Abstract
Purpose
We previously reported that autophagy in tumor cells plays a critical role in cross-presentation of tumor antigens and that autophagosomes are efficient antigen carriers for cross-priming of tumor-reactive CD8+ T cells. Here we sought to characterize further the autophagosome-enriched vaccine named DRibble (DRiPs-containing blebs), derived from tumor cells after inhibition of protein degradation and provide insights into the mechanisms responsible for their efficacy as a novel cancer immunotherapy.
Experimental Design
DRibbles were characterized by western blot and light or transmission electron microscopy. The efficiency of cross-presentation mediated by DRibbles was first compared with that of whole tumor cells and pure proteins. The mechanisms of antigen cross-presentation by DRibbles were analyzed and the anti-tumor efficacy of the DRibble vaccine was tested in 3LL Lewis lung tumors and B16F10 melanoma.
Results
The DRibbles sequester both long-lived and short-lived proteins, including defective ribosomal products (DRiPs), as well as damage-associated molecular pattern (DAMP) molecules exemplified by HSP90, HSP94, calreticulin, and HMGB1. DRibbles express ligands for CLEC9A, a newly described C-type lectin receptor expressed by a subset of conventional DCs (cDCs) and plasmacytoid DCs (pDCs) and cross-presentation was partially CLEC9A-dependent. Furthermore, this autophagy assisted antigen cross presentation pathway involved both caveolae- and clathrin-mediated endocytosis and ERAD machinery. It depends on proteasome and TAP1, but lysosome functions of APCs. Importantly, DC loaded with autophagosome-enriched DRibbles can eradicate 3LL Lewis lung tumors and significantly delay the growth of B16F10 melanoma.
Conclusion
These data documented the unique characteristics and potent anti-tumor efficacy of the autophagosome-based DRibble vaccine. The efficacy of DRibble cancer vaccine will be further tested in clinical trials.
Keywords: DRibble, autophagosome, cross-presentation, CLEC9A, tumor regression, cancer vaccine, immunotherapy
Introduction
Autophagy is a fundamental cellular process in which damaged cytosolic proteins and superfluous organelles are sequestered in autophagosomes and delivered to lysosomes for their clearance. Autophagy is seen as a double-edged sword in the development of tumortargeted therapies; it can be either a tumor suppression or survival mechanism in response to metastatic stress and anti-tumor therapy (1, 2). Emerging evidence suggests an important role for autophagy in both innate and adaptive immunity (3-5). It has been reported that autophagy enhances MHC I presentation of HSV-1 viral antigens via formation of autophagosomes in antigen presenting cells (6), and constitutively delivers antigens for MHC II presentation through autophagosomes (7). We recently showed that autophagy in tumor cells played a critical role in cross-presentation of tumor antigens and identified autophagosomes as the novel, efficient carriers for cross-presentation of tumor associated antigens (TAA) (8).
Based on these findings and the well-established role of cross-presentation in priming antitumor T-cell responses, we developed a novel tumor vaccine comprising enriched autophagosomes that favors cross-presentation of multiple tumor-associated antigens (TAA). TAAs are degraded by two major proteolysis pathways in the tumor cells. It is generally believed that the long-lived proteins are degraded by the lysosomes through the autophagy pathway (9), whereas short-lived proteins (SLiPs) including defective ribosomal products (DRiPs), are ubiqutinated and degraded by proteasomes (10, 11). With induction of autophagy and inhibition of lysosomal/proteosomal activity, a broad spectrum of cellular antigens, including long-lived proteins, short-lived proteins (SLiPs), as well as defective ribosomal products (DRiPs), is sequestered in autophagosomes (12). We refer these autophagosome-enriched, DRiPs-containing blebs as to DRibbles.
Efficient cross-presentation of TAA is pivotal for the success of cancer vaccines. The process of cross-presentation involves antigen internalization, processing, and presentation of peptides on MHC I molecules by pAPCs, such as DCs (13). What determines the efficiency of antigen cross-presentation is not well understood. Two mechanisms are known so far. First, specific cell surface receptors on DCs mediate distinct antigen endocytosis and intracellular routing for MHC I or MHC II presentation (14, 15). Mannose receptor (CD206)-mediated uptake of soluble ovalbumin (OVA) favors MHC I cross-presentation because it specifically targets OVA to early endosomes, but not lysosomes; whereas DC-SIGN (CD209A) routes antigens into lysosomes for MHC II-restricted presentation (15, 16). Recently, CLEC9A (DNGR-1), a newly identified C-type lectin receptor on cDCs, was found to be required for efficient cross-presentation of dead cell-associated materials (17). Like the mannose receptor, CLEC9A may also divert cargo away from lysosomes, since it localizes only in nonlysosomal compartments. Second, the size of the exogenous materials may dictate the route of endocytosis and direct cargo either to nonacidic compartments or to lysosomes (18, 19). Extracellular particulate materials were documented to enter cells either by clathrin-dependent endocytosis or caveolae-mediated endocytosis based on their size. Clathrin-mediated entry of particulate materials with a size of less than 200 nm was targeted for lysosomal degradation, whereas caveolae-mediated internalization of particles larger than 200 nm retained cargo in nonacidic compartments (19) and thus may lead to efficient cross-presentation (16).
In this study, we showed that tumor cell-derived DRibbles sequestered a broad range of antigens and were highly efficient at cross priming of antigen specific CD8+ and CD4+ T cells in vitro and in vivo. Interestingly, we found CELC9A was required for efficient cross-presentation of DRibble antigens. We also delineated mechanisms by which DRibbles were internalized and intracellular proteolysis pathways and machineries involved in cross-presentation of antigens delivered by DRibbles. Furthermore, we isolated DRibbles from a variety of tumor cell lines by augmenting autophagy and inhibiting protein degradation, and tested their therapeutic efficacy in mice bearing established B16F10 melanomas and 3LL Lewis lung carcinomas.
Materials and Methods
Mice, cell lines and cell culture
C57BL/6 mice were purchased from the Charles River Laboratories. OT-I T cell receptor (TCR) transgenic mice (recognize the H-2Kb-restricted OVA257-264 peptide) were obtained from the Jackson Laboratory. Pmel-1 transgenic mice (recognize mouse and human H-2Db restricted gp10025-33 peptide) were kindly provided by Dr. Nicholas P. Restifo (National Cancer Institute, NIH) and bread in our facility. All mice were used in accordance with the protocol approved by the Animal Care and Use Committee of the Earle A. Chiles Research Institute.
