Abstract
BACKGROUND:
The molecular adsorbent recirculating system (MARS) is an albumin-dialysis modality that has been investigated predominantly in patients with acute and acute-on-chronic liver failure.
OBJECTIVES:
To report the clinical efficacy and safety of MARS therapy for intractable pruritus in cholestasis patients with stable chronic liver disease, characterizing the impact of MARS on cytokine levels and on the transcriptome in the blood compartment.
METHODS:
MARS therapy was performed on three patients with cholestatic liver disease using 8 h runs for two consecutive days. The expression levels of 65 cytokines/chemokines and 24,000 genes were profiled by Luminex (Luminex Corporation, USA) and microarray, respectively.
RESULTS:
A quality-of-life assessment demonstrated a marked improvement during therapy, which was sustained in two of three patients. No bleeding or infectious complications were observed. Bile acid levels were markedly reduced following MARS (mean [± SD] pretreatment 478.9±112.2 μmol/L versus post-treatment 89.7±68.8 μmol/L). Concordant decreases in cytokine/chemokine levels were noted for interleukin (IL)-1beta, IL-2, IL-6, IL-8, IL-12 (p40), RANTES, tranforming growth factor-alpha, tumour necrosis factor-alpha and thrombopoietin following MARS. On microarray profiling, biologically relevant concordant changes among all patients were evident for 20 different genes (10 upregulated and 10 downregulated). The upregulation of several potentially immune suppressive/regulatory genes (eg, early growth response 3 [EGR-3], ephrin-A2 [EFNA2] and serum amyloid A1 [SAA1]), concurrent with downregulation of genes involved in innate immunity (eg, toll-like receptor 4 interactor with leucine-rich repeats [TRIL]) and inflammation (eg, ephrin receptor B1 [EPHB1]), was observed.
CONCLUSIONS:
This investigative approach offers new insights into intractable pruritus and suggests future therapeutic targets. The clinical benefit of MARS in cholestasis patients with intractable pruritus may not exclusively result from filtration of pruritogens, but also from systemic changes in cytokine/chemokine levels and changes in gene expression of blood cells.
Keywords: Cholestasis, Cytokine, Gene expression, Intractable pruritus, MARS
Abstract
HISTORIQUE :
Le système de recirculation adsorbant moléculaire (SRAM) est une modalité de dialyse de l’albumine qui a surtout été mis à l’essai chez les patients ayant une insuffisance hépatique aiguë ou aiguë à chronique.
OBJECTIFS :
Rendre compte de l’efficacité et de l’innocuité cliniques d’une thérapie par SRAM pour soigner un prurit réfractaire chez les patients ayant une cholestase et une maladie hépatique chronique stable, afin de caractériser les répercussions du SRAM sur les taux de cytokine et sur le transcriptome du compartiment sanguin.
MÉTHODOLOGIE :
Trois patients ayant une maladie hépatique cholostatique ont reçu un traitement par SRAM toutes les huit heures pendant deux jours consécutifs. Les chercheurs ont profilé le taux d’expression de 65 cytokines et chémokines et de 24 000 gènes par Luminex (Luminex Corporation, États-Unis) et par microréseaux, respectivement.
RÉSULTATS :
Une évaluation de la qualité de vie a démontré une amélioration marquée pendant le traitement, qui s’est maintenue chez deux des trois patients. Les chercheurs n’ont observé aucune complication hémorragique infectieuse. Les taux d’acide biliaire étaient considérablement plus faibles après le SRAM (moyenne [±ÉT] de 478,9±112,2 μmol/L avant le traitement par rapport à 89,7±68,8 μmol/L après le traitement). Les chercheurs ont constaté des diminutions concomitantes des taux de cytokine et de chémokine de l’interleukine (IL)-1bêta, de l’IL-2, de l’IL-6, de l’IL-8, de l’IL-12 (p40), des protéines RANTES, du facteur de croissance transformant alpha, du facteur de nécrose tumorale alpha et de la thrombopoïétine après le SRAM. Au profilage par microréseaux, tous les patients présentaient des changements à 20 gènes différents concordants sur le plan biologique (dix surexprimés et dix sous-exprimés). Ils ont observé la surexpression de plusieurs gènes au potentiel immunosuppressif ou régulateur (p. ex., réponse de croissance précoce 3 [EGR-3], éphrine A2 [EFNA2] et amyloïde sérique A1 [SAA1]), conjointement avec une sous-expression des gènes participant à l’immunité innée (p. ex., récepteur de type Toll 4 comportant des motifs répétés riches en leucine [TRIL]) et à l’inflammation (p. ex., récepteur d’éphrine B1 [EPHB1]).
