Abstract
Mesenchymal stem cells (MSCs) derived from bone marrow (BM), adipose tissue (AT), umbilical cord blood (CB), and umbilical cord tissue (CT) are increasingly being used to treat equine inflammatory and degenerative lesions. MSCs modulate the immune system in part through mediator secretion. Animal species and MSC tissue of origin are both important determinants of MSC function. In spite of widespread clinical use, how equine MSCs function to heal tissues is fully unknown. In this study, MSCs derived from BM, AT, CB, and CT were compared for their ability to inhibit lymphocyte proliferation and secrete mediators in response to activation. Five MSC lines from each tissue were isolated. Lymphocyte proliferation was assessed in a mixed leukocyte reaction, and mediator secretion was determined by ELISA. Regardless of tissue of origin, quiescent MSCs did not alter lymphocyte proliferation or secrete mediators, except for transforming growth factor-β (TGF-β1). When stimulated, MSCs of all tissue types decreased lymphocyte proliferation, increased prostaglandin (PGE2) and interleukin-6 (IL-6) secretion, and decreased production of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). BM-MSCs and CB-MSCs also produced nitric oxide (NO), while AT-MSCs and CT-MSCs did not. Equine MSCs did not produce indoleamine 2,3-dioxygenase (IDO). These data suggest that activated equine MSCs derived from BM, AT, CT, and CB secrete high concentration of mediators and are similar to MSCs from rodents and humans in their immunomodulatory profiles. These findings have implication for the treatment of inflammatory lesions dominated by activated lymphocytes and TNF-α and IFN-γ in vivo.
Keywords: Equine, Mesenchymal stem cells, Immunomodulation, Lymphocytes, Bone marrow, Umbilical cord blood, Adipose and umbilical cord tissue
INTRODUCTION
Equine multipotent mesenchymal stem cells (MSCs) are highly proliferative, plastic-adherent, fibroblast-like cells that are capable of osteogenic, chondrogenic, and adipogenic differentiation (10,31,50,52,56,57). Equine MSCs have been isolated from numerous tissues including bone marrow (BM) (3,17,52,55), adipose tissue (AT) (52,56,57), umbilical cord tissue (CT) (13,41,52), and umbilical cord blood (CB) (13,25,26,50). MSCs are utilized in human and veterinary medicine to aid in tissue regeneration and healing. MSCs are thought to assist in healing in part via modulation and down-regulation of the immune response including decreasing the cells and cytokines associated with acute inflammation and increasing blood flow to promote normal healing instead of scarring (36). Currently, the majority of research in adult-derived equine MSC therapy is focused on orthopedic injuries and the regeneration of bone, cartilage, and tendon (18).
Equine MSCs, similar to human and mouse MSCs, express major histocompatibility complex (MHC) class I but do not express MHCII or T-cell costimulatory molecules on their surface (13,28,29). In humans and rodents, MSCs do not stimulate a T-helper cell response but rather suppress T-cell proliferation in mixed leukocyte reactions (MLRs) in vitro (15,24,59). Numerous soluble mediators have been implicated in the inhibition of T-cell proliferation. These soluble factors include prostaglandin E2 (PGE2) (2), transforming growth factor-β (TGF-β1) (20), interleukin-6 (IL-6) (39), nitric oxide (NO) (47), indoleamine 2,3-dioxygenase (IDO) (37), and IL-10 (5). Mediator secretion by MSCs is thought to be stimulated by tumor necrosis factor-α (TNF-α) and interferon gamma (IFN-γ), mediators largely present in inflammatory environments (47). Murine MSCs exposed to IFN-γ become activated and are able to suppress graft versus host disease in vivo (42), while human AT-derived MSCs pretreated with IFN-γ and TNF-α inhibit T-cell proliferation more than non-pretreated MSCs (14). These data suggest that MSCs require activation by proinflammatory mediators to become immunosuppressive.
There may be important differences between species in terms of mechanisms of immunomodulation. For example, murine MSCs secrete NO as a T-cell suppressant, while human MSCs utilize IDO (46). Defining the “immunophenotype” (mediators secreted by MSCs that modulate the immune system) of equine MSCs will help guide the appropriate therapeutic use of MSC in equine medicine. In addition, a more complete understanding of the mechanisms equine MSCs use to modulate the immune system will help establish the best arenas for which horses can serve as a model for human disorders and tissue regeneration.
Interest in adult stem cells derived from CB and CT is increasing for a number of reasons. The collection of BM and AT for MSC isolation is relatively invasive. The number of BM-MSCs decreases with donor age (38) and equine BM-MSCs undergo earlier senescence (30 population doublings) when compared to AT-MSCs and CT-MSCs (60–80 population doublings), which would limit their use in tissue banking (54). Finally, MSCs derived from CB and CT may have increased pluripotentiality (23,27,50). Studies have compared the ability of MSCs isolated from a variety of tissues to modulate the immune system in humans (59), rodents (51), nonhuman primates (6), pigs (11), and dogs (22). Equine MSCs derived from BM, AT, CT, and CB appear to differ in their osteogenic and chondrogenic differentiation ability (7,57); however, to date, there are no equine studies that have compared the ability of MSCs derived from different tissue types to alter lymphocyte proliferation and secrete immunomodulatory mediators.
In this study, we compared the immunomodulatory properties of equine MSCs derived from AT, BM, CT, and CB. We hypothesized that MSCs, irrespective of tissue of origin, secrete immunomodulatory mediators and suppress T-cell proliferation in vitro.
MATERIALS AND METHODS
Tissue Collection
For this study, five samples from each tissue type were obtained from two sources, the Center for Equine Health (CEH) and the Regenerative Medicine Laboratory (RML). Some samples were obtained from adult horses (BM and AT) and foals (CT and CB) housed at the CEH at the University of California, Davis (UCD) according to approved animal care and use protocols. Tissue samples were also obtained from the RML at the William R. Pritchard Veterinary Medical Teaching Hospital, School of Veterinary Medicine, UCD. These tissues were initially submitted to expand MSCs for autologous patient treatment. After full treatment, the remaining MSCs were donated for research (written owner consent obtained). All samples were collected as previously described (4,13,50,52,54,56).
Isolation and Culture of MSCs
CB, CT, BM, and AT were processed exactly as previously described (13,50,52,54). After processing, cells were plated in a tissue culture flask in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 10% equine serum (Hyclone), 1% penicillin-streptomycin (Gibco), and 0.1% Fungizone (Gibco), referred to as MSC medium. Tissue culture was maintained at 37°C in 5% CO2, ambient O2. Cells were passaged at 70% confluence using 0.05% trypsin/EDTA (Gibco), neutralized with MSC medium, and replated in tissue culture flasks. Low passage MSCs were cryopreserved in liquid nitrogen using standard cryopreservation protocols (48).
For experimental use, cryopreserved MSCs were thawed quickly in a 37°C water bath, washed with MSC medium, centrifuged, plated in MSC medium without added Fungizone and expanded at 37°C, 5% CO2 (48). For all experiments, MSCs were between passages 3 and 7 and highly proliferative and did not show the morphological signs associated with senescence.