HEK 293T cells were maintained in DMEM supplemented with 2mM L-glutamine, 1mM sodium pyruvate, and 10% FBS. Murine B16-F10 melanoma was obtained from ATCC and clone 3 was derived from an in vivo passage. The F10 cell line was characterized for expression of the melanoma-associated gp100 and envelope protein of endogeneous retrovirus melREV as determined by flow cytometry analysis. The F10 cell line was also shown to stimulate the proliferation of pmel-1 naïve T cells in vivo. The Lewis lung 3LL tumor cells were obtained from ATCC. These cells have not undergone further testing. These cell lines were cultured in RPMI1640 with 2mM L-glutamine, 1mM sodium pyruvate, and 10% FBS.
DNA construction and generation of cell lines
HEK 293T cells that expressed the M-GFP-OVA or gp100 model antigens, GFP-ubiquitin (Ub), or tdTamato-LC3, were generated as described before (8). To characterize the antigens sequestered in DRibbles, we used the following different forms of OVA: Ub-M-GFP-OVA (long-lived cytosolic protein), Ub-R-GFP-OVA (short-lived cytosolic protein), Ub-M-GFP-TfR-OVA (long-lived, membrane-bound protein), and Ub-R-GFP-TfR-OVA (short-lived, membrane-bound protein). M, methionine; R, arginine; V, valine; TfR, transmembrane domain of the transferrin receptor.
Carboxy-fluorescein diacetate succinimidyl ester (CFSE)-labeling and in vitro and in vivo CFSE dilution assay
Cross-presentation of antigens to naive OT-I or pmel-1 T cells was measured by CFSE dilution of labeled T cells by flow cytometry analysis. Flt3L DCs were isolated from spleens of C57BL6 mice at day 15 after sequential intravenous injection of plasmid DNA encoding murine Flt3 ligand (2 μg DNA in 2ml PBS, day 1) and GM-CSF (Day 10) (8). For the in vivo CFSE assay, DRibbles were injected subcutaneously into both flanks either directly or after loading onto DCs in vitro for 6 hrs, or injected directly into both inguinal lymph nodes of C57BL6 mice. At the same day, 3×106 CFSE-labeled Thy1.1+ OT-I T cells were adoptively transferred into these mice. Lymph nodes were collected 4 or 5 days later, single cell suspensions were prepared and analyzed by flow cytometry after staining with antibodies against thy1.1 and CD8.
Preparation of DRibbles
Autophagosome-enriched DRibbles were prepared as described previously (8). Briefly, tumor cells were treated with Bortezomib (velcade, 100 nM) and NH4Cl (10 mM) for 24-48 hours, cells were disrupted by mild sonication at 115 V, 56-60HZ using the G112SP1G Special Ultrasonic Cleaner (Laboratory Supplies CO., Inc.). The resulting suspension was pre-cleared by centrifugation at 300×g for 10min, and was then separated into the crude autophagosome-containing large vesicles (DRibbles) and the supernatant consisting of cytosolic components by a 15-min centrifugation at 10,000×g. DRibbles were stored in PBS at 4°C for short-term (less than one month) or −80°C for long-term.
Western blotting
Western blot was performed as previously described (8). The primary antibodies included mouse anti-GFP (1:1000, StressGen), rabbit anti-ubiquitin (1:1000, Upstate), rabbit anti-LC3 (1:1000, Novus), rabbit anti-HS90α (1:1000, Chemicon), rabbit anti-HSP94, rabbit anti-calreticulin (1:500, Upstate), and rabbit anti-HMGB 1 (1:1000, Abcam). The secondary antibodies were goat-anti rabbit-HRP (1:10,000, Jackson ImmunoResearch), and goat anti-mouse-HRP (1:10,000, Jackson ImmunoResearch).
Fluorescent light microscopy
Images of live cells were taken using a Zeiss inverted microscope capable of digital epifluorescence imaging. A GFP filter (excited at 470/40, dichromatic mirror at 495, and long pass emission filter 500LP) and an Orange filter (excited at 525/50, dichromatic mirror at 555, and band pass emission filter 590/50) were used to capture fluorescent images. Cell images were processed with Photoshop. Pseudo green and red colors were applied to GFP- and tomato- positive cells respectively and resulting images were overlaid to show co-localization of GFP and tomato colors.
Transmission electron microscopy
DRibbles containing autophagosomes were prepared from the culture media of murine Lewis lung 3LL tumor cells treated with 100 nM bortezomib and 10 mM ammonium chloride for 48 hours. DRibble samples were fixed in 100 mM sodium cacodylate (pH 7.2), 2.5% glutaraldehyde, 1.6% paraformaldehyde, 0.064% picric acid, 0.1% ruthenium red, gently washed, and post-fixed for 1 h in 1% osmium tetroxide plus 0.8% potassium ferricyanide in 100 mM sodium cacodylate, pH 7.2. After thorough rinsing in water, samples were dehydrated, infiltrated overnight in 1:1 acetone:Epon 812, infiltrated 1 h with 100% Epon 812 resin, and embedded in the resin. After polymerization, 60- to 80-nm thin sections were cut on a Reichert ultramicrotome, stained 5 min in lead citrate, rinsed, post-stained for 30 min in uranyl acetate, rinsed, and dried. EM was performed at 60 kV on a Philips Morgagne TEM, equipped with a CCD, and images were collected at original magnifications of 1,000-37,000X.
Detection of CLEC9A ligand and CLEC9A expression
To detect the expression of the CLEC9A ligand, a recombinant soluble FcmILZ-CTLD9A receptor that contains a c-type lectin dormain (CTLD) fused to C-terminal of human IgG and trimerization isoleucine zipper (ILZ) was made. The human Fc fragment of the soluble fusion receptor was mutated at the Fc receptor-binding site to avoid soluble CLEC9A-binding to DC membrane through the Fc and Fc receptor interaction. DRibbles harvested from HEK 293T cells were incubated with the soluble fusion receptor FcmILZ-CTLD9A for 1 hour. The soluble FcmILZ OX40 ligand fusion protein (constructed in our laboratory) was used as negative control. DRibbles were washed extensively to remove excess free soluble receptor. PE-conjugated anti-human Fc Fab fragment was used to detect ligand expression on DRibbles by flow cytometry. A sensitive photo multiplier tube (PMT) was added on the Forward Side Scatter parameter of a FACS Aria II cell sorter, which allow detection of small size particles to 200nm or greater. To detect CLEC9A on DCs, a single cell suspension of splenic DCs was stained with APC-conjugated anti-CLEC9A antibody (7H11 mAb specific for mCLEC9A, kindly provided by Dr. Caetano Reis e Sousa at London Research Institute, UK) and PE-conjugated anti-CD11c antibody. Flow cytometry was performed to detect CD11c and CLEC9A. All events were gated on CD11c+ population.