CONCLUSIONS :
Cette démarche de recherche donne un nouvel éclairage sur le prurit réfractaire et ouvre la voie à de futures cibles thérapeutiques. Les bienfaits cliniques du SRAM chez les patients ayant une cholestase et un prurit réfractaire ne résultent peut-être pas exclusivement du filtrage des pruritogènes, mais également de changements systémiques aux taux de cytokine et de chémokine ainsi que de l’expression génique des globules sanguins.
Pruritis (itch) is one of the most common debilitating symptoms in patients with cholestatic liver disease. In these patients, pruritus has a significant impact on quality of life (QoL), mental health and, in severe, otherwise intractable cases, may require assessment for liver transplantation. However, these patients are often not prioritized for organ allocation in systems based on the current listing criteria (ie, Model for End-stage Liver Disease score).
The physiopathology of cholestatic pruritus has not been completely elucidated. Current treatment options for intractable pruritus often target underlying cholestatic liver disease (ursodeoxycholic acid) or the removal of bile acids (1–6). Anion exchange resins (cholestyramine and colesevelam) and enzyme-inducing agents (rifampin) act mainly by increasing the clearance of bile acids (7–9). However, cholestatic pruritus may not improve despite marked reduction in bile acid levels; conversely, improvement achieved with these medications may be sustained regardless of rebound increases in bile acids on cessation of therapy. Other therapies (naloxone, naltraxone, nalmefene) prevent the binding of endogenous opioid agonists, the levels of which are elevated in cholestasis (10–13). Recently, the pruritogenic potential of lysophosphatidic acid has been examined in intrahepatic cholestasis of pregnancy and primary biliary cirrhosis (PBC) (14). To date, medical therapies have not been developed to specifically target this pathway.
More invasive therapeutic modalities have been used when conventional approaches have proven ineffective. Results for plasmapheresis have been positive but lack large patient numbers (15–17). Albumin dialysis-based therapies, such as the molecular adsorbent recirculating system (MARS, Gambro, Sweden), have previously been investigated predominantly in acute and acute-on-chronic liver failure, in which the removal of both albumin-bound (ie, bilirubin, bile acids, middle- and short-chain fatty acids) and small water-soluble molecules (ie, ammonia, creatinine, cytokines, urea) have been characterized. MARS has also been used in patients with cholestatic chronic liver disease for relief of intractable pruritus. Several case series have shown improvement in biochemical parameters (bile acids) and in QoL by visual analogue scores in these patients without, nonetheless, offering new insights on how the clinical benefit may be obtained.
While MARS therapy appears to have a significant effect on bile acids, we hypothesized that there may be novel pruritogenic pathways that may be modified by albumin dialysis therapy. In the present pilot study, we report the clinical efficacy and safety of MARS therapy for intractable pruritus in three cholestasis patients with stable chronic liver disease, characterizing the changes in cytokine and chemokine levels, and the impact of MARS in gene expression observed in the blood compartment.
METHODS
Inclusion criteria
The study was approved by the Health Research Ethics Board of the University of Alberta (Edmonton, Alberta). Adult patients (>18 years of age) with PBC or benign recurrent intrahepatic cholestasis (BRIC) with intractable pruritus, defined by bile acid levels >300 μmol/L and a European Quality of Life-5 Dimensions (EQ-5D) visual analogue score ≤30/100 (0 = worst) were considered eligible. All patients were receiving ursodeoxycholic acid. To be considered eligible, patients were required to be either refractory to or intolerant of medical therapy consisting of cholestyramine (8 g/day), rifampin (300 mg/day to 600 mg/day) and naltrexone (50 mg/day). Patients were excluded if they had other causes of intractable pruritus (eg, drug reactions).