MSC Characterization
Flow cytometric analysis of surface markers CD29 (Beckman Coulter, Inc., Brea, CA, clone 4B4LDC9LDH8), CD44 (AbD Serotek, Raleigh, NC, USA, clone CVS18), CD90 (VMRD, Inc., Pullman, WA, clone DH24A), MHCI (AbD Serotek, clone CVS22), MHCII (AbD Serotek, clone CVS20), CD86 (BD Pharmigen, clone IT2.2), and F6B (a pan leukocyte antibody, (Dr. Jeffrey Stott, UCD, School of Veterinary Medicine) (32) were completed exactly as previously described (13). Flow cytometry data were analyzed using FlowJo flow cytometry software (Tree Star, Inc., Ashland, OR, USA).
Mixed Leukocyte Reaction (MLR)
Peripheral Blood Mononuclear Cell (PBMC) Isolation
Equine peripheral blood was collected into tubes containing acid-citrate dextrose (ACD; BD Biosciences, Franklin Lakes, NJ) via jugular venipuncture. PBMCs were obtained by mixing 20 ml blood with 15 ml of Dulbecco's phosphate-buffered saline (DPBS, Gibco), then underlaying with 10 ml Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). The blood was centrifuged (500×g, 20 min, no brake), and PBMCs were isolated, washed with DPBS (300×g, 10 min), resuspended in MLR medium (DMEM+10% FBS), and kept on ice until plating.
T-Cell Enrichment
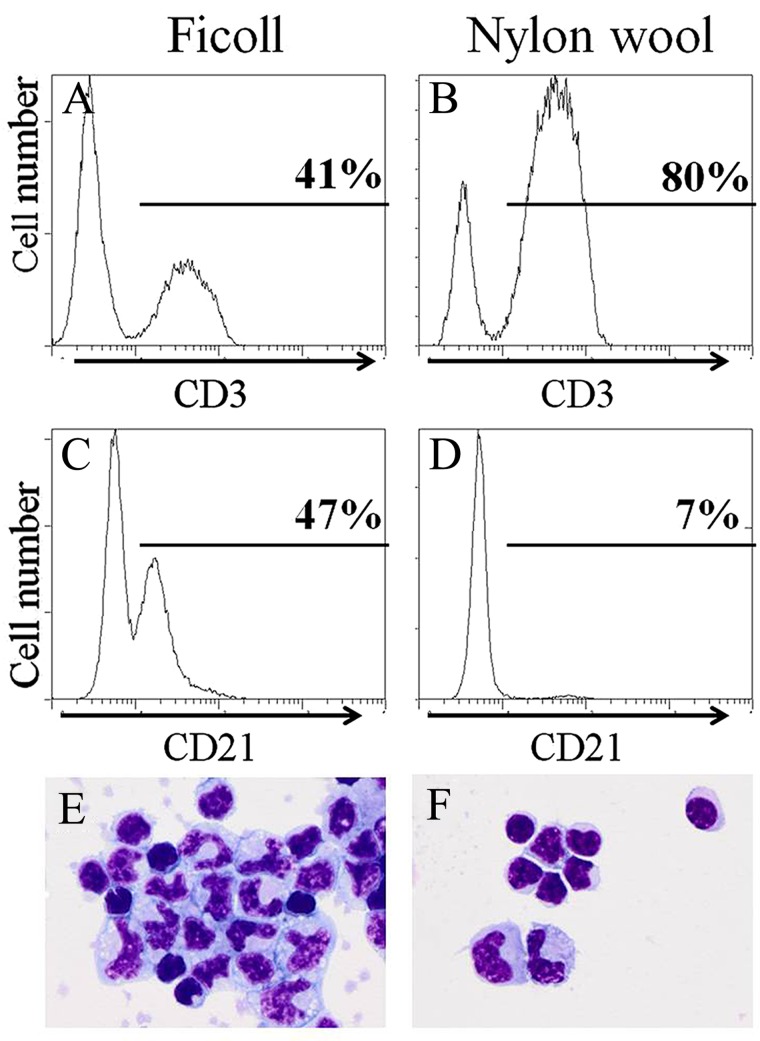
Nylon wool (0.5 g; Polysciences, Inc., Warrington, PA, USA) was loaded into a 12-ml syringe, autoclaved, then soaked with 37°C MLR medium for 1 h prior to the addition of PBMCs. PBMCs were layered over the nylon wool in 2 ml of media and incubated at 37°C for 1 h. The nylon wool was then washed with MLR medium. The flow-through was centrifuged (300×g, 10 min). T-cell-enriched PBMCs were resuspended in 5 ml of cold (4°C) MLR medium and kept on ice until plating. Composition of the cells obtained after T-cell enrichment was not measured for each assay however nylon wool enriched CD3+ T cells from <10% in whole blood (data not shown) to <80–85% post-nylon wool isolation as determined by flow cytometry (Fig. 1A, B; equine CD3+ clone UC-F6G, Dr. Jeffrey Stott, UCD, School of Veterinary Medicine). CD21+ B cells decreased from ∼47% post-Ficoll to ∼7% post-nylon wool treatment (Fig. 1C, D; anti-human CD21, clone B-ly4, BD Pharmigen) (8,16). The remaining CD3-CD21− cells were identified as monocytes (Fig. 1E, F). In one study, T-cell enrichment by nylon wool was shown to alter T-cell activation status through decreased cytokine production (58). Appropriate controls in each experiment were designed (T cells alone) to correct for any baseline activation.
Figure 1.
Representative flow cytometric histograms and cell images depicting T-cell enrichment and B-cell depletion by nylon wool. (A) CD3+ T cells post-Ficoll peripheral blood mononuclear cell (PBMC) isolation. (B) CD3+ T cells post nylon wool isolation. (C) CD21+ B cells post-Ficoll PBMC isolation. (D) CD21+ B cells post-nylon wool isolation. (E) Cells present post-Ficoll PBMC isolation (100×). (F) Cells present post-nylon wool T cell isolation (100×).
Irradiation
To prevent MSCs and PBMCs proliferation, stimulator allogeneic PBMCs and all MSCs were plated at 1×106 cells/ml in cell culture flasks and irradiated (10 Gy, Varian 2100C linear accelerator, Varian Medical Systems, Inc., Palo Alto, CA). Post irradiation, PBMCs and MSCs were washed (300×g, 10 min), resuspended in MLR medium, and kept on ice until plating.