Investigation of the pathways involved in cross-presentation of DRibble antigens
To examine which endocytic pathways that are involved in DRibble cross-presentation, DCs were pretreated with chlorpromazine (10 μg/ml, Sigma) to inhibit clathrin-mediated endocytosis, or filipin III (5 μg/ml, Sigma) to block caveolae-mediated endocytosis, or both, for 30 minutes before loading with DRibbles isolated from OVA-expressing cells (OVA-DRibble). DCs loaded with SIINFEKL peptide (250ng/ml) were used as a control. Antigen-pulsed DCs were then incubated with CFSE-labeled OT-I T cells for 4 days. Division of OT-I T cells was monitored by flow cytometry analysis. To assess the requirement of the ERAD pathway, proteasomes, and lysosomal activity, DCs were pretreated with exotoxin A (10 μg/ml, Sigma), Bortezomib (velcade, 100 nM), or NH4Cl (10 mM, Sigma), respectively, for 30 minutes before OVA-DRibble or SIINFEKL peptide was loaded. To test the role of TAP1 in peptide -loading, DCs from TAP1 knockout mice were used as APCs in parallel with wild type DCs.
Immunotherapy experiments
For treatment of 3LL primary tumor, C57BL/6 mice were subcutaneously injected 2×105 3LL tumor cells. Subsequently, DRibbles-pulsed DCs with or without adjuvant were injected subcutaneously into both flanks of C57BL/6 mice on days 6, 8 and 10. Tumor growth was measured thrice a week. Five or six mice were included in each group. In the 3LL lung metastases model, 2×105 3LL tumor cells were injected i.v. into C57BL/6 mice. Mice were then treated with s.c DCs loaded with 3LL-derived DRibbles, dead cells, and tumor cell lysate in the presence of adjuvant at day 3. All mice were sacrificed and the metastases in the lungs were enumerated on day 28.
Statistical analysis
Log-rank nonparametric analysis was used to analyze the tumor-free survival data. Each group consisted of at least five mice, and no animal was excluded from the statistical evaluation. Student’s t test was used to analyze the number of T cells and tumor metastases. A two-tail P < 0.05 was considered significant.
Results
Generation and characterization of DRibbles from HEK 293T cells
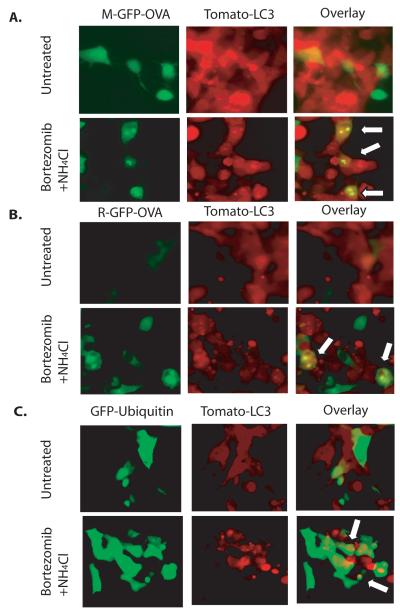
We previously reported that tumor cell autophagy was required for efficient cross-presentation of TAA (8). We also demonstrated that treatment of tumor cells with Bortezomib, a proteasome inhibitor, induced autophagy in tumor cells and enhanced cross-presentation of TAA. Moreover, treatment of tumor cells with NH4Cl, which blocks lysosomal degradation, also augmented cross-presentation (8). Yewdell and his colleagues recently reported that cross-presentation favored long-lived proteins, whereas SLiPs including DRiPs were not cross-presented because they were quickly degraded by proteasomes (20). To show whether cross-presentation of SLiPs and DRiPs were shunted into autophagosomes and cross-presented, we generated stable HEK 293T cells that expressed the short-lived Ub-R-GFP-OVA or long-lived Ub-M-GFP-OVA fusion proteins (9). Consistent with Yewdell’s observations, proteosome inhibition resulted in accumulation o f Ub-R-GFP-OVA protein and increased their cross-presentation to the level induced by long-lived M-GFP-OVA (Supplementary Fig. S1). When HEK 293T cells were treated with Bortezomib and NH4Cl concurrently, a large number of tomato-LC3+ blebs, indicating autophagosomes, were accumulated in the cells. Interestingly, we also observed that many tomato-LC3+ blebs were also released into the culture media after prolonged treatment (Fig. 1). To determine whether protein antigens or ubiqutinated proteins were sequestered into autophagosomes, we transfected HEK 293T cells that expressed tomato-LC3 with plasmid DNA encoding M-GFP-OVA, R-GFP-OVA, or GFP-Ub fusion proteins. Both the long-lived Ub-M-GFP-OVA (Fig. 1A) and the short-lived UB-R-GFP-OVA (Fig. 1B) were sequestered in punctuates and colocalized with tomato-LC3 after treatment with Bortezomib and NH4Cl. Interestingly, GFP-Ub also colocalized with tomato-LC3 in the punctuates, suggesting that ubiqutinated proteins, the substrates of proteosome degradation, were also packaged in the autophagosomes (Fig. 1C).
Figure 1.
Colocalization of the long-lived M-GFP-OVA, short-lived R-GFP-OVA, and GFP-ubiquitin with tomato-LC3 fusion protein in punctuates after proteasome and lysosome inhibition. (A). Colocalization of M-GFP-OVA and Tomato-LC3 in punctuates. (B). Colocalization of R-GFP-OVA and Tomato-LC3 in punctuates. (C). Colocalization of GFP-Ubiquitin and Tomato-LC3 in punctuates. HEK 293T cells that are stably expressing Tomato-LC3 were transfected with plasmids that express M-GFP-OVA, R-GFP-OVA, or GFP-ubiquitin separately and cultured for 48 hours. Cells were then treated with 100 nM bortezomib and 10 mM NH4Cl for 24 hours. Untreated cells were used as control. Arrows denote the spots of colocalization.
We isolated DRibbles from HEK 293T cells that expressed the different forms of OVA fusion proteins by differential centrifugation as described before (8). Western blot analysis of these DRibbles showed the presence of not only long-lived proteins, but also short-lived proteins, short protein fragments, possibly DRiPs, as well as large amounts of ubiqutinated proteins (Supplementary Fig. S2A). The model antigens including M (methionine)-GFP-OVA (long-lived, cytosolic), R (arginine)-GFP-OVA (short-lived, cytosolic), M-GFP-TfR-OVA (long-lived, membrane-bound), and R-GFP-TfR-OVA (short-lived, membrane-bound), were all found in DRibbles.