MARS
MARS therapy was performed in the General Systems Intensive Care Unit at the University of Alberta Hospital. Before commencing MARS, each patient received a temporary 16 cm (12 Fr) hemodialysis catheter (Mahurkar/Coviden, USA) inserted in the right internal jugular vein under ultrasound guidance. MARS therapy was performed in conjunction with intermittent hemodialysis (Integra, Gambro, Sweden). Bloodlines were primed with 2 L of 0.9% normal saline and 5000 units/L heparin. Blood flow rates were 250 mL/min. Four hundred millilitres of 25% albumin was used in the albumin dialysate, which was run at a rate of 250 mL/min countercurrent to the blood circuit. The albumin dialysate was recirculated through charcoal and anion-exchange columns before undergoing standard hemodialysis. Bicarbonate dialysis (standard composition: sodium 140 mmol/L, potassium 4 mmol/L, sodium bicarbonate 30 mmol/L) was run at 500 mL/min. Patients targeted an even fluid balance while on MARS. Heparin was used for anticoagulation while on MARS therapy (bolus 500 to 1000 units, infusion 250 units/h to 1000 units/h) and was run to target an activated clotting time of between 180 s and 200 s. Each patient underwent two MARS runs consisting of 8 h per run on consecutive days (total of 16 h). During MARS, a dedicated certified MARS trained nurse was responsible for operating the device while a second nurse was responsible for other aspects of patient care.
Sample collection and storage
Blood samples were collected from indwelling venous lines immediately before the first treatment (ie, pre-MARS) and immediately at the end of the second MARS run (ie, post-MARS). Serum samples were obtained by centrifugation within 1 h of blood collection, aliquoted and stored at −80°C until use. PAXgene RNA tubes (PreAnalytiX GmbH, Switzerland) were used for prompt RNase inactivation of whole blood specimens. Blood in PAXgene tubes were stored at −80°C until use.
Microarray analysis
Nimblegen Gene Expression 4×72K Hs18.0 arrays (Roche NimbleGen, USA) containing 24,000 different probes for human genes were used to profile messenger RNA (mRNA) expression in peripheral blood specimens. RNA extraction and microarray processing were performed at the Alberta Transplant Applied Genomics Centre (University of Alberta). Briefly, RNA was extracted using the PAXgene Blood RNA Kit (PreAnalytix, Switzerland) and 200 ng of total RNA was used for preparation of a complementary DNA (cDNA) library that was subjected to unbiased linear preamplification over 17 polymerase chain reaction cycles (WTA2 kit, Sigma Aldrich, USA). Labelling of 1 μg of the resulting cDNA with Cy3 was performed using the One-Color DNA Labeling kit (Roche Nimblegen, Sweden) according to manufacturer’s instructions. These were subsequently hybridized onto the microarray slides and scanned on a microarray scanner (MS200, Roche Nimblegen). Data extraction was performed using Nimblescan software (Roche Nimblegen), having arrays being robust multi-array average summarized. Each patient’s pre- and post-MARS samples were run concurrently on the same array chip.
Cytokine analysis
A panel of 65 cytokines and chemokines (EGF, Eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO, interferon [IFN]-alpha 2, IFN-gamma, interleukin [IL]-10, IL-12 [p40], IL-12 [p70], IL-13, IL-15, IL-17, IL-1ra, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, MCP-1, MCP-3, MDC [CCL22], MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, regulated on activation, normal T cell expressed and secreted [RANTES], transforming growth factor [TGF]-alpha, tumour necrosis factor [TNF]-alpha, TNF-beta, VEGF, sCD40L, sIL-2Rα, MCP-2, MCP-4, ENA-78/CXCL5, SDF-1α+β/CXCL12, BCA-1/CXCL13, I-309/CCL1, MIP-1δ/MIP-5/CCL15, TARC/CCL17, 6Ckine/CCL21, EOTAXIN-2/CCL24, EOTAXIN-3/CCL26, CTACK/CCL27, IL-23, LIF, TPO, TRAIL/TNFSF10, SCF, TSLP, IL-20, IL-21, IL-28A, IL-16, IL-33/NF-HEV) were simultaneously measured in plasma samples using a MILLIPLEX MAP Human Cytokine/Chemokine kits (Millipore, USA) according to the manufacturer’s protocol. The multiplexing analysis was performed using the Luminex 100 system (Luminex, USA) by Eve Technologies Corporation (Canada).
Autotaxin
Serum autotaxin activity was determined with a choline-release assay using lipophosphatidylcholine as a substrate, as described in detail elsewhere (18) and performed at the Tytgat Institute for Liver and Intestinal Research at the University of Amsterdam (Rotterdam, The Netherlands).
Data analysis
Microarray data analysis was performed using GeneSpring GX 11 software (Agilent Technologies, USA). Arrays were baseline transformed to the expression of beta-actin (ACTB, Gene ID 60) on each chip. Fold-change was used to characterize differences in gene expression in each patient individually, excluding the 20% of probes with the lowest raw intensity values. Cytokine and chemokine changes were also expressed as fold-change increase (when values post/pre-MARS >1.0) or decrease (−1/[values post/pre-MARS]).
