Abstract
Purpose.
To quantify and compare the structural and functional changes in subjects with early age-related macular degeneration (AMD), using spectral-domain optical coherence tomography (SD-OCT) and microperimetry.
Methods.
Twenty-one eyes of 21 subjects with early AMD were examined. MP-1 10-2 visual fields (VFs) and SD-OCT line and detail volume scans were acquired. The thicknesses of the outer segment (OS; distance between inner segment ellipsoid band and upper retinal pigment epithelium [RPE] border) and RPE layers and elevation of the RPE from Bruch's membrane were measured using a computer-aided manual segmentation technique. Thickness values were compared with those for 15 controls, and values at locations with VF total deviation defects were compared with values at nondefect locations at equivalent eccentricities.
Results.
Sixteen of 21 eyes with AMD had VF defects. Compared with controls, line scans showed significant thinning of the OS layer (P = 0.006) and thickening and elevation of the RPE (P = 0.037, P = 0.002). The OS layer was significantly thinner in locations with VF defects compared with locations without defects (P = 0.003). There was a negligible difference between the retinal layer thickness values of the 5 eyes without VF defects and the values of normal controls.
Conclusions.
In early AMD, when VF defects were present, there was significant thinning of the OS layer and thickening and elevation of the RPE. OS layer thinning was significantly associated with decreased visual sensitivity, consistent with known photoreceptor loss in early AMD. For AMD subjects without VF defects, thickness values were normal. The results highlight the clinical utility of both SD-OCT retinal layer quantification and VF testing in early AMD.
In early AMD, thinning of the OS layer and thickening and elevation of the RPE were found. OS layer thinning was significantly associated with decreased MP-1 visual sensitivity, consistent with known photoreceptor loss in early AMD.
Introduction
Age-related macular degeneration (AMD) is the third leading cause of blindness worldwide, accounting for 8.7% of blindness in the global population.1 Due to the increase in the average lifespan of the elderly population, it is expected that 17.8 million individuals in the United States will be affected by 2050.2 In view of the relevance of AMD as a public health issue, it is important to understand the changes that occur in the early stages of disease, which may lead to improved future intervention strategies.
There has been recent interest in the use of fundus perimetry, also known as microperimetry, as an outcome measure in clinical trials of macular disease.3–7 Functional loss due to AMD was previously demonstrated by findings of reduced visual field sensitivity in retinal locations with drusen compared with nondrusen locations, using scanning laser ophthalmoscope (SLO) perimetry and microperimetry.8–11 In contrast, other studies reported no difference in sensitivity between drusen and nondrusen areas.12
Optical coherence tomography (OCT) is a noninvasive imaging technique that provides cross-sectional images of retinal layer structure, useful for visualization of pathology.13 Spectral-domain OCT (SD-OCT) has been demonstrated as a useful technique in the characterization of AMD.14–16 Previous investigations of the relationship between these structural and functional measures in patients with early and late AMD, using SLO microperimetry combined with SD-OCT, have shown significant correlations between retinal sensitivity and drusen volume11 and between sensitivity and integrity of the inner segment/outer segment junction11,17 (herein called the inner segment ellipsoid [ISe] band18). Another study described an association between retinal thinning and rod-mediated light sensitivity in nonexudative AMD.19 Others reported moderate correlation between segmented OCT scans and visual acuities in subjects with early and advanced dry AMD.20
AMD is a disease primarily affecting the outer retina.21 The sequence of events in AMD is said to begin with preliminary damage to the outer retina and related structures: the retinal pigment epithelium (RPE), photoreceptors, and choroid.22,23 Therefore, our measures targeted outer retinal structure, specifically the RPE and outer segment (OS) layers.
The aim of the study was to quantify the changes in outer retinal structure associated with visual field defects in subjects with early AMD. Areas in the central macula with visual field defects identified using microperimetry were compared with those without defects.