As expected, the typical autophagosome marker, LC3-II, was present in DRibbles. Compared to vesicles isolated from untreated cells (Natural DRibbles), DRibbles from Bortizomib-treated cells were enriched in antigens. These data suggest that DRibbles packaged a broad range of cellular antigens capable of priming a broad repertoire of T cells that would be specific for both long- and short-lived TAAs. Furthermore, heat shock proteins, such as HSP 90 and HSP94, were also present in the DRibbles (Supplementary Fig. S2B). Heat shock proteins have been shown to chaperone protein fragments and to augment antigen cross-presentation by binding to HSP receptors on DCs (21, 22). Interestingly, calreticulin, a calcium-binding chaperone located in the ER, and the high mobility group box 1 protein (HMGB1), were also found in DRibbles. The presence of damage-associated molecular pattern (DAMP) signals, such as HSP, HMGB1, and calreticulin, in the DRibbles suggests that DRibbles may be able to activate the innate pattern-recognition receptors and bridge innate and adaptive immune responses (23).
DRibbles from 293T cells were efficient in activating naïve CD8+ T cells when loaded onto DCs
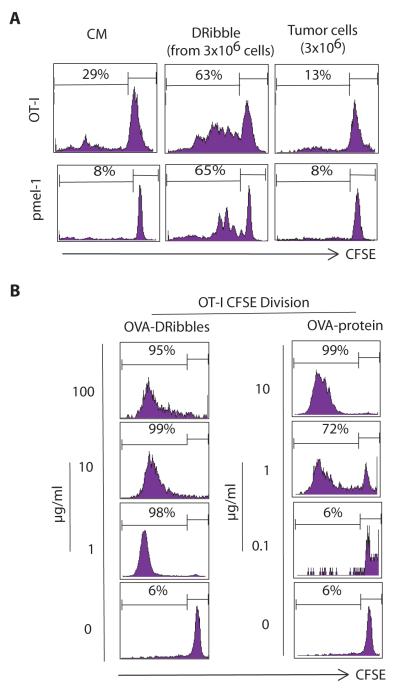
To determine whether DRibbles could efficiently stimulate naïve CD8+ T cells, we loaded DRibbles from HEK 293T cells that expressed the M-GFP-OVA or gp100 onto induced splenic DCs generated by sequential i.v. injection of Flt3-ligand and GM-CSF encoding plasmid DNA (8). Subsequently, we examined the activation and proliferation of naive OT-I T cells or pmel-1 T cells by flow cytometry analyssi. DCs loaded with DRibbles containing the M-GFP-OVA were highly efficient at stimulating OT-I T cell proliferation (63% T cells divided) (Fig. 2A). In contrast, DCs pulsed with an equivalent number of irradiated whole cells barely stimulated OT-I T cells. Similarly, DCs pulsed with gp100-DRibbles showed robust capacity at activating naïve pmel-1 T cells, whereas DCs loaded with irradiated whole cells failed to do so. Surprisingly, when loaded onto DCs, DRibbles at a concentration of 1 μg/ml total protein could drive 98~99% OT-I T cells to proliferate, while a much higher concentration of the pure OVA protein (10 μg/ml) was needed to drive a similar level of T-cell proliferation (Fig. 2B). These results showed that when loaded onto DCs, DRibbles, as a source of tumor antigens, were superior in activating antigen-specific CD8+ T cells as compared to irradiated whole cells or even pure proteins.
Figure 2.
DRibbles were highly efficient antigen carriers for cross-presentation of antigens to naïve CD8+ T cells. DRibbles were collected from HEK 293T cells that expressed M-GFP-OVA protein or the human melanoma antigen gp100. DCs were loaded with the indicated amount of antigen-expressing DRibbles, irradiated antigen-expressing cells, or pure OVA/gp100 protein. CFSE-labeled OT-I, or pmel-1 transgenic T cells were added. Activation of T cells was assessed by dilution of CFSE in the labeled T cells at day 4 or 6. (A). DCs pulsed with DRibbles were superior to DCs pulsed with irradiated 6 whole tumor cells at activating OT-I and pmel-1 CD8+ T cells. DRibbles from 3×106 HEK 293T cells that expressed OVA or gp100, or equivalent number of antigen-expressing irradiated cells were loaded onto DCs. (B). DCs pulsed with DRibbles were more efficient (on a μg basis) than pure proteins in stimulating OT-I CD8+ T cells. Data are representative of 3 independent experiments with similar results.
Cross-presentation of DRibble antigens involved CD8+ DC-specific C-type lectin receptor, CLEC9A
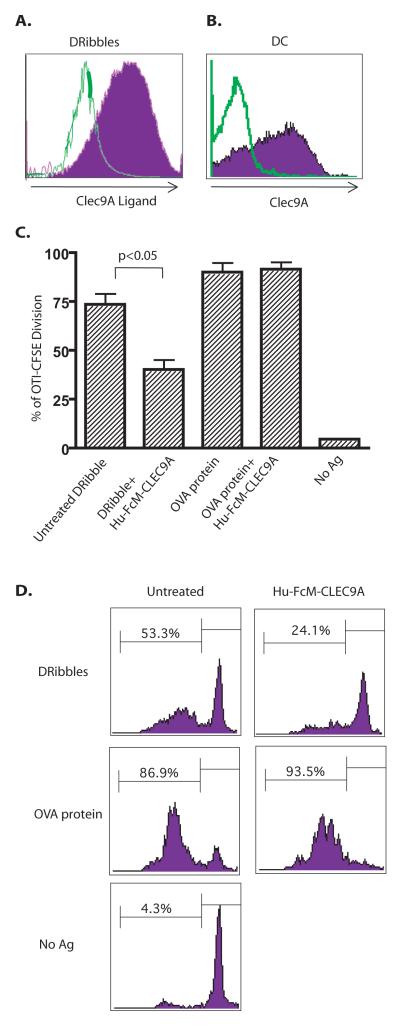
Recently, a novel C-type lectin receptor, CLEC9A, was shown to be critical for efficient cross-presentation of dead cell materials by binding to a preformed ligand that is exposed only when cells undergo necrosis (17). Because DRibbles were released from Bortezomib and NH4Cl-treated dead or dying tumor cells, we hypothesized that DRibbles could express CLEC9A ligand and CLEC9A may be a receptor for efficient cross-presentation of DRibble antigens. To test this hypothesis, we prepared a soluble receptor fusion protein by fusing the mutated human IgG1 Fc domain with isolucine zipper and the C-type lectin domain of CLEC9A (FcmILZ-CTLD9A). The size of DRibbles was evaluated by using microbeads with standard sizes on a modified flow cytometer (Supplementary Figure S3). We found abundant expression of the CLEC9A ligand on our DRibble preparation (Fig. 3A). Meanwhile, CLEC9A receptor is expressed by the majority of the Flt3L-induced splenic DCs that were used in the cross-presentation assay with anti-CLEC9A antibody (Fig. 3B). Caetano Reis e Sousa’s and Mireille H. Lahoud’s groups reported CLEC9A expression on the CD24high subset DCs that were generated by culturing bone marrow cells in media supplemented with recombinant Flt3L. CD24+ DCs were the precursors of CD8α+ DCs. Consistent with their reports, our unpublished data showed that the majority of our Flt3L-induced splenic DCs expressed CD24, and these CD24+ DCs were CLEC9A positive. Most importantly, we also found that pre-incubation of DRibbles with the soluble FcmILZ-CTLD9A but not the FcmILZ-OX40L (control fusion protein) significantly inhibited cross-presentation (Fig. 3C and D). In contrast, the same FcmILZ-CTLD9A fusion protein did not affect cross-presentation of the soluble OVA protein, which is known to be endocytosed through the mannose receptor (24).