RESULTS
Effectiveness and safety
Two adult PBC patients and one adult BRIC patient with intractable pruritus underwent two sessions of MARS therapy each during the study period. All patients had failed standard medical therapy before referral for MARS therapy. MARS was administered in 8 h runs for two consecutive days for all patients. Clinical and biochemical outcomes are shown in Table 1. QoL assessment using the EQ-5D visual analogue scale demonstrated the marked improvement obtained during therapy in all three patients (Figure 1: range pre 10 to 20 versus post 70 to 90), which was sustained for the short term (two weeks) in two of three patients. Reductions in mean (± SD) hemoglobin levels (pre 100.0±5.29 g/L versus post 90±8.54 g/L) and platelet levels (pre 194.7±78.6×109/L versus post 153.3±75.1×109/L) post-MARS were not associated with requirement for blood transfusion. No bleeding or infectious complications associated with catheter insertion or therapy were apparent.
TABLE 1.
Quality of life and biochemical characteristics of patients pre- and post-MARS therapy
|
Patient
|
Fold change, mean | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Diagnosis | PBC | PBC | BRIC | |
| MELD score | 11 | 7 | 20 | |
| EQ-5D score (VAS) | 5.33↑ | |||
| Pre | 10 | 20 | 20 | |
| Post | 90 | 70 | 70 | |
| Bile acids, μmol/L | 5.43↓ | |||
| Pre | 555.1 | 531.5 | 350.1 | |
| Post | 48.2 | 169.1 | 51.8 | |
| Ammonia, μmol/L | 1.11↑ | |||
| Pre | 24 | 44 | 27 | |
| Post | 42 | 37 | 20 | |
| Alanine aminotransferase, U/L | 1.05↑ | |||
| Pre | 151 | 42 | 59 | |
| Post | 152 | 46 | 62 | |
| Aspartate aminotransferase, U/L | 1.12↑ | |||
| Pre | 173 | 56 | 69 | |
| Post | 159 | 65 | 88 | |
| Total bilirubin, μmol/L | 1.48↓ | |||
| Pre | 208 | 28 | 565 | |
| Post | 138 | 23 | 303 | |
| Conjugated bilirubin, μmol/L | 1.69↓ | |||
| Pre | 125 | 14 | 377 | |
| Post | 79 | 10 | 161 | |
| Alkaline phosphatase, U/L | 1.11↓ | |||
| Pre | 481 | 255 | 532 | |
| Post | 445 | 239 | 448 | |
| Gamma-glutamyl transpeptidase, U/L | 1.18↓ | |||
| Pre | 73 | 212 | 51 | |
| Post | 60 | 190 | 42 | |
| International normalized ratio | 1.07↑ | |||
| Pre | 1.1 | 1 | 1.7 | |
| Post | 1 | 1 | 2.2 | |
| Partial thromboplastin time, s | 2.26↑ | |||
| Pre | 31 | 31 | 55 | |
| Post | 57 | 126 | 48 | |
| Hemoglobin, g/L | 1.11↓ | |||
| Pre | 96 | 98 | 106 | |
| Post | 81 | 91 | 98 | |
| Platelets, ×109/L | 1.30↓ | |||
| Pre | 199 | 114 | 271 | |
| Post | 148 | 81 | 231 | |
| Creatinine, μmol/L | 1.26↓ | |||
| Pre | 47 | 80 | 47 | |
| Post | 45 | 63 | 30 | |
| Urea, mmol/L | 2.94↓ | |||
| Pre | 4.4 | 5.2 | 2.6 | |
| Post | 1.1 | 2 | <1.0 | |
Fold-changes are given, with arrows representing fold-change increase (↑) or decrease (↓). BRIC Benign recurrent intrahepatic cholestasis; EQ-5D European Quality of Life-5 Dimensions questionnaire; MARS Molecular adsorbent recirculating system; MELD Model for End-stage Liver Disease; PBC Primary biliary cirrhosis; VAS Visual analogue score
Figure 1).