Methods
Subjects
Twenty-four subjects with a diagnosis of early AMD were recruited for the study. Digital color fundus photographs were obtained and graded according to the definitions of the International Classification and Grading System,24 and the stage of disease was determined.25 Each subject had one of the following stages of severity of AMD: stage 1a (soft distinct drusen ≥ 63 μm only), stage 1b (pigmentary abnormalities only, no soft drusen ≥ 63 μm), stage 2a (soft indistinct drusen ≥ 125 μm or reticular drusen only), stage 2b (soft distinct drusen ≥ 63 μm, with pigmentary abnormalities), or stage 3 (soft indistinct drusen ≥ 125 μm or reticular drusen with pigmentary abnormalities).25 Two subjects had reticular pseudodrusen. In addition, all subjects had steady foveal fixation, as assessed by the MP-1 (Nidek Instruments, Inc., Padua, Italy). Subjects were excluded if they had unsteady central or eccentric fixation, refractive errors exceeding ±5.00 diopters (D) sphere or −2.00 D cylinder, significant lenticular opacities (graded at NC3, NO3, C1, P1, or worse, according to the Lens Opacity Classification System III26), pseudophakia, any other ocular pathology such as an epiretinal membrane, any systemic or neurological disease, a history of any other ocular disease or ocular surgery, or if they were taking medications known to affect the visual field. Of the 24 subjects, 3 were unable to complete the visual field tests reliably and had to be excluded. All subjects had received an eye examination less than 3 months prior to their study visit. Test procedures were performed on the dominant eye of each subject, as determined by the hole-in-the-card test.27 Briefly, the subject holds a card with a small hole, through which a distant object is viewed with both eyes open. Then by alternately closing each eye, the dominant eye is determined. Fifteen age-similar healthy subjects (i.e., subjects without any retinal signs of AMD, as confirmed by fundus examination and SD-OCT imaging), who conformed to the above criteria were recruited as controls.
The study adhered to the tenets of the Declaration of Helsinki for research involving human subjects, and the protocol was approved by the Columbia University Medical Center Institutional Review Board. Each participant gave informed consent prior to enrollment in the study.
Optical Coherence Tomography
Structural changes to the central macula were evaluated with SD-OCT (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany), using high-resolution settings and automated tracking (ART). For each study eye, a 9-mm line scan along the horizontal meridian centered at the fovea was obtained as an average of 100 scans, for maximal signal-to-noise ratio. Detail volume scans of the central retina (4.3 mm × 2.8 mm or 15° × 10°) comprising 25 to 49 B-scans (40–51 averaged frames per B-scan) were also acquired. To ensure image quality, scans were excluded if the signal-to-noise ratio was less than 25 dB. Examples of SD-OCT scans are shown in Figure 1.
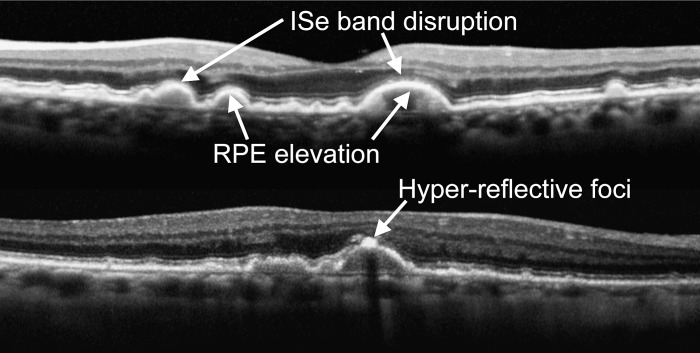
Figure 1. .
OCT images in eyes with AMD. Characteristics of early AMD are shown in two different subjects (top, stage 2a; bottom, stage 3).
SD-OCT images were segmented using a computer-aided, manual segmentation technique, described previously.28,29 The segmented borders were the following: ISe: marked by a line through the center of the hyperreflective band defined as the ISe; OS/RPE: border between OS and RPE, the upper border of the hyperreflective band defined as the RPE; lower RPE border: the lower border of the RPE band; BM/choroid: the border between Bruch's membrane (BM) and the choroid. Using the locations of these boundaries, we defined two retinal layers as shown in Figure 2: receptor outer segment (OS), the distance between ISe and OS/RPE; and the RPE, the distance between OS/RPE and the lower RPE border. In areas without drusen, the lower RPE border was equivalent to the BM/choroid border, because the resolution of SD-OCT does not allow distinction between BM and the lower RPE border. In areas with drusen, where there were elevations of the RPE and BM was visible, we measured the elevation of the RPE from BM, the distance between the lower RPE border and BM/choroid.
Figure 2. .
Segmented OCT images from a normal eye (top) and an eye with AMD (bottom). Segmented images are shown in (A, B), in which the following layers have been demarcated (from inner to outer retina): ISe, the line through the center of the hyperreflective band defined as the inner segment ellipsoid band (blue line); OS/RPE, border between outer segment and retinal pigment epithelium, the upper border of the hyperreflective band defined as the RPE (green line); lower RPE border, the lower border of the RPE band (yellow line); BM/choroid: the border between Bruch's membrane and the choroid (red line). The following retinal layers were measured: receptor OS, the distance between ISe and OS/RPE; and the RPE, the distance between OS/RPE and the lower RPE border. In areas of drusen, we measured the elevation of the RPE from BM, the distance between the lower RPE border and BM/choroid. (C–F) shows an enlarged view of the sections in (A, B) outlined by white broken lines. (C, D) are raw images; (E, F) are segmented images. The eye with AMD (bottom) was graded at stage 2a.