Figure 3.
The involvement of CLEC9A in cross-presentation of DRibbles in vitro. (A). The majority of DRibbles expressed a ligand for the CLEC9A receptor. DRibbles harvested from HEK 293T cells were incubated with the soluble FcmILZ-CTLD9A fusion protein (filled histogram) for 1 hour. The soluble FcmILZ OX40 ligand fusion protein was used as negative control (open histogram). PE-conjugated anti-human Fc Fab was used to binding by flow cytometry. (B). CLEC9A was detected on the surface of the majority of splenic DCs from mice that had received sequential injection of plasmid DNA encoding Flt3 ligand and GM-CSF. All events were gated on CD11c+ population. The open histogram shows the isotype control. The filled histogram shows expression of CLEC9 A. (C) (bar graph)&(D) (histogram). Cross-presentation of DRibble-OVA, but not soluble OVA protein, was significantly reduced by incubating DRibbles with soluble FcmILZ CTLD9A. DRibbles were pretreated with soluble FcmILZ-CTLD9A overnight at 4°C and then used to pulse DC for 6 hours. Percentages of divided OT-I T cells are shown as mean±SEM. Data are representative of results from 2-4 independent experiments.
Cross-presentation of OVA antigen in DRibbles by DCs involved both caveolae and clathrin-mediated endocytosis, ERAD, and proteasomes/TAP1 pathways
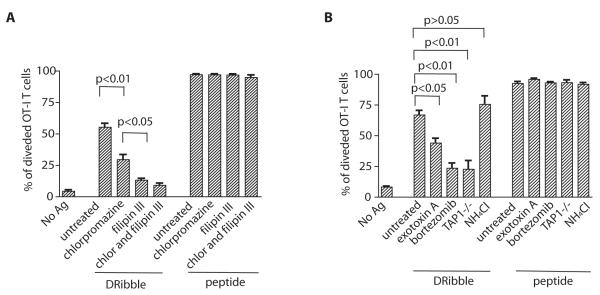
The diameter of DRibbles, as measured by EM, varied from 300 nm to 900 nm. The sizes are in the range of typical of autophagosmes of mammalian cells (25). Thus, it is most likely the phagocytosis pathway is involved in the cross-presentation of DRibbles. First, we examined the contribution of clathrin- and caveolae-dependent pathways in the internalization of DRibbles with selective inhibitors of these two pathways. We pretreated DCs with either chlorpromazine, which inhibits clathrin-mediated endocytosis by perturbing clathrin processing, or filipin III, which complexes with membrane cholesterol and blocks caveolae-mediated endocytosis before they were pulsed with OVA-DRibbles or the SIINFEKL peptide (18). Cross-presentation of OVA-DRibbles was significantly reduced when DCs were pretreated with either chlorpromazine or filipin III (Fig. 4A). In contrast, these inhibitors did not affect the ability of peptide-pulsed DCs to stimulate OT-I T cells. The data suggested involvement of both pathways in DRibble cross presentation. Moreover, inhibition by filipin III was more effective than chlorpromazine, suggesting cross-presentation of DRibbles by DCs predominantly utilize the caveolae-mediated endocytosis pathway. Because caveolae-mediated endocytosis routes antigens away from lysosomes, its involvement could contribute to the high efficiency of antigen cross-presentation mediated by DRibbles.
Figure 4.
Multiple pathways are involved in cross-presentation of DRibble antigens. (A). Inhibition of caveolae (filipin III) and/or clathrin-mediated (chlorpromazine) endocytosis diminished cross-presentation of ovalbumin by DRibble-pulsed DCs. DCs loaded with SIINFEKL peptides were used as a control. Division of CFSE-labeled OT-I T cells was monitored by flow cytometry. (B). Blockade of ERAD-mediated retrotranslocation, proteosome activity, or knockout of TAP1, but not blockade of lysosomal activity markedly reduced cross-presentation of OVA- DRibble. The results shown in the bar graph are expressed as the percentage±SEM of OT-I T cells that had undergone at least one division 4 days after coculture with DCs loaded with antigens. Data are representative of three independent experiments.
The intracellular routes of cross-presentation after antigen uptake include the proteosome/TAP-independent peptide loading on MHC I in ER-phagosomes (16, 26, 27), and the most common proteosome/TAP-dependent peptide loading on MHC I in ER, endosomes, or phagosomes (20, 28, 29). Next we explored whether cross-presentation of DRibble antigens requires proteosome, TAP1, or lysosomal activity in DCs. As shown in Fig. 4B, the OVA antigen in DRibbles was cross-presented via a TAP-dependent intracellular pathway. TAP1-deficient DCs loaded with OVA-DRibble barely activated OT-I T cells. Inhibition of proteosome activity in DCs dramatically diminished cross-presentation of the OVA-DRibbles. The data demonstrated that like most exogenous antigens, proteosome degradation and TAP1-dependent peptide loading onto MHC I in DCs were critical for cross-presentation of antigens in DRibbles. These results indicate that antigens inside DRibbles either managed to exit the double membrane of autophagosomes or the autophagosomes involved in cross-presentation were not yet fully closed. Although the mechanisms by which the exogenous antigens exit the endosomes and enter the cytosol are still controversial, some studies have shown that ER-associated degradation (ERAD) machinery, present in the endosomes/phagosomes, is involved in transporting proteins to the cytosol (30). To assess whether the ERAD pathway is also involved in the cross-presentation of antigens in DRibble, we pretreated DCs with exotoxin A (ExoA) before pulsing with DRibbles. ExoA is a toxin derived from Pseudomonas aerugenosa. Peter Cresswell’s group have used ExoA to successfully block translocation of ERAD substrates into the cytosol through the Sec61 channel and demonstrated that ERAD was critical for cross-presentation of soluble OVA protein or OVA-IgG immune complexes (31, 33). Using this approach, we found that pretreatment of DC with ExoA partially inhibited T-cell activation by DRibble-loaded DCs (Fig. 4B), suggesting that ERAD-mediated translocation was involved in the cross-presentation of OVA-DRibbles. Treatment of DCs with NH4Cl, which inhibits lysosomal-mediated degradation, did not decrease cross-presentation of OVA-DRibbles. The ability of peptide-loaded DC to activate OT-I T cells was not affected by treatment with ExoA, bortezomib, and NH4Cl (Fig. 4B). In short, cross-presentation of DRibble antigens involved the ERAD pathway, required proteosome activity and TAP1, but not lysosomal degradation.
