrin
- RYR2
cardiac ryanodine receptor
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- ICD
implantable cardioverter-defibrillator
- NSVT
non-sustained VT
- VPB
ventricular premature beats
- VT
ventricular tachycardia
References
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–9. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–34. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 5.Lahat H, Pras E, Olender T, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–84. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, et al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54:2065–74. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang D, Xiao B, Yang D, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–7. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.di Barletta MR, Viatchenko-Karpinski S, Nori A, et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114:1012–9. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 9.Knollmann BC, Chopra N, Hlaing T, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerrone M, Noujaim SF, Tolkacheva EG, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007;101:1039–48. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauce B, Rampazzo A, Basso C, et al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: early diagnosis of asymptomatic carriers. J Am Coll Cardiol. 2002;40:341–9. doi: 10.1016/s0735-1097(02)01946-0. [DOI] [PubMed] [Google Scholar]
- 12.Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postma AV, Denjoy I, Kamblock J, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–70. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahat H, Eldar M, Levy-Nissenbaum E, et al. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13–21. Circulation. 2001;103:2822–7. doi: 10.1161/01.cir.103.23.2822. [DOI] [PubMed] [Google Scholar]
- 15.Swan H, Piippo K, Viitasalo M, et al. Arrhythmic disorder mapped to chromosome 1q42–q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–42. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 16.Haugaa KH, Leren IS, Berge KE, et al. High prevalence of exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia mutation-positive family members diagnosed by cascade genetic screening. Europace. 2010;12:417–23. doi: 10.1093/europace/eup448. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed U, Gollob MH, Gow RM, Krahn AD. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm. 2006;3:1486–9. doi: 10.1016/j.hrthm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:1319–21. doi: 10.1111/j.1540-8167.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 20.Odero A, Bozzani A, De Ferrari GM, Schwartz PJ. Left cardiac sympathetic denervation for the prevention of life-threatening arrhythmias: the surgical supraclavicular approach to cervicothoracic sympathectomy. Heart Rhythm. 2010;7:1161–5. doi: 10.1016/j.hrthm.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Makanjee B, Gollob MH, Klein GJ, Krahn AD. Ten-year follow-up of cardiac sympathectomy in a young woman with catecholaminergic polymorphic ventricular tachycardia and an implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2009;20:1167–9. doi: 10.1111/j.1540-8167.2009.01441.x. [DOI] [PubMed] [Google Scholar]
- 22.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–9. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilliard FA, Steele DS, Laver D, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastasiou-Nana MI, Anderson JL, Stewart JR, et al. Occurrence of exercise-induced and spontaneous wide complex tachycardia during therapy with flecainide for complex ventricular arrhythmias: a probable proarrhythmic effect. Am Heart J. 1987;113:1071–7. doi: 10.1016/0002-8703(87)90914-8. [DOI] [PubMed] [Google Scholar]
- 26.Katritsis D, Rowland E, O’Nunain S, Shakespeare CF, Poloniecki J, Camm AJ. Effect of flecainide on atrial and ventricular refractoriness and conduction in patients with normal left ventricle. Implications for possible antiarrhythmic and proarrhythmic mechanisms. Eur Heart J. 1995;16:1930–5. doi: 10.1093/oxfordjournals.eurheartj.a060850. [DOI] [PubMed] [Google Scholar]
- 27.Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149–54. doi: 10.1016/j.hrthm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Roden DM, Woosley RL. Drug therapy. Flecainide N Engl J Med. 1986;315:36–41. doi: 10.1056/NEJM198607033150106. [DOI] [PubMed] [Google Scholar]
- 29.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93:531–40. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 30.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–80. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 31.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77:274–84. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
- 32.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–9. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 33.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 34.Sherrid MV, Daubert JP. Risks and challenges of implantable cardioverter-defibrillators in young adults. Prog Cardiovasc Dis. 2008;51:237–63. doi: 10.1016/j.pcad.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Swan H, Laitinen P, Kontula K, Toivonen L. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol. 2005;16:162–6. doi: 10.1046/j.1540-8167.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosso R, Kalman J, Rogowsky O, et al. Long-Term Effectiveness of Beta Blocker and Calcium Blocker Combination Therapy in Patients With CPVT. Heart Rhythm. 2010;7:S423. “abstr”. [Google Scholar]
- 37.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 38.Postma AV, Denjoy I, Hoorntje TM, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]