All segmentation was performed by a trained observer masked to the visual field results and the severity of disease based on fundus grading, although in some cases the severity of disease was obvious to the observer based on the appearance of the OCT image. This technique was previously shown to have good reliability.29 All scans were also inspected by a second observer for agreement of border placement. Where disruptions of the ISe band interfered with accurate border placement—for example, where the ISe band appeared to disappear over large drusen or in association with hyperreflective foci—the OS layer thickness values were treated as missing data.
Microperimetry
Visual field sensitivities, preferred retinal locus (PRL), and fixation stability were assessed in all subjects with AMD, using the MP-1 (NAVIS software version 1.7.3; Nidek Instruments, Inc.). Subjects were tested following pupil dilation of the dominant eye with 1% tropicamide and a 15-minute adaptation period to the 1.27 cd/m2 background. The nontested eye was occluded. Identical instructions were given to each subject, and one examiner conducted all testing. A 10-2 pattern similar to the 10-2 pattern of the Humphrey visual field was used to assess visual field sensitivities. The pattern consisted of 68 test locations in the central 20°, with a separation of 2°. White test lights (stimulus size Goldmann III, 200 ms in duration) were presented on a 1.27 cd/m2 white background using a 4-2 threshold strategy. Subjects were asked to maintain fixation on a 2° red cross and fixation was monitored by an infrared fundus tracking device of the MP-1 to ensure central fixation during testing. Catch trials were performed during testing, in which a presentation is made to the physiologic blind spot and all visual fields had fewer than 15% false positives. All subjects had recent experience of at least one visual field test performed on a microperimeter within the last 6 months and were given a brief practice session prior to the start of testing. Results were compared with a recently collected normative database consisting of 50 subjects (age range: 18–68 years), from which prediction limits were calculated using a linear Bayesian model,30 to derive total deviation (TD) defects. The TD analysis represents the sensitivity difference between the measured sensitivity value and the age-corrected normal value, for each location in the visual field. TD defects represent the values that have a probability of occurring in 5%, 2%, and 1% of the age-similar population. Global indices were calculated using the sensitivity values at each location in the 10-2 pattern: mean sensitivity (MS; the average sensitivity value across the 10-2 visual field), mean deviation (MD; the average sensitivity difference between the measured value and the age-corrected normal value at each location), and pattern standard deviation (PSD; the SD around the mean that constitutes the MD, a measure of variability, sensitive to localized loss).31
Analysis
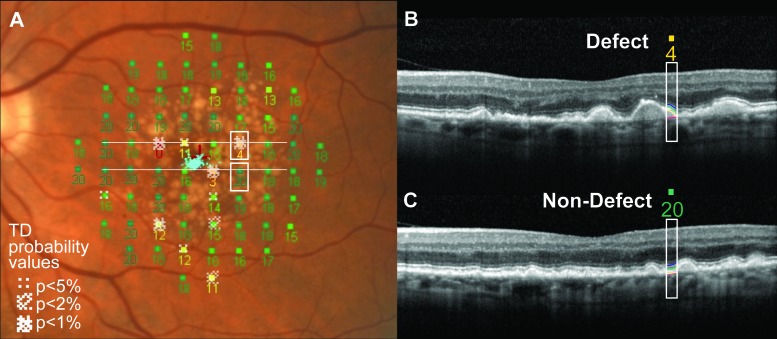
Mean retinal layer thicknesses of the foveal line scans were compared between subjects with AMD and control subjects without AMD. In volume scans, RPE and OS layer thicknesses at locations with visual field defects were compared with thicknesses at nondefect locations at equivalent retinal eccentricities, to account for changes in retinal thickness measures with eccentricity. Pairs of defect and nondefect locations were selected randomly at parafoveal locations, given that previous studies have noted that the greatest deficit in retinal function in AMD occurs in the parafovea at 2 to 5° eccentricity,32,33 and this is also consistent with the retinal location of photoreceptor loss in AMD.21 MP-1 visual field data were mapped to SD-OCT data, assuming 289 μm equals 1° (Fig. 3).
Figure 3. .
MP-1 visual field with SD-OCT scans superimposed. The MP-1 visual field sensitivity values and TD probability map are shown in (A). Two SD-OCT slices of a volume scan are shown in (B, C) and correspond to the upper (B) and lower (C) white horizontal lines depicted in (A). The pair of stimulus locations compared is marked by white boxes in (A) to show one location with a TD defect and one location of normal sensitivity, at equivalent eccentricities. The white boxes in (B, C) indicate the sections of the scans corresponding to the stimulus positions (sensitivity values 4 and 20, corresponding to white boxes in [A]) and diameter (0.43°) and show the retinal layer segmentations. The eye was graded at stage 3. The red cross at the fovea in (A) shows the fixation cross during MP-1 testing and the blue points overlying the cross represent the fixation pattern during testing.