Tumor cell-derived DRibbles contained large amount of autophagosomes
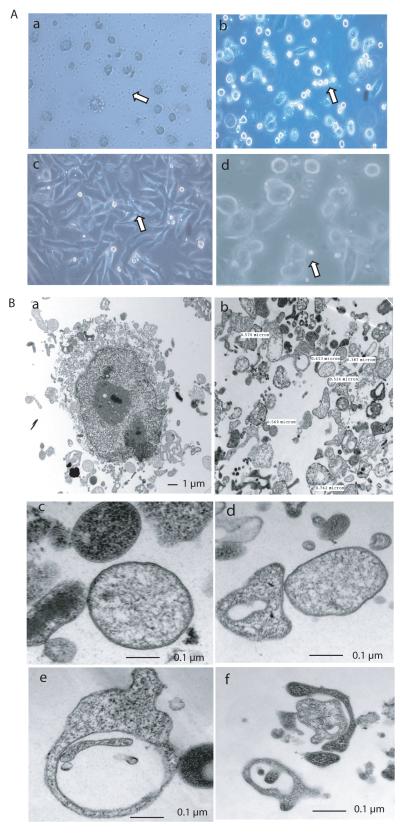
Next we investigated the feasibility of making DRibbles from a variety of tumor cell lines. DRibbles were visualized by light microscopy after tumor cells were treated with Bortezomib and NH4Cl. These included human (non-small cell lung cancer and melanoma) and mouse (3LL and B16F10) cell lines (Fig. 5A). DRibbles derived from 1×106 cells typically contained 10~100 μg proteins depending on the cell line used. To visualize the fine structure of DRibbles, we examined DRibbles from the 3LL mouse Lewis lung cancer cells with the transmission electron microscope (Fig. 5B). Many vesicles were released from the treated cells. DRibbles were harvested from the culture supernatant of the treated cells using differential centrifugation. A large number of vesicles with a unique double-membrane structure were found in the DRibble preparation. The majority of them were 300 nm~1 μm in size, which falls in the range of the typical size of autophagosomes from mammalian cells (25). The light density of these vesicles varied too, indicating that while some of the vesicles were autophagosomes, others maybe amphisomes (Figure 5B-c,d) (32). Some of the vesicles included multiple smaller double-membrane structures (Figure 5B-e). A phagophore that was still packaging cellular materials was also found in DRibble preparations (Figure 5B-f).
Figure 5.
DRibbles harvested from tumor cells were enriched in autophagosomes and induced highly efficient cross-priming of CD8+ T cells in vitro. (A). DRibbles were induced from a variety of tumor cell lines, including human non-small lung cancer cells (a), mouse Lewis lung cancer 3LL cells (b), human melanoma FEMX cells (c), and mouse melanoma B16F10 (d). Images were taken with a light microscope. Small vesicles indicated by the arrowheads were distinctive morphologically from large cells. (B). Transmission electron micrographs of autophagosome-rich DRibbles harvested from mouse Lewis lung 3LL tumor cells. a. A large tumor cell is surrounded by a number of vesicles. b. Multiple vesicles with a variety of morphology in DRibbles. Their sizes ranged between 100 nm and 1μm. c. Fine structure of autophagosomes with the typical double-membrane structure and differential light density. d. Display of autophagosomes with different morphology. e. An autophagosome with a clear vacuole inside. f. A phagophore in the process of packaging a small vesicle. TEM images were collected at original magnifications of 1,000-37,000x.
Anti-tumor efficacy of DCs loaded with 3LL-dervied DRibbles in 3LL Lewis lung cancer model
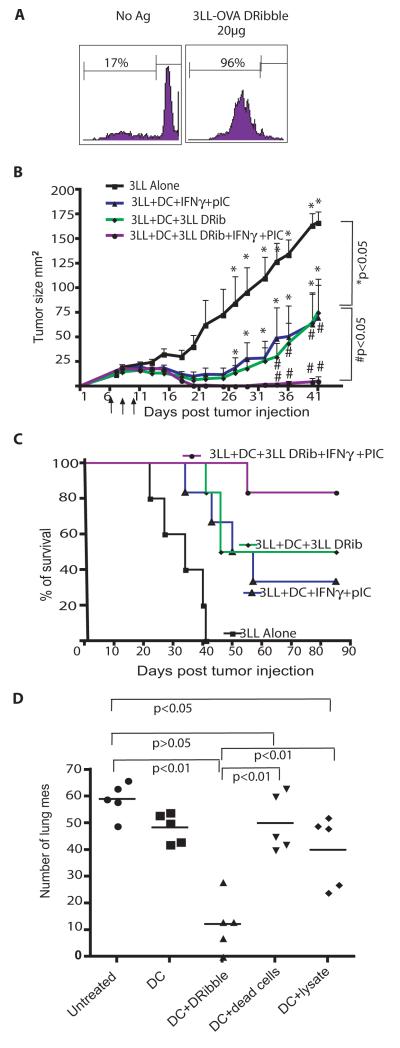
First, we assessed whether 3LL-derived DRibbles could cross-prime T cells. DRibbles were prepared from 3LL tumor cells that expressed the Ub-M-GFP-OVA model antigen. When DCs were loaded with 20 μg 3LL-OVA DRibbles before they were used to stimulate naïve OT-I T cells, 96% of the OT-I T cells proliferated in vitro (Fig. 6A). IFN-γ induces DC to produce IL-12 p70, immunoproteasomes and polarizes the immune response to Th1 type (33, 34). Toll-like receptor (TLR) agonists engage TLR on DC to induce vigorous inflammatory responses and set the stage for later adaptive immune responses (35, 36). Hence in this tumor model, we used TLR3 agonist poly [I:C] in combination with IFN-γ to treat DCs during antigen loading to improve the efficacy of DRibble vaccine. Mice bearing 6-day established 3LL tumor (s.c.) were vaccinated with DCs pulsed with DRibbles collected from parental 3LL tumor cells, or DCs loaded with DRibble in the presence of IFN-γ and poly [I:C]. Untreated mice or mice treated with unloaded DCs served as controls. Vaccination with DCs loaded with DRibbles alone inhibited tumor growth (Fig. 6B) and significantly improved survival (Fig. 6C) (P<0.05) compared to untreated control. The treatmen of DCs with IFN-γ and poly [I:C] remarkably enhanced the therapeutic efficacy of the DRibble-pulsed DC vaccine; five out of six treated mice were rendered tumor-free and survived more than 80 days.
Figure 6.