Experimental Setup, Tritiated Thymidine
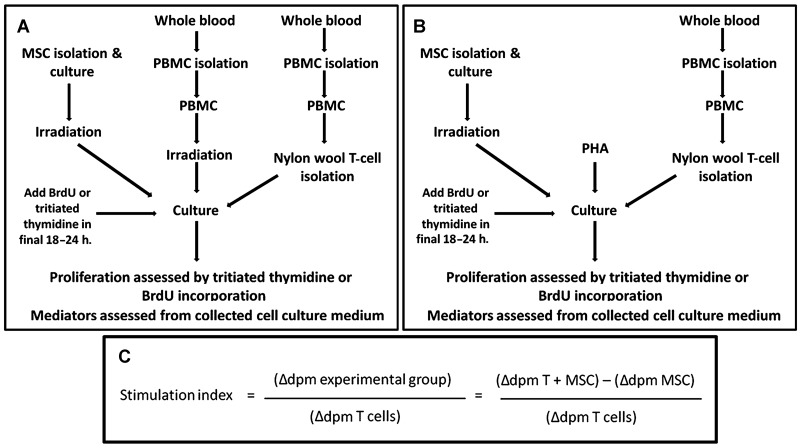
The experimental design for the MLR is depicted in Figure 2. Enriched T cells from experimental horses, allogeneic PBMCs, and MSCs were plated in12-well plates (CellStar, Greiner Bio-one North America, Inc., Monroe, NC) at the ratio of 1:1:10, MSC/irradiated PBMCs/enriched T cells and were incubated at 37°C, 5% CO2 for 4 days. A cell ratio of 1:10 was selected as our initial work determined that a cell ratio of 1 MSC:100 T cells lessened the antiproliferative effects of MSCs on T cells. However, there was no difference in lymphocyte proliferation in cell ratios of 1:1, 1:5, and 1:10 (data not shown). This cell ratio was comparable to the ratios used by others (35,39). In the final 18 h of coculture, cells were treated with 1.5×10−6 Ci/ml of tritiated thymidine (GE Healthcare, Waukesha, WI). Adherent cells were collected with cell scrapers (Costar, Corning, Lowell, MA). All media containing cells were removed, and the cells were pelleted (8,000×g, 5 min). The supernatant was removed, and DNA was isolated according to manufacturer's directions with a prolonged lysis step of 1 h (DNeasy Blood and Tissue Kit, Qiagen, Inc., Valencia, CA). Purified DNA (170 µl) was added to 20 ml of scintillation counting solution (Scintisafe, Fisher Scientific, Pittsburgh, PA) and counted on a liquid scintillation counter (Beckman Coulter) (Fig. 2 A,B).
Figure 2.
Mixed leukocyte reaction (MLR) flow chart and stimulation index calculation. (A) MLR with alloantigen stimulation. (B) MLR with phytohemagglutin (PHA) stimulation. (C) Stimulation index calculation. dpm, disintegrations per minute; BrdU, bromodeoxyuridine; MSC, mesenchymal stem cell.
A stimulation index was calculated by the following formula: mean Δ disintegrations per minute (dpm) of experimental group/mean dpm of experimental enriched T cells alone. Δ dpm was calculated to determine proliferation of experimental enriched T cells without influence of other cells and was defined as dpm of experimental group (e.g., T+MSC)-mean dpm of the nonexperimental factor (e.g., MSCs alone) (9) (Fig. 2C).
Experimental Setup, Bromodeoxyuridine (BrdU) Incorporation
Enriched T cells from experimental horses, allogeneic PBMCs, and MSCs were plated in 24-well plates (CellStar) at a ratio of 1:10 MSCs/enriched T cells and were incubated at 37°C, 5% CO2 for 4 days. In the final 24 h of coculture, cells were treated with BrdU (1 mM) (BD Biosciences). Cells were collected and processed as per manufacturer directions (FITC BrdU Flow Kit, BD Biosciences) and analyzed on a flow cytometer (Cytomics FC500, Beckman Coulter) (Fig. 2A, B).
Mediator Secretion Assay
T-cell-enriched PBMCs stimulated with either allogeneic PBMCs or phytohemagglutin (PHA, Sigma) were incubated with MSCs at a 5:1 T cell/MSC ratio. Cells were plated in12-well plates (CellStar) and incubated at 37°C, 5% CO2. After 4 days of coculture, the media supernatant was removed. Any contaminating cells were pelleted (300×g, 10 min), and the supernatant was aliquoted and frozen at −80° for further analysis.
ELISAs for PGE2 (R&D Systems, Prostaglandin E2 Parameter Assay Kit, Minneapolis, MN) (19), TGF-β1 (R&D Systems, Human TGF-β1 Immunoassay) (33), IFN-γ (R&D Systems, Equine IFN-γ Duoset), and TNF-α (Thermo Scientific, Equine TNFα Screening Set, Waltham, MA) (34) were completed per manufacturer instructions. IL-6 was measured via ELISA exactly as previously described (12). All ELISA plates were read spectrophotometrically on a microplate reader (Synergy HT Multi-Mode, Biotek, Winooski, VT) with Gen5 software (Biotek).
Assay for NO Production
Nitric oxide is readily converted to NO2 in media. Nitrite (NO2 −), the ion of NO2, was measured in media using a Griess reagent system, modified to follow a published technical bulletin (Griess Reagent System, Promega Corporation) (49,53). Briefly, 1 volume of media was combined with 1 volume of 1% sulfanilamide (in 5% phosphoric acid) (Sigma). After 5–10 min, 1 volume of 0.1% napthylethylenediamine dihydrochloride (Sigma) was added. Wells were incubated for at least 5 min and read within 30 min at 540 nm on a microplate reader (Thermomax, Molecular Devices, Inc., Menlo Park, CA). NO2 − concentration was determined using a standard curve.
IDO Assay
IDO catalyses the conversion of tryptophan to N-formyl kynurenine, which is then catabolized to kynurenine. Kynurenine levels are directly proportional to IDO activity. A mediator secretion assay (described above) was performed, with MSC media supplemented with l-tryptophan (Sigma) to a final concentration of 600 µM. Two volumes of cultured media were treated with 1 volume of 30% trichloroacetic acid (Sigma) and centrifuged. Equal parts of trichloroacetic acid-treated supernatant and Ehrlich's reagent (1% p-dimethylaminobenzaldehyde in glacial acetic acid, Sigma) were mixed and read at 490 nm on a microplate reader (Synergy HT Multi-Mode Gen5 software) (1,44).
Statistical Analyses
Results are expressed as median and range. Data were analyzed using Mann-Whitney-Wilcoxon tests with Bonferroni correction to adjust for multiple comparisons (α = 0.05) (StatXact 9, Cytel, Inc., Cambridge, MA, USA).
RESULTS
MSC Characterization
MSCs derived from AT, BM, CT, and CB were uniformly positive for CD29 (average 92.27%, range 89.45–94.04%), CD44 (average 94.68%, range 93.03–96.49%), CD90 (average 91.34%, range 77.18–96.86%), MHCI (average 69.69%, range 49.04–95.82%) and negative for CD86, F6B (equine panleukocyte marker), and MHCII (data not shown). Trilineage differentiation capacity was not assessed for the specific MSCs used in these studies, although our laboratory, along with collaborators, have previously performed differentiation studies on equine MSCs derived in the same manner (13,50,52,55,56).