Results
The 21 subjects with AMD (mean age: 72.8 ± 6.6 years; range: 58–81 years; 4 males, 17 females) had best-corrected visual acuities (BCVAs) ranging from 20/20 to 20/40 in the test eye. Eight eyes were graded at stage 1a, 2 at stage 1b, 7 at stage 2a, and 4 at stage 3. The 5 AMD eyes without MP-1 visual field defects were all stage 1a. The 15 control subjects (mean age: 63.3 ± 10.7 years; range: 51–80 years; 4 males, 11 females) had BCVAs of 20/20 or better. There was a significant difference in age between the two groups of subjects (unpaired t-test: t = 3.21, P = 0.003).
MP-1 Findings
Sixteen eyes had visual field defects and a mean MS of 14.9 ± 2.4 dB, and 5 eyes had no defects and a mean MS of 19.5 ± 0.4 dB. The mean MDs for eyes with defects and eyes without defects were −4.2 ± 2.4 and 0.32 ± 0.5 dB, respectively, and the mean PSDs were 2.9 ± 1.0 and 1.3 ± 0.1 dB. For the 16 eyes with defects, the mean number of TD defects was 17.8 ± 14.2. The number of TD defects was significantly correlated with logMAR (logarithm of the minimum angle of resolution) visual acuity (Pearson's r = 0.549, P = 0.012, r2 = 0.300).
SD-OCT
The following changes characteristic of early AMD were observed in the line and volume scans (see Fig. 1): RPE elevations corresponding to drusen were seen in all 21 eyes with AMD; disruptions of the ISe band were seen in 17 of 21 eyes; hyperreflective foci were seen in 11 eyes; and the RPE appeared to be thickened and uneven in all 21 eyes. All SD-OCT scans in the 15 control eyes were unremarkable.
OS and RPE Thickness Measurements and RPE Elevation
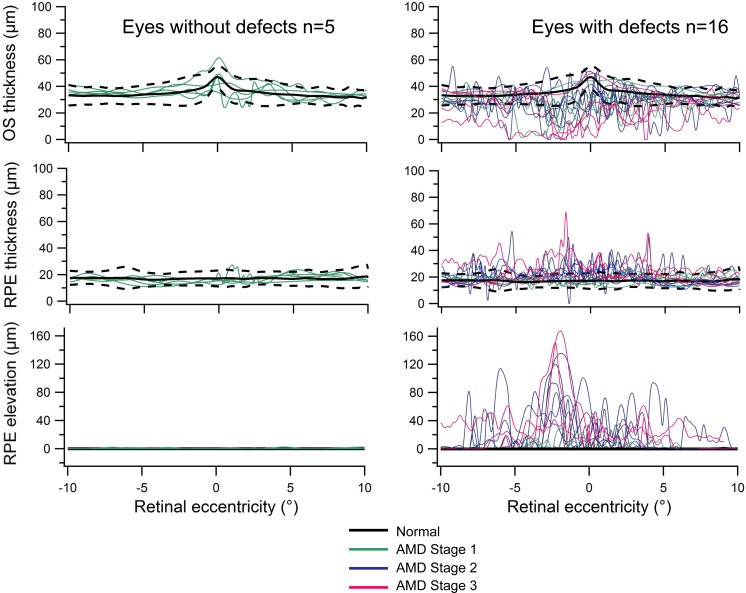
Figure 4 shows the thickness values for the 21 eyes with AMD in foveal line scans. In the 5 eyes without MP-1 visual field defects, thickness values were within the 95% confidence interval (CI) for normal subjects. However, for the 16 eyes with defects, these values tended to fall outside the 95% CI for normal subjects; the OS layer was abnormally thinned and the RPE was abnormally thickened and elevated.
Figure 4. .
OS and RPE thickness plots. Foveal line scan thicknesses of the RPE and OS layers, as a function of retinal eccentricity for normal subjects (black lines) and for each subject with AMD (colored lines). The black dotted lines indicate the 95% confidence interval around the mean normal values (for n = 15 normal controls). The left panel shows eyes without MP-1 visual field defects (n = 5) and the right panel shows eyes with defects (n = 16).
When eyes were grouped according to the stage of disease, eyes at stages 2 and 3 tended to show more OS thinning, more RPE thickening, and greater RPE elevation than eyes graded at stage 1. Notably, there were eyes at stage 1b that demonstrated thinning in the OS layer, in the absence of RPE changes.