Vaccination with DCs pulsed with 3LL tumor cell-derived DRibbles showed significant anti-tumor effect in mice bearing 3LL tumors. (A). DRibbles derived from 3LL cells that expressed OVA stimulated proliferation of transgenic OT-I T cells in vitro. (B and C). The anti-tumor efficacy of DRibble vaccine in mice bearing s.c. tumors. The vaccination with DRibble-loaded DCs plus poly [I:C] and IFN-γ mediated tumor regression (B) and cured 5 out of 6 mice with 6-day 3LL s.c. lung tumors (C). Data are representative of three independent experiments. (D). Therapeutic efficacy of DCs loaded with DRibbles, lysates, or whole tumor cells. This is representative of two experiments with similar results.
To compare the anti-tumor effect of DRibbles with other forms of tumor antigens, we i.v injected 3LL tumor cells into C57BL/6 mice, and vaccinated these mice with DCs loaded with 3LL DRibbles, dead cells, cell lysate in the presence of IFN-γ and poly [I:C] 3 days later. Vaccination with DRibble-loaded DCs showed significantly better ability in reducing the number of lung metastases as compared to DCs loaded with dead tumor cells and lysates (Figure 6D).
We also evaluated the antitumor efficacy of DRibble vaccination in B16F10 tumor model. C57BL/6 mice bearing 6-day B16F10 tumor were irradiated at 500 cGy, and then were adoptively transferred with 5×106 naïve spleen cells from pmel-1 TCR transgenic mice. These mice received four s.c. vaccinations at days 7, 10, 13, and 20 with 3×106 DCs loaded with DRibbles made from HEK 293T cells that expressed gp100. IFN-γ was used to treat DCs to improve DC function. We observed that IFN-γ increased production of IL-12 p70 (Supplementary Fig. S4A) by DCs. Vaccination with DCs loaded with gp100-DRibbles without IFN-γ slightly increased the number of pmel-1 T cells in the peripheral blood. DC loaded with DRibbles and IFN-γ induced a dramatic expansion of pmel-1 T cells (Supplementary Fig. S4B). Accordingly, DCs pulsed with DRibble alone delayed tumor growth (p<0.05) (Supplementary Fig. S4C) and prolonged the median survival time of mice (38 days for vaccinated group vs. 26 days for control) (Supplementary Fig. S4D), whereas DCs pulsed DRibbles and IFN-γ markedly enhanced median survival time of mice (from 38 to 59 days). DCs alone did not have any anti-tumor effect in this model (data not shown).
Discussion
In the past decade, a number of cancer vaccines have been used in specific active immunotherapy trials for patients with melanoma, breast, and prostate cancers (37). These vaccines are based on peptides, partial or full-length proteins, whole tumor cells, and TAA-loaded DCs (38, 39). The overall clinical response rate in cancer patients following active immunotherapy with vaccine was reported to be only 3.3%, using conventional response criteria (40). These unsatisfactory clinical outcomes may be a consequence of one or more of the followings: 1) poor immunogenicity, likely due to poor cross-presentation of TAAs in vaccines 2) tumor immune escape due to antigen loss or defects in the antigen processing machinery, and 3) immune suppression induced by myeloid-derived suppressor cells (MDSCs) and T regulatory (Treg) cells. Therefore, efficient cross-presentation, inclusion of multiple tumor antigens, and reversal of immune suppression are important elements for the success of cancer vaccines.
We developed a novel autophagosome-based DRibble vaccine that facilitates efficient cross-presentation of multiple antigens to CD8+ T cells. Four unique characteristics of this novel vaccine could explain the high efficiency of cross-presentation of antigens in DRibbles and their superior antitumor activity. First, the DRibble vaccine incorporated SLiPs and DRiPs in autophagosomes. These SLiPs and DRiPs are not efficiently cross-presented by DCs under normal conditions. After inhibition of proteosome- and lysosome-mediated degradation, SLiPs and DRiPs became available for cross-presentation because they are shunted into autophagosomes when their degradation was blocked. Cross-presentation of SLiPs or DRiPs may be particularly beneficial for anti-tumor response, because they may give rise to most of the peptides presented on MHC class I molecules expressed on tumor cells (41). Cross-priming of CD8+ T cells specific for tumor SLiPs could increase the efficiency of tumor recognition and destruction. Second, an immune response to a diverse repertoire of TAA in the vaccine may avoid immune escape via differential or altered expression of a particular antigen or MHC I molecule. Vaccines consisting of multiple epitopes or undefined antigens derived from tumor cells have given better clinical responses than singe peptide or protein (42). Third damage-associated molecular pattern (DAMP) molecules, such as HSP90, HSP94, calreticulin, and HMGB1 were also present in the DRibbles. Heat shock proteins, best known as chaperones, have been shown to play a critical role in preserving the antigen repertoire and augmenting cross-presentation of antigens (43). Calreticulin, an ER calcium-binding chaperone, was shown to be essential for triggering immunogenic responses by dying tumor cells (23). HMGB1, a DAMP molecule released by necrotic cells, augments cross-presentation through interacting with RAGE (receptors for advanced glycosylation), TLR2, TLR4 on DCs (44, 45). Thus, these DAMP molecules in the DRibble preparation could serve as natural adjuvant during the vaccination for cancer therapy. DCs also express a variety of C-type lectin proteins which function as TLR independent pathogen pattern recognition receptors (PRR) and recognize viruses, bacteria, fungi, and even necrotic cell corpses. These receptors include DC-SIGN (CD209), langerin (CLEC4K, CD207), Dectin 1 (CLEC7A), Dectin 2 (CLEC6A), MICL (CLEC12A), DNGR 1 (CLEC9A), DCIR (CLEC4A), Dec 205 (CD205), etc. C-type lectin receptor-mediated signaling after pattern recognition induces differential cytokine production from DCs and thereby helps shape the immune response (46). CLEC9A, a member of C-type lectin family, is expressed on CD8α+ DCs and their precursors (CD8 α− CD24+ DCs), which are best known for cross-presenting cell-associated antigens (47, 48). Recently, CLEC9A was shown to recognize dead cell remnants and play a critical role in cross-presentation of antigens from necrotic cells (17). These features suggested that targeting CLEC9A could be a novel strategy in future DC-based vaccine design (17). Interestingly, DRibbles could directly bind to CLEC9A, and we showed that CLEC9A was involved in cross-presentation of DRibble antigens. These results suggested that involvement of CLEC9A in recognition or subsequent processing of DRibbles by DCs. Consistent with Caetano Reis e Sousa group’s findings (47), we observed that CLEC9A did not affect the uptake of DRibbles. CLEC9A may facilitate cross-presentation at the intracellular routing or processing level. Because of these unique characteristics and to distinct it from conventional pathways, we refer this novel autophagosome mediated cross-presentation pathway as autophagy-assisted antigen cross-presentation pathway.