In the Absence of Activation, MSCs Neither Stimulate Nor Inhibit Baseline Lymphocyte Proliferation
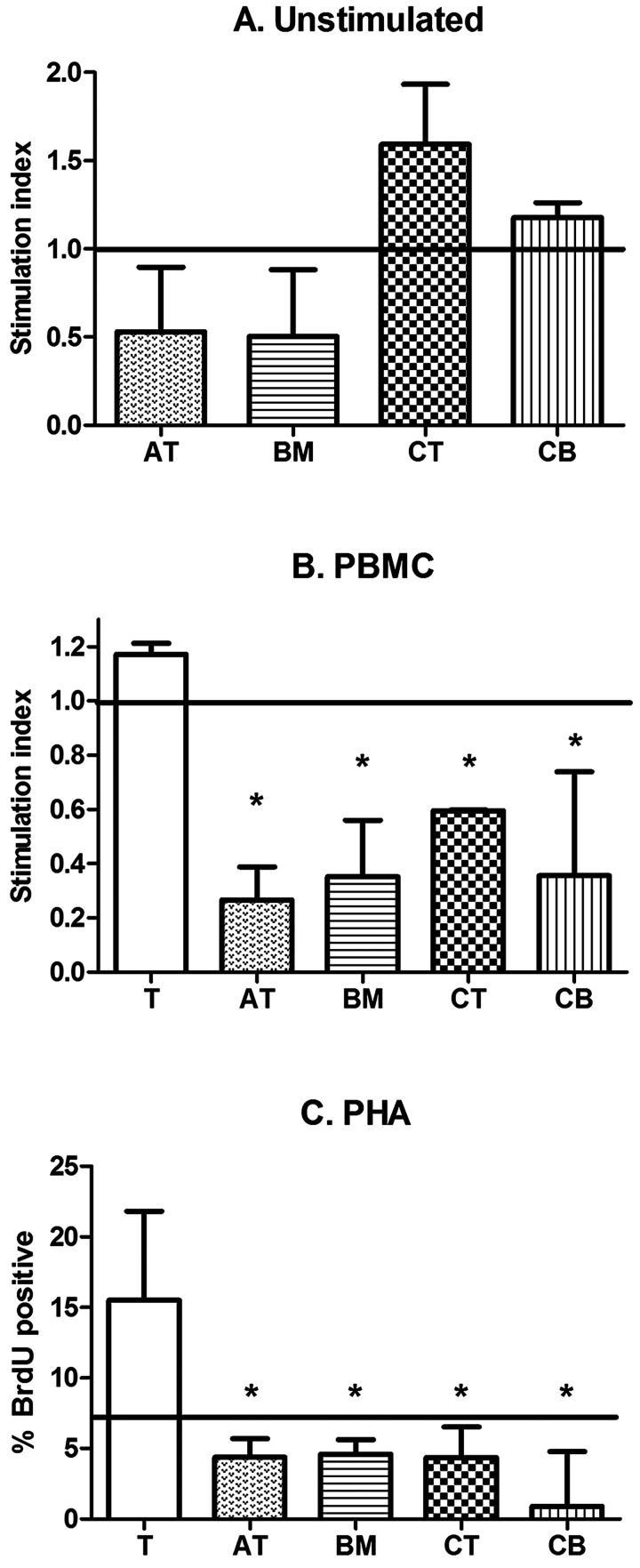
One-way MLRs were performed to determine if MSCs derived from different tissues influenced baseline lymphocyte proliferation. In the absence of stimulation, BM-MSCs and AT-MSCs inhibited T-cell proliferation to approximately half of baseline proliferation (Fig. 3A). CT-MSCs and CB-MSCs neither inhibited nor stimulated baseline T-cell proliferation (Fig. 3A). Although a trend was noted, there was no statistical significance between MSCs derived from different tissues (p=0.06) and there was no statistical difference between MSCs and baseline T-cell proliferation (p>0.05, all comparisons).
Figure 3.
Mixed leukocyte reactions. (A) Equine adipose tissue (AT)-MSCs, bone marrow (BM)-MSCs, cord tissue (CT)-MSCs, and cord blood (CB)-MSCs neither stimulate nor inhibit T-cell proliferation in an unstimulated environment. Equine AT-MSCs, BM-MSCs, CT-MSCs, and CB-MSCs inhibit T-cell proliferation in response to (B) allogeneic PBMCs or (C) mitogen (PHA) stimulation. Data in (A) and (B) are presented as median and range stimulation index value. Baseline unstimulated T-cell proliferation is indicated by the solid horizontal line at index value 1.0. Bars with an asterisk are significantly different in median stimulator index value when compared to PBMC stimulated T cells (p<0.05, Mann-Whitney-Wilcoxon). Data in (C) are presented as median and range % BrdU positive. Baseline unstimulated T-cell proliferation is indicated by the solid horizontal line at 7.0%. Bars with an asterisk are significantly different in median % BrdU-positive cells when compared to PHA-stimulated T cells (p<0.05, Mann-Whitney-Wilcoxon)
MSCs Inhibit Proliferation of Stimulated Lymphocytes
MSCs significantly suppressed T-cell proliferation when stimulated by allogeneic PBMCs (Fig. 3B, p<0.05) or PHA (Fig. 3C, p<0.05). Activated equine BM-, AT-, CT-, or CB-derived MSCs all reduced T-cell proliferation in vitro regardless of stimulatory agent. Lymphocyte proliferation, as measured by tritiated thymidine and BrdU incorporation, was very similar; however, greater variability was noted using tritiated thymidine. As such, we validated BrdU incorporation and switched over completely to this nonradioactive methodology.
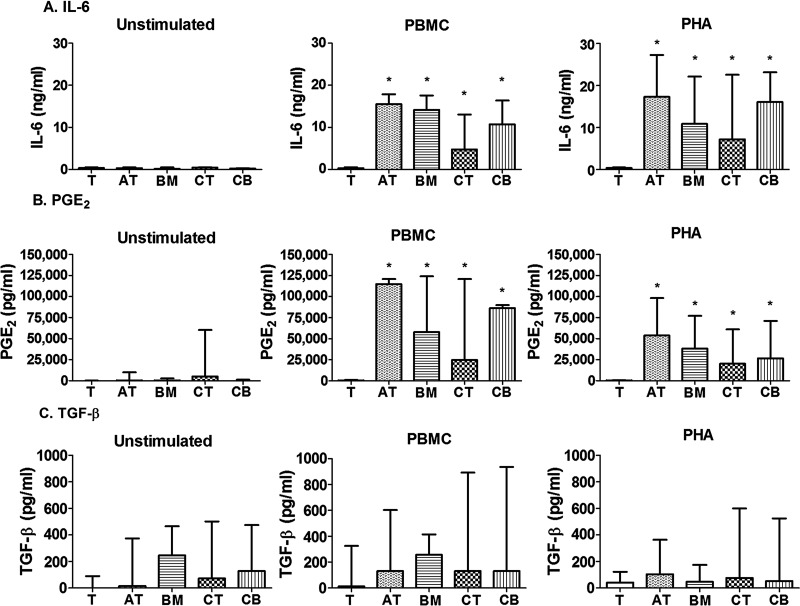
Stimulated MSCs Secrete IL-6 and PGE2 Whereas TGF-β1 Is Constitutively Expressed by All MSCs
Unstimulated T cells, AT-MSCs, BM-MSCs, CT-MSCs, and CB-MSCs did not produce IL-6 (Fig. 4A). Overall, the pattern of mediator secretion in response to PHA and alloantigens (PBMCs) was similar. In alloantigen (PBMC)-stimulated assays, AT-MSCs, BM-MSCs, CT-MSCs, and CB-MSCs secreted significantly more IL-6 when compared to alloantigen-stimulated T cells (Fig. 4A, p<0.05). After incubation with PHA-stimulated T cells, all tissue-derived MSCs secreted significantly more IL-6 when compared to PHA-stimulated T cells (Fig. 4A, p<0.05).