The mean thickness values for the RPE and OS retinal layers and RPE elevation are shown in the Table for normal eyes, AMD eyes without visual field defects, and AMD eyes with defects. A statistically significant variation between these three groups of subjects was found in RPE layer thickness (one-way ANOVA: F = 4.784, P = 0.015), OS thickness (F = 8.447, P = 0.001), and RPE elevation (F = 12.461, P < 0.001). Post hoc analysis (Games–Howell test) revealed that, in AMD eyes with defects, the RPE was thickened (P = 0.037) and elevated (P = 0.002), and the OS layer was thinned (P = 0.006), compared with normal eyes. In AMD eyes without defects, the RPE elevation was significantly greater than that in normal eyes (P = 0.005), but the RPE and OS layer thicknesses were not significantly different from those in normal eyes (P = 0.866; P = 0.718).
Table. .
RPE and OS Layer Thicknesses and RPE Elevation Measurements in Foveal Line Scans for Normal Eyes, AMD Eyes with Visual Field Defects, and AMD Eyes without Defects
|
|
Mean Thickness, μm |
SD, μm |
| RPE | ||
| Normal eyes | 16.6 | 1.8 |
| AMD eyes with defects | 19.9 | 3.3 |
| AMD eyes without defects | 16.7 | 1.2 |
| OS | ||
| Normal eyes | 34.9 | 2.8 |
| AMD eyes with defects | 28.5 | 6.4 |
| AMD eyes without defects | 36.2 | 3.5 |
| RPE elevation | ||
| Normal eyes | 0 | 0 |
| AMD eyes with defects | 12.0 | 10.5 |
| AMD eyes without defects | 0.2 | 0.1 |
Relationship between Retinal Layer Thicknesses and the MP-1 Global Visual Field Indices
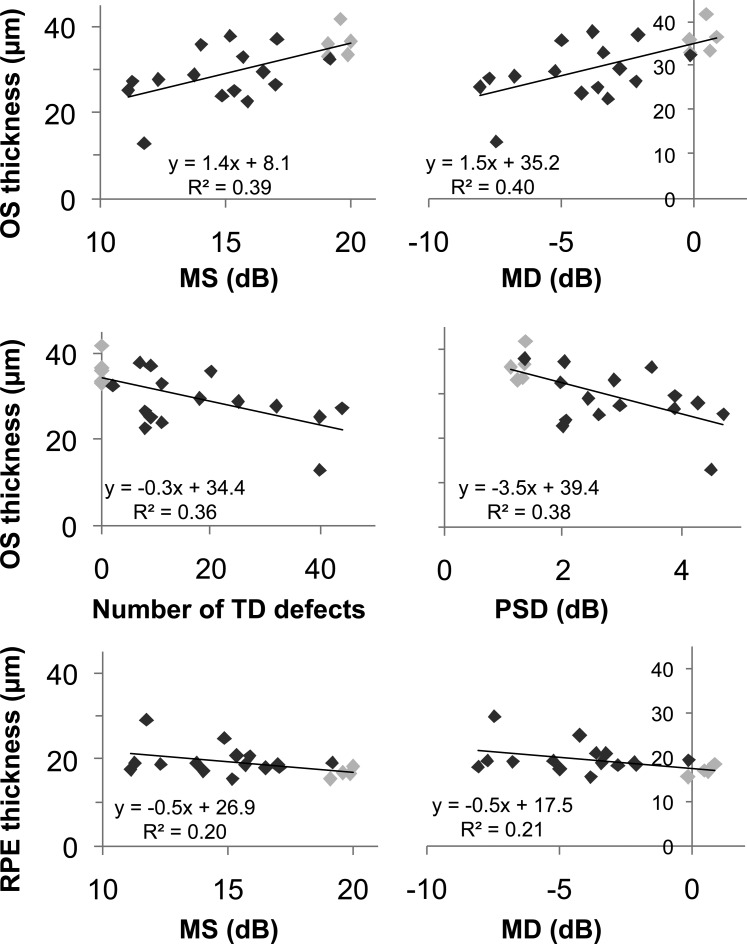
The correlation between retinal layer thicknesses from foveal line scans and global visual field indices was examined (see Fig. 5) for all 21 eyes. The number of TD defects represents the proportion of the 68 visual field locations in which there were defects (i.e., the extent of the scotoma). As expected, the data points (pale gray) for the 5 eyes without TD defects had the least severe values in each scatterplot. There were significant relationships between thinning of the OS layer and worsening of the visual field, where significant correlations were found between the OS layer thickness and the MS (Pearson's r = 0.622, P = 0.003), the MD (r = 0.633, P = 0.003), and the PSD (r = −0.617, P = 0.004). Around 40% of the proportion of variance in OS thickness was associated with the global indices. Thickening of the RPE was significantly correlated with worsening MS (r = −0.448, P = 0.047) and MD (r = −0.454, P = 0.045), and 20% of the proportion of variance in RPE thickness was attributed to the MS and the MD. The magnitude of RPE elevation did not have any significant relationships to the visual field measures.