Imaging by light microscopy and transmission electron microscopy revealed that autophagosomes are the major component of the DRibbles. Autophagosomes are characterized by their double-membrane structure. Our data show that successful cross-presentation of antigens in DRibbles requires intact caveolae and clathrin-mediated endocytosis, as well as proteosome, and TAP. ERAD pathway was also involved. Nevertheless, lysosomal activity appeared not to be necessary. Based on these findings, we envision the following pathway for cross-presentation of autophagosome antigens: Initially, the autophagosomes enter cells via caveolae-dependent and clathrin-dependent endocytosis. During entry, the CLEC9A receptor binds to autophagosomes and directs them into non-acidic compartments, e.g. early phagosomes/endosomes or other intracellular compartments suitable for efficient cross-presentation. Subsequently, the antigens are first released from autophagosomes into phagosomes/endosomes and translocated into the cytosol through the ERAD machinery, and degraded by the proteosome. The peptide products are imported into either the ER or endosomes/phasosomes, loaded onto MHC I complex, and presented on the cell surface (Supplementary Fig. S5).
Finally we showed that DRibbles, or DRibbles loaded onto DCs, in combination with IFN-γ and TLR agonist, induced robust anti-tumor responses against established 3LL lung carcinoma and B16F10 melanoma. Combining DRibble vaccine with agents that reverse the immune suppression in tumor-bearing hosts could further enhance the anti-tumor effect. Based on these findings, we begun a phase I/II clinical trial of the DRibble vaccine in patients with non-small cell lung cancer. We are planning a similar trial in breast cancer patients. Future studies will be focused on analysis of potential DAMP molecules, the nature of tumor antigens, CLEC9A ligands that are involved in cross-presentation, and the detailed analysis of immune responses induced by DRibble vaccines.
Supplementary Material
Translational Relevance.
A perfectly designed cancer vaccine should target antigens that are abundantly present on the surface of tumor cells and have not been exposed to immune system. These targets would be packaged with the appropriate chaperones, include TLR signals and be directed to the most efficient APCs, such as dendritic cells that can migrate into or reside in lymph nodes, so that vaccines would stimulate potent therapeutic activity. This report identifies these properties in “DRibble” autophagosome-enriched vaccines that we have recently described as an effective immunotherapy agent. In the accompanying paper we report (Twitty etal.,) that this vaccination strategy, unlike vaccination with whole tumor cells, primes T cells which recognize a spectrum of related cancer cells and provides cross-protection of chemically-induced tumors. Together these two reports provide insights into why this vaccine strategy, already in a clinical trial, efficiently cross-presents tumor/tumor-associated antigens. This is an important step, but effective immunotherapy will likely require a multimodality combination therapy approach.
Acknowledgements
We thank Drs. Caminshi, Reis e Sousa, and Bown for their general gift of anti-CLEC9A antibody. We thank Eric Barklis, Mike Webb and the OHSU EM Core Facility for electron microscopy sample preparation and imaging. We thank E. Akporiaye, S. Shu, and C. Twitty for their input.
Grant Support This work was supported by NIH grants CA107243 (HMH), CA141278 (HMH) and CA 123864 (WJU), the Susan G. Komen Breast Cancer Foundation, the Providence Portland Medical Foundation, and the Safeway Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest H-M. Hu and B.A. Fox are co-founders of UbiVac, which has licensed the autophagosome intellectual property.
References
- 1.Dikic I, Johansen T, Kirkin V. Selective autophagy in cancer development and therapy. Cancer Res. 2010;70(9):3431–4. doi: 10.1158/0008-5472.CAN-09-4027. [DOI] [PubMed] [Google Scholar]
- 2.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nedjic J, Aichinger M, Mizushima N, Klein L. Macroautophagy, endogenous MHC II loading and T cell selection: the benefits of breaking the rules. Curr Opin Immunol. 2009;21:92–97. doi: 10.1016/j.coi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Heath RJ, Xavier RJ. Autophagy, immunity and human disease. Curr Opin Gastroenterol. 2009;25:512–520. doi: 10.1097/MOG.0b013e32833104f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippe R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gueguen M, Long EO. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci U S A. 1996;93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yewdell JW, Anton LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- 11.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, Urba WJ, Hu HM. Cross-presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy. 2009;5:576–577. doi: 10.4161/auto.5.4.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 15.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 16.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20:89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norbury CC, Basta S, Donohue KB, Tscharke DC, Princiotta MF, Berglund P, Gibbs J, Bennink JR, Yewdell JW. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–1321. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 21.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 22.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 24.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 25.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, Sahara H, Torigoe T, Hiratsuka H, Sunakawa H, Hirata K, Sato N. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 27.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, Houde M, Desjardins M, Sher A, Sacks D. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 28.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 29.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci U S A. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 33.Groettrup M, van den Broek M, Schwarz K, Macagno A, Khan S, de Giuli R, Schmidtke G. Structural plasticity of the proteasome and its function in antigen processing. Crit Rev Immunol. 2001;21:339–358. [PubMed] [Google Scholar]
- 34.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 35.Zheng R, Cohen PA, Paustian CA, Johnson TD, Lee WT, Shu S, Koski GK. Paired Toll-like receptor agonists enhance vaccine therapy through induction of interleukin-12. Cancer Res. 2008;68:4045–4049. doi: 10.1158/0008-5472.CAN-07-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittendorf EA, Peoples GE, Singletary SE. Breast cancer vaccines: promise for the future or pipe dream? Cancer. 2007;110:1677–1686. doi: 10.1002/cncr.22978. [DOI] [PubMed] [Google Scholar]
- 38.Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, Hortobagyi GN, Kudelka AP, Grabstein KH, Cheever MA, Ioannides CG. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369-377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002;8:3407–3418. [PubMed] [Google Scholar]
- 39.Avigan D, Vasir B, Gong J, Borges V, Wu Z, Uhl L, Atkins M, Mier J, McDermott D, Smith T, Giallambardo N, Stone C, Schadt K, Dolgoff J, Tetreault JC, Villarroel M, Kufe D. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 40.Curigliano G, Spitaleri G, Dettori M, Locatelli M, Scarano E, Goldhirsch A. Vaccine immunotherapy in breast cancer treatment: promising, but still early. Expert Rev Anticancer Ther. 2007;7:1225–1241. doi: 10.1586/14737140.7.9.1225. [DOI] [PubMed] [Google Scholar]
- 41.Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Neller MA, Lopez JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–295. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccines. 2008;7:1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 44.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 45.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Shin Teh J, Lo JC, Rizzitelli A, Wu L, Vremec D, van Dommelen SL, Campbell IK, Maraskovsky E, Braley H, Davey GM, Mottram P, van de Velde N, Jensen K, Lew AM, Wright MD, Heath WR, Shortman K, Lahoud MH. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–16701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.