Figure 4.
MSC mediator secretion. (A) interleukin (IL)-6, (B) prostaglandin E2 (PGE2), and (C) transforming growth factor (TGF)-β1 secretion by unstimulated T cells, unstimulated MSCs, stimulated T cells, and stimulated T cells in the presence of MSCs after alloantigen (PBMC) or mitogen (PHA) stimulation. Data are presented as median and range mediator secretion. Bars indicated with an asterisk are significantly different in median mediator secretion from the T-cell control (p<0.05, Mann-Whitney-Wilcoxon).
Unstimulated T cells, AT-MSCs, BM-MSCs, and CB- MSCs did not produce PGE2 (Fig. 4B). Quiescent CT-MSCs produced measurable amounts of PGE2; however, this difference was not statistically significant compared to unstimulated T cells (Fig. 4B). MSCs, regardless of tissue of origin, secreted significantly more PGE2 when incubated with activated T cells compared to activated T cells alone (Fig. 4B, all comparisons, p<0.05). TGF-β1 was constitutively produced by all MSC lines (Fig. 4C), although AT-MSCs did not produce more TGF-β1 than T cells alone (p=0.37). Although TGF-β1 production was generally higher in MSC culture conditions when compared to stimulated T cells alone, a significant increase was not noted (Fig. 4C, p>0.05). TGF-β1 was the only cytokine measured that was not produced in higher quantities by activated MSCs compared to baseline MSCs (Fig. 4C).
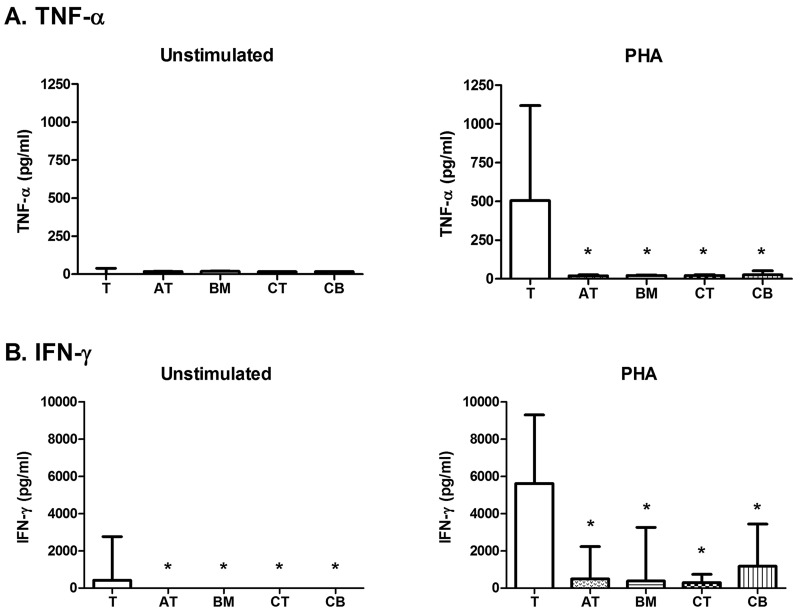
MSCs Decrease TNF-α and IFN-γ Produced by Activated T-Cell-Enriched PBMCs
T cells stimulated with PHA produced high concentrations of TNF-α and IFN-γ when compared to baseline T cells (Fig. 5). MSCs significantly and equally decreased the production of TNF-α by PHA-stimulated T cells, regardless of MSC tissue of origin (Fig. 5A, p<0.05).
Figure 5.
MSC mediator inhibition. (A) tumor necrosis factor (TNF)-α and (B) interferon (IFN)-γ production by unstimulated T cells, unstimulated MSCs, stimulated T cells, and stimulated T cells in the presence of MSCs after mitogen (PHA) stimulation. Data are presented as median and range mediator production. Bars indicated with an asterisk are significantly different in median mediator production from the T-cell control (p<0.05, Mann-Whitney-Wilcoxon).
At baseline, T cells produced a small but measureable amount of IFN-γ whereas unstimulated MSCs did not (Fig. 5B, p<0.05). Stimulated T cells produced increased IFN-γ compared to T cells alone (Fig. 5B). In cultures of MSCs and PHA-stimulated T cells, IFN-γ production by T cells was significantly reduced (Fig. 5B, p<0.01, all comparisons). TNF-α and IFN-γ concentration obtained by PBMC stimulation of T cells was similar to but lower than the amount secreted after PHA stimulation (data not shown).
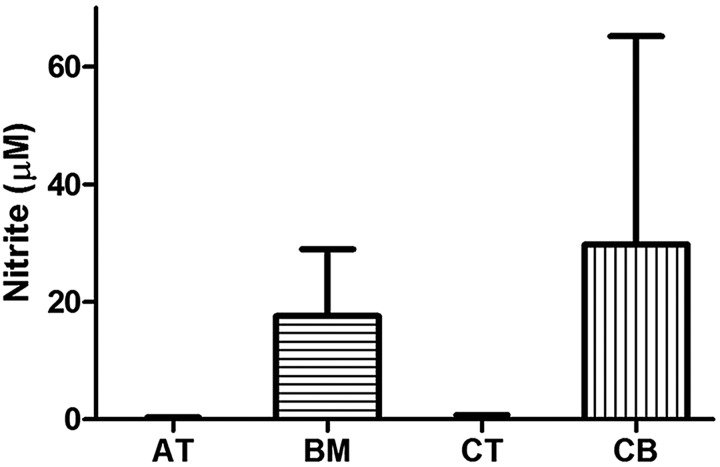
NO Is Produced by Activated BM-MSCs and CB-MSCs, But Not by AT-MSCs or CT-MSCs
Nitrite was only detectable in cultures including BM- and CB-MSCs and not in cultures including AT-MSCs or CT-MSCs (Fig. 6). CB-MSCs and BM-MSCs produced significantly more nitrite than AT- and CT-MSCs (Fig. 6, p<0.001). NO was not produced by T cells stimulated with PHA (data not shown). Production of NO by MSCs was similar with alloantigen (PBMC) stimulation (data not shown).
Figure 6.
Nitrite (NO2 −) production by MSCs after mitogen (PHA) stimulation of T cells. Data are presented as median and range NO2 − production.
IDO Is Not Produced by Equine AT-, BM-, CT-, or CB-MSCs
Kynurenine, a downstream product of IDO-induced tryptophan catabolism, was not produced by AT-, BM-, CT-, or CB-MSCs with or without stimulation by alloantigen or PHA-stimulated T cells (data not shown).