Figure 5. .
Relationship between layer thicknesses and MP-1 indices. Scatterplots showing the significant relationships between retinal layer thicknesses and the MP-1 visual field. The 16 AMD eyes with TD defects are shown in dark gray, and the 5 AMD eyes without TD defects are shown in light gray.
The TD defects used in the above analyses were defined as having a probability of 5% or worse. To compare the effect of the depth of TD defect, we compared OS thickness values with the number of TD defects with a probability of <2% and <1%. There were significant correlations between the OS layer thickness and the number of TD defects <2% (Pearson's r = −0.617, P = 0.004, r2 = 0.380) and the number of TD defects <1% (r = −0.648, P = 0.002, r2 = 0.420).
Comparison between Locations with and without Visual Field Defects
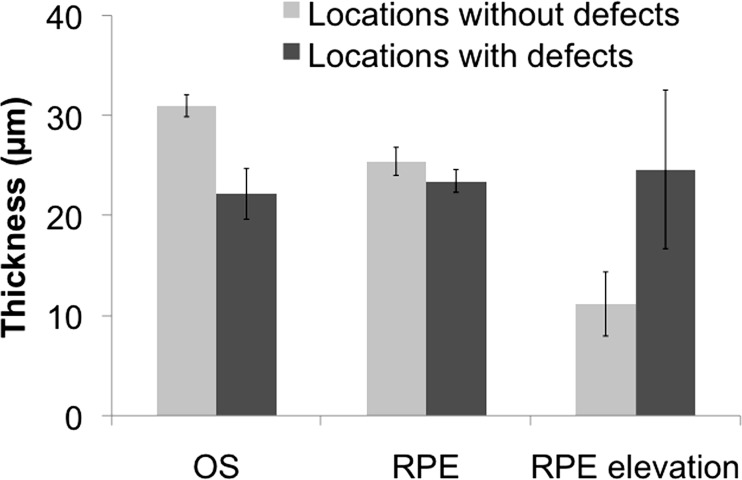
In volume scans, the thicknesses of the RPE and OS layers at locations with defects were compared with those without visual field defects at equivalent eccentricities in the 16 eyes with defects. An example of the locations of comparison in one subject is shown in Figure 3. The OS layer was thinner in locations with defects in 14 of 16 eyes, and this difference (mean difference = 9 μm) was statistically significant (independent samples t-test: t = 3.20, P = 0.003).
In locations with defects, the RPE was thicker in 7 eyes and showed greater elevation from Bruch's membrane in 8 eyes. However, these differences (RPE mean difference = 2 μm; RPE elevation mean difference = 13 μm) did not reach statistical significance (RPE: t = 1.09, P = 0.286; RPE elevation: t = −1.573, P = 0.126; Fig. 6).
Figure 6. .
Comparison of mean retinal layer thicknesses between locations with and without visual field defects. Error bars represent 1 SE.
Discussion
In this study of subjects with early AMD, we quantified structural changes in the thickness of the outer retina and evaluated these changes in association with functional loss in the visual field. An obvious pattern emerged in our data, in which eyes with visual field defects had different structural findings when compared with eyes without visual field defects, and this pattern was in agreement with the stages of severity of AMD. In eyes with visual field defects and early AMD, we demonstrated abnormal thinning of the OS layer and a thickening and elevation of the RPE. In addition, when we compared locations with visual field defects to locations without defects, the OS layer was thinner in defect locations. However, in eyes without visual field defects, there were negligible differences in retinal layer thickness values from normal.
Histopathologic studies of tissue sections in the macular region have shown that RPE and photoreceptor cell changes arise early in the sequence of events in AMD.21,34 The structural changes associated with drusen are decreased photoreceptor density over the drusen35 and deflected and shortened photoreceptor outer segments overlying drusen.34 Our OCT results are in agreement with these histopathologic findings. A possible explanation for our finding of thickening of the RPE in early AMD could be due to the presence of basal laminar deposits beneath the RPE36; however, these may also result in differing reflectivity levels adjacent to the RPE and may have confounded segmentation of the lower RPE border. Alternatively, the RPE layer itself could thicken if there were changes in cell shape. The presence of heaped and sloughed RPE cells has been reported in a recent study of a grading system for RPE degeneration based on donor eyes with geographic atrophy.37 Our findings are also consistent with a previous clinical study, in which thinning of the photoreceptor layer overlying drusen was found on SD-OCT imaging in patients with AMD.16 In this previous study, the authors measured the photoreceptor layer, defined as the distance between the top of RPE and the outer plexiform layer, as opposed to OS thickness (distance between the upper RPE border and the ISe) in our present study.
We observed a range of functional deficits within the same stage of disease. Despite the relatively good visual acuities of all subjects in our study, their visual field results ranged from normal to significant defects. This finding emphasizes the inadequacy of visual acuity as an assessment of visual loss and the clinical importance of additional functional testing, such as microperimetry. Clinically, our technique may be useful to differentiate between subjects within the same stage of disease, but with differing functional losses.
When we compared the OCT findings with microperimetry, we found significant relationships between OS layer thickness values and visual field loss and stronger relationships were present for more severe visual field defects. This is in agreement with previous studies that have described a relationship between retinal sensitivity and SD-OCT changes in AMD.11,17,19,38 However, these earlier studies did not quantify retinal layer thicknesses or evaluate visual field defects in comparison with a normative database.
Several previous studies determined that rod system sensitivity loss exceeded that of cones in AMD21,32,33,39; however, other studies also found significant cone dysfunction in early AMD.40,41 At the mesopic background luminance of the MP-1 (1.27 cd/m2) increment thresholds may be mediated by mixed rod–cone system responses, or by a mainly cone system response (Crossland MD, et al. IOVS 2012;53:ARVO E-Abstract 4822). The nature of the response will also vary depending on damage due to retinal disease and intricate photoreceptor interactions.42–44 It will vary further with stimulus spectral, spatial, and temporal properties as well as with retinal eccentricity according to rod and cone distributions.42,45
The limitations of our study include the small number of subjects in each group, the difference in mean age between subjects with AMD and controls, and the lack of follow-up of subjects over time. Longitudinal evaluation of a larger number of subjects in the subcategory of AMD subjects without functional loss would be of interest to confirm whether thinning of the OS layer or other structural change precedes visual loss.
In summary, in early AMD subjects with visual field defects, we observed significant thinning of the OS layer and a thickening and elevation of the RPE. Thinning of the OS layer was significantly associated with decreased visual sensitivity. The results suggest that comparisons between outer retinal layer thickness measurements and microperimetry have potential clinical utility for monitoring progression in early AMD.
Acknowledgments
The authors thank Beulah Abraham for preliminary segmentation work and Jennifer Dalberth for editorial assistance.
Footnotes
Supported by National Eye Institute/National Institutes of Health Grants R01 EY02115, R01 EY09076, and R01 EY015520; The New York Community Trust; Research to Prevent Blindness (New York, New York); and Topcon, Inc., Tokyo, Japan (DCH).
A portion of this work has been published previously as an ARVO abstract: Acton JH, et al. IOVS 2012: ARVO E-Abstract 4379 and Acton JH, et al. IOVS 2011: ARVO E-Abstract 4466.
Disclosure: J.H. Acton, None; R.T. Smith, None; D.C. Hood, Topcon, Inc. (F, C); V.C. Greenstein, None
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 2.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–540 [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich R, Mawer NP, Mody CH, Brand CS, Squirrell D. Visual function following photodynamic therapy for central serous retinopathy: a comparison of automated macular microperimery versus best corrected visual acuity. Clin Exp Ophthalmol. 2012;40:e32–e39 [DOI] [PubMed] [Google Scholar]
- 4.Finger RP, Charbel Issa P, Hendig D, Scholl HP, Holz FG. Monthly ranibizumab for choroidal neovascularizations secondary to angioid streaks in pseudoxanthoma elasticum: a one-year prospective study. Am J Ophthalmol. 2011;152:695–703 [DOI] [PubMed] [Google Scholar]
- 5.Genead MA, McAnany JJ, Fishman GA. Topical dorzolamide for treatment of cystoid macular edema in patients with choroideremia. Retina. 2012;32:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Wang W, Yu S, et al. Functional recovery after intravitreal bevacizumab treatments for idiopathic choroidal neovascularization in young adults. Retina. 2012;32:679–686 [DOI] [PubMed] [Google Scholar]
- 7.van Zeeburg EJ, Maaijwee KJ, Missotten TO, Heimann H, van Meurs JC. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am J Ophthalmol. 2012;153:120–127 [DOI] [PubMed] [Google Scholar]
- 8.Midena E, Vujosevic S, Convento E, Manfre A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remky A, Elsner AE. Blue on yellow perimetry with scanning laser ophthalmoscopy in patients with age related macular disease. Br J Ophthalmol. 2005;89:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takamine Y, Shiraki K, Moriwaki M, Yasunari T, Miki T. Retinal sensitivity measurement over drusen using scanning laser ophthalmoscope microperimetry. Graefes Arch Clin Exp Ophthalmol. 1998;236:285–290 [DOI] [PubMed] [Google Scholar]