DISCUSSION
MSCs have been shown to have immunomodulatory effects and are currently being used clinically to treat degenerative, immune-mediated, and inflammatory disorders in humans and degenerative and inflammatory lesions in horses. A number of studies have compared the immunomodulatory functions of human MSCs derived from different tissues in vitro (23,24,59). Our data suggest that equine AT-MSCs, BM-MSCs, CT-MSCs, and CB-MSCs are quite similar in terms of in vitro function with only subtle differences in secretion of certain mediators. Our study found that, at baseline, BM-MSC, AT-MSCs, CT-MSCs, and CB-MSCs neither stimulate nor inhibit lymphocyte proliferation and, for the most part, do not secrete measurable concentrations of mediators. These data are compatible with data in other species that suggest that MSCs require ongoing activation or stimulation to modulate immune cells. When activated, equine MSCs, regardless of tissue of origin, increased secretion of PGE2 and IL-6 and exerted an inhibitory effect on T-cell proliferation and cytokine production (TNF-α and IFN-γ). The one parameter by which MSCs differed was the production of NO: BM- and CB-MSCs produced NO, while AT- and CT-MSCs did not. If in vitro data comparing MSC function between MSC types correlate with in vivo function, the selection of tissue from which to derive MSCs will be dependent on the practicality of obtaining, culturing, and banking each tissue or factors, such as differentiation ability, rather than the immunomodulatory mediators measured in this study.
PGE2, IL-6, NO, and IDO have been implicated as factors that decrease T-cell proliferation (2,39,47). Species differences have been noted (46); human MSCs produce IDO, and mouse MSCs produce NO to limit T-cell proliferation. Our work implies that, with the production of NO, horse MSCs may modulate one arm of the immune response similar to mouse MSCs, with NO produced by BM-MSCs and CB-MSCs. The divergence between blood-derived MSCs and solid tissue-derived MSCs, with regards to NO production, has not been previously described. As NO is a short-lived mediator with paracrine effects, lymphocytes must be in close proximity to be affected. NO may play a role in the vasoactive and angiogenic affects noted for MSCs.
TGF-β1 was the single cytokine measured that was constitutively produced by all of our equine MSCs and was not increased upon activation. TGF-β1 concentrations mimicked those produced by unactivated human and mouse BM-MSCs, although it is not yet known whether the TGF-β1 produced by equine MSCs is physiologically relevant (20,21). Similar to our study, TGF-β secretion by human MSCs was not increased by MSC activation. In addition, these authors showed that blocking TGF-β restored T-cell proliferation in an MLR assay (20). Others have shown even lower TGF-β1 concentrations in cultures of human MSCs incubated with stimulated T cells (8 and 60 pg/ml for AT-MSCs and BM-MSCs, respectively), indicating that even constitutive low production of TGF-β1 may be sufficient to alter T-cell proliferation (45). Although not yet demonstrated in horse MSCs, these results suggest that basal secretion of TGF-β1 by MSCs may be sufficient to inhibit T-cell proliferation seen in vitro.
The immunomodulatory capability of all of our MSCs was greatly amplified when incubated with activated T cells. The production of mediators was enhanced in the presence of either allo-activated or mitogen- stimulated T cells when compared to nonactivated T cells. In our assays, MSCs were in contact with lymphocytes. Investigation of the importance of cell-cell contact for the inhibition of lymphocyte proliferation in the equine model has not been investigated. We hypothesize that, in inflammation, activated PBMCs produce TNF-α and IFN-γ. This results in T-cell proliferation and augmented cytokine release. These cytokines stimulate MSC mediator secretion. Mediators secreted by MSCs then act on cells of the immune system and result in decreased lymphocyte proliferation. For in vitro assays, differences in the immunomodulatory function of MSCs exist with the use of purified T cells versus PBMCs as responder cells. Human MSCs did not decrease proinflammatory cytokine production when purified T cells were used in the MLRs (30). T-cell-enriched PBMCs in our MLRs allowed for a more global look at the interaction of other immune cells (including B cells, NK cells, and monocytes) with MSCs in a manner more reflective of the environment encountered by MSCs in vivo.
The known interactions between MSCs and lymphocytes has provided some of the rationale for the use of MSCs with T-cell-mediated diseases (e.g., graft vs. host disease and Crohn's disease). There is currently no information on the efficacy of equine MSCs to treat “autoimmune” or immune-mediated diseases. Our data suggest that MSCs entering an inflammatory niche dominated by TNF-α and IFN-γ may become activated and down-regulate the lymphocyte response to promote healing. It is not yet known if the concentrations of mediators produced by equine MSCs are physiologically relevant or active. This distinction is important as there are discrepancies in animal studies between in vitro immunomodulation and in vivo efficacy (2,40,43). Limitations to our study include a small sample size and narrow scope of reagents available to the equine research market which limit mediator analyses. Further studies are needed to (1) delineate the mechanisms of action for each mediator, (2) determine if in vitro functions correlate with in vivo efficacy, (3) determine the necessity of cell-to-cell contact between MSCs and T cells for immunomodulatory function, and (4) expand our understanding of equine MSC interaction with other cells of the immune system. This is the first study of equine MSC immunophenotype in vitro. These data provide a critical starting point for beginning to dissect out how equine MSCs respond to and alter the inflammatory niche and how the horse may best serve as a large animal model for human diseases.
ACKNOWLEDGMENTS
This project was supported in part by the National Institutes of Health on Aging grant number R43AG033965 under the Small Business Innovation Research (SBIR) Program (ThermoGenesis Corp., Rancho Cordova, CA, USA). Financial support was also provided by the Center for Equine Health and a gift from Mr. Dick and Carolyn Randall. The authors would like to thank Dr. Philip Kass (UCD, Department of Population Health and Reproduction) for assistance with statistical analysis, Dr. Jeffrey Stott and the Stott Lab (UCD, Department of Pathology, Microbiology and Immunology) for the generous gift of CD3 and F6B antibodies, Dr. Jennifer L. Granick for assistance with flow cytometry, and the members of the Veterinary Regenerative Medicine Laboratory at the William R. Pritchard Veterinary Medical Teaching Hospital. The authors declare no conflicts of interest.