- 11.Hartmann KI, Bartsch DU, Cheng L, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–1331 [DOI] [PubMed] [Google Scholar]
- 12.Sunness JS, Johnson MA, Massof RW, Marcus S. Retinal sensitivity over drusen and nondrusen areas—a study using fundus perimetry. Arch Ophthalmol. 1988;106:1081–1084 [DOI] [PubMed] [Google Scholar]
- 13.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman SR, Kozak I, Cheng LY, et al. Optical coherence tomography: raster scanning and manual segmentation in determining drusen volume in age-related macular degeneration. Retina. 2010;30:431–435 [DOI] [PubMed] [Google Scholar]
- 15.Khanifar AA, Koreishi AF, Izatt JA, Toth CA. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology. 2008;115:1883–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009;116:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landa G, Su E, Garcia PM, Seiple WH, Rosen RB. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina. 2011;31:364–370 [DOI] [PubMed] [Google Scholar]
- 18.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark ME, McGwin G Jr, Neely D, et al. Association between retinal thickness measured by spectral-domain optical coherence tomography (OCT) and rod-mediated dark adaptation in non-exudative age-related maculopathy. Br J Ophthalmol. 2011;95:1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappuru RR, Ouyang Y, Nittala MG, et al. Relationship between outer retinal thickness substructures and visual acuity in eyes with dry age related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:6743–6748 [DOI] [PubMed] [Google Scholar]
- 21.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249 [PubMed] [Google Scholar]
- 22.Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442 [DOI] [PubMed] [Google Scholar]
- 23.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732 [DOI] [PubMed] [Google Scholar]
- 24.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374 [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–526 [DOI] [PubMed] [Google Scholar]
- 26.Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–836 [DOI] [PubMed] [Google Scholar]
- 27.Durand AC, Gould GM. A method of determining ocular dominance. J Am Med Assoc. 1910;55:369–370 [Google Scholar]
- 28.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2328–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood DC, Cho JS, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acton JH, Bartlett NS, Greenstein VC. Comparing the Nidek MP-1 and Humphrey field analyzer in normal subjects. Optom Vis Sci. 2011;88:1288–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heijl A, Lindgren G, Olsson J. A package for the statistical analysis of visual fields. Doc Ophthalmol Proc Ser. 1987;49:153–168 [Google Scholar]
- 32.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273 [PubMed] [Google Scholar]
- 33.Chen C, Wu L, Wu D, et al. The local cone and rod system function in early age-related macular degeneration. Doc Ophthalmol. 2004;109:1–8 [DOI] [PubMed] [Google Scholar]
- 34.Johnson PT, Lewis GP, Talaga KC, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–4488 [DOI] [PubMed] [Google Scholar]
- 35.Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005;46:4788–4795 [DOI] [PubMed] [Google Scholar]
- 36.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968–977 [DOI] [PubMed] [Google Scholar]
- 37.Vogt SD, Curcio CA, Wang L, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011;93:413–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Querques L, Querques G, Forte R, Souied EH. Microperimetric correlations of autofluorescence and optical coherence tomography imaging in dry age-related macular degeneration. Am J Ophthalmol. 2012;153:1110–1115 [DOI] [PubMed] [Google Scholar]
- 39.Sallo FB, Rechtman E, Peto T, et al. Functional aspects of drusen regression in age-related macular degeneration. Br J Ophthalmol. 2009;93:1345–1350 [DOI] [PubMed] [Google Scholar]
- 40.Gerth C, Delahunt PB, Alam S, Morse LS, Werner JS. Cone-mediated multifocal electroretinogram in age-related macular degeneration: progression over a long-term follow-up. Arch Ophthalmol. 2006;124:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phipps JA, Guymer RH, Vingrys AJ. Loss of cone function in age-related maculopathy. Invest Ophthalmol Vis Sci. 2003;44:2277–2283 [DOI] [PubMed] [Google Scholar]
- 42.Stockman A, Sharpe LT. Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt. 2006;26:225–239 [DOI] [PubMed] [Google Scholar]
- 43.Latch M, Lennie P. Rod-cone interaction in light adaptation. J Physiol. 1977;269:517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frumkes TE, Sekuler MD, Barris MC, Reiss EH, Chalupa LM. Rod-cone interaction in human scotopic vision. 1. Temporal analysis. Vision Res. 1973;13:1269–1282 [DOI] [PubMed] [Google Scholar]
- 45.Wyszecki G, Stiles WS. Color Science. 2nd ed. New York: Wiley; 2000 [Google Scholar]