REFERENCES
- 1. Agaugue S.; Perrin-Cocon L.; Coutant F.; Andre P.; Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J. Immunol. 177(4):2061–2071; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal S.; Pittenger M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822; 2005. [DOI] [PubMed] [Google Scholar]
- 3. Arnhold S. J.; Goletz I.; Klein H.; Stumpf G.; Beluche L. A.; Rohde C.; Addicks K.; Litzke L. F. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am. J. Vet. Res. 68(10):1095–1105; 2007. [DOI] [PubMed] [Google Scholar]
- 4. Bartholomew S.; Owens S. D.; Ferraro G. L.; Carrade D. D.; Lara D. J.; Librach F. A.; Borjesson D. L.; Galuppo L. D. Collection of equine cord blood and placental tissues in 40 thoroughbred mares. Equine Vet. J. 41(8):724–728; 2009. [DOI] [PubMed] [Google Scholar]
- 5. Batten P.; Sarathchandra P.; Antoniw J. W.; Tay S. S.; Lowdell M. W.; Taylor P. M.; Yacoub M. H. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue Eng. 12(8):2263–2273; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Beggs K. J.; Lyubimov A.; Borneman J. N.; Bartholomew A.; Moseley A.; Dodds R.; Archambault M. P.; Smith A. K.; McIntosh K. R. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 15(8–9):711–721; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Berg L.; Koch T.; Heerkens T.; Bessonov K.; Thomsen P.; Betts D. Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet. Comp. Orthop. Traumatol. 22(5):363–370; 2009. [DOI] [PubMed] [Google Scholar]
- 8. Blanchard-Channell M.; Moore P. F.; Stott J. L. Characterization of monoclonal antibodies specific for equine homologues of CD3 and CD5. Immunology 82(4):548–554; 1994. [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley L. M. Cell Proliferation. In: Mishell B. B.; Shiigi S. M., eds. Selected methods in cellular immunology. San Francisco, CA: W. H. Freeman & Co.; 1980:153–166. [Google Scholar]
- 10. Braun J.; Hack A.; Weis-Klemm M.; Conrad S.; Treml S.; Kohler K.; Walliser U.; Skutella T.; Aicher W. K. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am. J. Vet. Res. 71(10):1228–1236; 2010. [DOI] [PubMed] [Google Scholar]
- 11. Brunswig-Spickenheier B.; Boche J.; Westenfelder C.; Peimann F.; Gruber A. D.; Jaquet K.; Krause K.; Zustin J.; Zander A. R.; Lange C. Limited immune-modulating activity of porcine mesenchymal stromal cells abolishes their protective efficacy in acute kidney injury. Stem Cells Dev. 19(5):719–729; 2010. [DOI] [PubMed] [Google Scholar]
- 12. Burton A. B.; Wagner B.; Erb H. N.; Ainsworth D. M. Serum interleukin-6 (IL-6) and IL-10 concentrations in normal and septic neonatal foals. Vet. Immunol. Immunopathol. 132(2–4):122–128; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Carrade D. D.; Owens S. D.; Galuppo L. D.; Vidal M. A.; Ferraro G. L.; Librach F.; Buerchler S.; Friedman M. S.; Walker N. J.; Borjesson D. L. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 13(4):419–430; 2011. [DOI] [PubMed] [Google Scholar]
- 14. Crop M. J.; Baan C. C.; Korevaar S. S.; Ijzermans J. N.; Pescatori M.; Stubbs A. P.; van Ijcken W. F.; Dahlke M. H.; Eggenhofer E.; Weimar W.; Hoogduijn M. J. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin. Exp. Immunol. 162(3):474–486; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deuse T.; Stubbendorff M.; Tang-Quan K.; Phillips N.; Kay M. A.; Eiermann T.; Phan T. T.; Volk H. D.; Reichenspurner H.; Robbins R. C.; Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 20(5):655–667; 2011. [DOI] [PubMed] [Google Scholar]
- 16. Flaminio M. J.; Ibrahim S.; Lunn D. P.; Stark R.; Steinbach F. Further analysis of anti-human leukocyte mAbs with reactivity to equine leukocytes by two-colour flow cytometry and immunohistochemistry. Vet. Immunol. Immunopathol. 119(1–2):92–99; 2007. [DOI] [PubMed] [Google Scholar]
- 17. Fortier L. A.; Nixon A. J.; Williams J.; Cable C. S. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am. J. Vet. Res. 59(9):1182–1187; 1998. [PubMed] [Google Scholar]
- 18. Fortier L. A.; Smith R. K. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. North Am. Equine Pract. 24(1):191–201; 2008. [DOI] [PubMed] [Google Scholar]
- 19. Frisbie D. D.; Al-Sobayil F.; Billinghurst R. C.; Kawcak C. E.; McIlwraith C. W. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthr. Cartilage 16(10):1196–1204; 2008. [DOI] [PubMed] [Google Scholar]
- 20. Groh M. E.; Maitra B.; Szekely E.; Koc O. N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp. Hematol. 33(8):928–934; 2005. [DOI] [PubMed] [Google Scholar]
- 21. Han K. H.; Ro H.; Hong J. H.; Lee E. M.; Cho B.; Yeom H. J.; Kim M. G.; Oh K. H.; Ahn C.; Yang J. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl. Immunol. 25(1):7–15; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Kang J. W.; Kang K. S.; Koo H. C.; Park J. R.; Choi E. W.; Park Y. H. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 17(4):681–693; 2008. [DOI] [PubMed] [Google Scholar]
- 23. Kern S.; Eichler H.; Stoeve J.; Kluter H.; Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301; 2006. [DOI] [PubMed] [Google Scholar]
- 24. Keyser K. A.; Beagles K. E.; Kiem H. P. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant. 16(5):555–562; 2007. [DOI] [PubMed] [Google Scholar]
- 25. Koch T. G.; Heerkens T.; Thomsen P. D.; Betts D. H. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 7:26; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch T. G.; Thomsen P. D.; Betts D. H. Improved isolation protocol for equine cord blood-derived mesenchymal stromal cells. Cytotherapy 11(4):443–447; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Kogler G.; Sensken S.; Airey J. A.; Trapp T.; Muschen M.; Feldhahn N.; Liedtke S.; Sorg R. V.; Fischer J.; Rosenbaum C.; Greschat S.; Knipper A.; Bender J.; Degistirici O.; Gao J.; Caplan A. I.; Colletti E. J.; Almeida-Porada G.; Müller H. W.; Zanjani E.; Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 200(2):123–135; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krampera M.; Franchini M.; Pizzolo G.; Aprili G. Mesenchymal stem cells: From biology to clinical use. Blood Transfus. 5(3):120–129; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krampera M.; Pasini A.; Pizzolo G.; Cosmi L.; Romagnani S.; Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr. Opin. Pharmacol. 6(4):435–441; 2006. [DOI] [PubMed] [Google Scholar]
- 30. Kronsteiner B.; Wolbank S.; Peterbauer A.; Hackl C.; Redl H.; Griensven M. V.; Gabriel C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 20(12):2115–2126; 2011. [DOI] [PubMed] [Google Scholar]
- 31. Lettry V.; Hosoya K.; Takagi S.; Okumura M. Coculture of equine mesenchymal stem cells and mature equine articular chondrocytes results in improved chondrogenic differentiation of the stem cells. Jpn. J. Vet. Res. 58(1):5–15; 2010. [PubMed] [Google Scholar]
- 32. Lunn D. P.; Holmes M. A.; Antczak D. F.; Agerwal N.; Baker J.; Bendali-Ahcene S.; Blanchard-Channell M.; Byrne K. M.; Cannizzo K.; Davis W.; Hamilton M. J.; Hannant D.; Kondo T.; Kydd J. H.; Monier M. C.; Moore P. F.; O'Neil T.; Schram B. R.; Sheoran A.; Stott J. L.; Sugiura T.; Vagnoni K. E. Report of the Second Equine Leucocyte Antigen Workshop, Squaw Valley, California, July 1995. Vet. Immunol. Immunopathol. 62(2):101–143; 1998. [DOI] [PubMed] [Google Scholar]
- 33. McCarrel T.; Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 27(8):1033–1042; 2009. [DOI] [PubMed] [Google Scholar]
- 34. McFarlane D.; Holbrook T. C. Cytokine dysregulation in aged horses and horses with pituitary pars intermedia dysfunction. J. Vet. Intern. Med. 22(2):436–442; 2008. [DOI] [PubMed] [Google Scholar]
- 35. McIntosh K.; Zvonic S.; Garrett S.; Mitchell J. B.; Floyd Z. E.; Hammill L.; Kloster A.; Di Halvorsen Y.; Ting J. P.; Storms R. W.; Goh B.; Kilroy G.; Wu X.; Gimble J. M. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 24(5):1246–1253; 2006. [DOI] [PubMed] [Google Scholar]
- 36. Meirelles Lda S.; Fontes A. M.; Covas D. T.; Caplan A. I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20(5–6):419–427; 2009. [DOI] [PubMed] [Google Scholar]
- 37. Meisel R.; Zibert A.; Laryea M.; Gobel U.; Daubener W.; Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase- mediated tryptophan degradation. Blood 103(12):4619–4621; 2004. [DOI] [PubMed] [Google Scholar]
- 38. Mueller S. M.; Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell. Biochem. 82(4):583–590; 2001. [DOI] [PubMed] [Google Scholar]
- 39. Najar M.; Rouas R.; Raicevic G.; Boufker H. I.; Lewalle P.; Meuleman N.; Bron D.; Toungouz M.; Martiat P.; Lagneaux L. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: The importance of low cell ratio and role of interleukin-6. Cytotherapy 11(5):570–583; 2009. [DOI] [PubMed] [Google Scholar]
- 40. Nauta A. J.; Westerhuis G.; Kruisselbrink A. B.; Lurvink E. G.; Willemze R.; Fibbe W. E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108(6):2114–2120; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Passeri S.; Nocchi F.; Lamanna R.; Lapi S.; Miragliotta V.; Giannessi E.; Abramo F.; Stornelli M. R.; Matarazzo M.; Plenteda D.; Urciuoli P.; Scatena F.; Coli A. Isolation and expansion of equine umbilical cord-derived matrix cells (EUCMCs). Cell Biol Int. 33(1):100–105; 2009. [DOI] [PubMed] [Google Scholar]
- 42. Polchert D.; Sobinsky J.; Douglas G.; Kidd M.; Moadsiri A.; Reina E.; Genrich K.; Mehrotra S.; Setty S.; Smith B.; Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 38(6):1745–1755; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poncelet A. J.; Vercruysse J.; Saliez A.; Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 83(6):783–790; 2007. [DOI] [PubMed] [Google Scholar]
- 44. Prasanna S. J.; Gopalakrishnan D.; Shankar S. R.; Vasandan A. B. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 5(2):e9016; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Puissant B.; Barreau C.; Bourin P.; Clavel C.; Corre J.; Bousquet C.; Taureau C.; Cousin B.; Abbal M.; Laharrague P.; Penicaud L.; Casteilla L.; Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 129(1):118–129; 2005. [DOI] [PubMed] [Google Scholar]
- 46. Ren G.; Su J.; Zhang L.; Zhao X.; Ling W.; L'Huillie A.; Zhang J.; Lu Y.; Roberts A. I.; Ji W.; Zhang H.; Rabson A. B.; Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27(8):1954–1962; 2009. [DOI] [PubMed] [Google Scholar]
- 47. Ren G.; Zhang L.; Zhao X.; Xu G.; Zhang Y.; Roberts A. I.; Zhao R. C.; Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2(2):141–150; 2008. [DOI] [PubMed] [Google Scholar]
- 48. Rubinstein P.; Dobrila L.; Rosenfield R. E.; Adamson J. W.; Migliaccio G.; Migliaccio A. R.; Taylor P. E.; Stevens C. E. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc. Natl. Acad. Sci. USA 92(22):10119–10122; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1. Sato K.; Ozaki K.; Oh I.; Meguro A.; Hatanaka K.; Nagai T.; Muroi K.; Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109(1):228–234; 2007. [DOI] [PubMed] [Google Scholar]
- 50. Schuh E. M.; Friedman M. S.; Carrade D. D.; Li J.; Heeke D.; Oyserman S. M.; Galuppo L. D.; Lara D. J.; Walker N. J.; Ferraro G. L.; Owens S. D.; Borjesson D. L. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am. J. Vet. Res. 70(12):1526–1535; 2009. [DOI] [PubMed] [Google Scholar]
- 51. Sudres M.; Norol F.; Trenado A.; Gregoire S.; Charlotte F.; Levacher B.; Lataillade J. J.; Bourin P.; Holy X.; Vernant J. P.; Klatzmann D.; Cohen J. L. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J. Immunol. 176(12):7761–7767; 2006. [DOI] [PubMed] [Google Scholar]
- 52. Toupadakis C. A.; Wong A.; Genetos D. C.; Cheung W. K.; Borjesson D. L.; Ferraro G. L.; Galuppo L. D.; Leach J. K.; Owens S. D.; Yellowley C. E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 71(10):1237–1245; 2010. [DOI] [PubMed] [Google Scholar]
- 53. Tung J. T.; Venta P. J.; Caron J. P. Inducible nitric oxide expression in equine articular chondrocytes: Effects of antiinflammatory compounds. Osteoarthr. Cartilage 10(1):5–12; 2002. [DOI] [PubMed] [Google Scholar]
- 54. Vidal M.; Walker N. J.; Napoli E.; Borjesson D. L. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue and umbilical cord tissue. Stem Cells Dev. 21(2):273–283; 2012. [DOI] [PubMed] [Google Scholar]
- 55. Vidal M. A.; Kilroy G. E.; Johnson J. R.; Lopez M. J.; Moore R. M.; Gimble J. M. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: Adipogenic and osteogenic capacity. Vet. Surg. 35(7):601–610; 2006. [DOI] [PubMed] [Google Scholar]
- 56. Vidal M. A.; Kilroy G. E.; Lopez M. J.; Johnson J. R.; Moore R. M.; Gimble J. M. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 36(7):613–622; 2007. [DOI] [PubMed] [Google Scholar]
- 57. Vidal M. A.; Robinson S. O.; Lopez M. J.; Paulsen D. B.; Borkhsenious O.; Johnson J. R.; Moore R. M.; Gimble J. M. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet. Surg. 37(8):713–724; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wohler J. E.; Barnum S. R. Nylon wool purification alters the activation of T cells. Mol. Immunol. 46(5):1007–1010; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoo K. H.; Jang I. K.; Lee M. W.; Kim H. E.; Yang M. S.; Eom Y.; Lee J. E.; Kim Y. J.; Yang S. K.; Jung H. L.; Sung K. W.; Kim C. W.; Koo H. H. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell. Immunol. 259(2):150–156; 2009. [DOI] [PubMed] [Google Scholar]