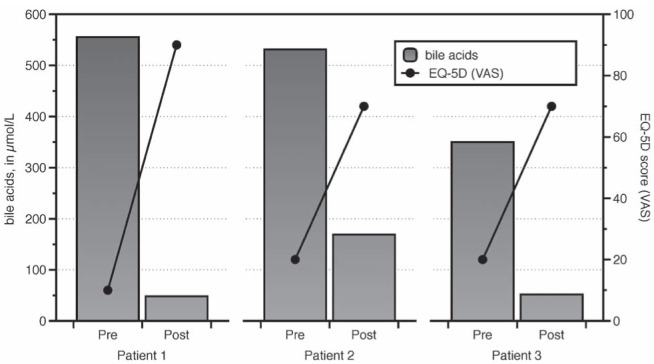
Bile acid and European Quality of Life-5 Dimensions (EQ-5D) questionnaire changes in patients treated with the molecular adsorbent recirculating system (MARS). The bars indicate bile acid levels while the lines represent scores for the EQ-5D test (visual analogue scale [VAS] component) in relation to MARS treatment (ie, pre/post)
Biochemical response to MARS therapy
Biochemical data are also presented in Table 1. Bile acid levels were markedly reduced with MARS therapy (Figure 1: mean pre-MARS 478.9 μmol/L versus post-MARS 89.7 μmol/L). Bilirubin levels were decreased in two of three patients (each had abnormal bilirubin levels pre-MARS treatment). No changes in ammonia levels were observed.
Cytokine and chemokine
Patterns of change in most cytokines and chemokines were complex and largely distinct among the patients. Concordant decreases were recorded for IL-1ß, IL-2, IL-6, IL-8, IL-12 (p40), RANTES, TGF-alpha, TNF-alpha and thrombopoetin, in which levels were reduced post-therapy in all three patients, although by low magnitudes (Table 2). No concordant increases were observed.
TABLE 2.
Baseline serum cytokine and chemokine levels and concordant fold-changes in patients who underwent MARS therapy
| Protein | Gene symbol | Baseline,mean, pg/mL |
Fold change
|
||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | |||
| IL-1β | IL1B | 2.72 | ↓ | −2.87 | −1.54 |
| IL-2 | IL2 | 1.05 | −1.15 | ↓ | −1.68 |
| IL-6 | IL6 | 5.30 | ↓ | −1.52 | −1.11 |
| IL-8 | IL8 | 95.01 | −1.38 | −1.26 | −1.62 |
| IL-12 (p40) | IL12B | 19.84 | −1.15 | −1.44 | −1.19 |
| RANTES | CCL5 | 815.37 | −1.43 | −1.51 | −1.38 |
| Transforming growth factor-α | TGFA | 3.20 | −1.27 | −1.65 | −1.15 |
| Tumour necrosis factor-α | TNF | 5.40 | −1.34 | −1.20 | −1.10 |
| Thrombopoietin | TPO | 452.45 | −1.29 | −1.07 | −1.02 |
Individual patient fold-changes and mean baseline levels of cytokine/chemokine were determined for values obtained within the linear range of detection. Arrows indicate one of the results obtained outside of the linear range of the assay but yet allowing analysis of the trend (ie, decrease [↓]). Negative values denote fold-change decrease. IL Interleukin; MARS Molecular adsorbent recirculating system; RANTES Regulated on activation, normal T cell expressed and secreted
Autotaxin activity levels
The activity levels of ectonucleotide pyrophosphatase/phosphodiesterase (autotaxin) were discordant among the patients. While patients 1 and 3 experienced a marked reduction of 37% (17.5 nmol/mL*min to 12.8 nmol/mL*min) and 21% (13.3 nmol/mL*min to 11.0 nmol/mL*min) respectively, patient 2 had low baseline values and showed a mild increase of 16% (7.3 nmol/mL*min to 8.4 nmol/mL*min) in the activity levels of this enzyme associated with MARS therapy.
Genome-scale transcriptome analysis
A comprehensive analysis of mRNA in blood samples was performed through microarray profiling. Up- or downregulation of gene expression for each individual patient was considered biologically relevant when the fold-change was ≥2.0. Concordant changes among the three patients were apparent for 20 different genes (10 upregulated and 10 downregulated) (Table 3). No transcripts corresponding to profiled serum cytokines or chemokines were found to be affected by MARS therapy in blood cells. Upregulation of several potentially immune suppressive/regulatory genes, such as early growth response 3 (EGR-3), ephrin-A2 (EFNA2) and serum amyloid A1 (SAA1), concurrent with downregulation of genes involved in innate immunity, such as toll-like receptor 4 interactor with leucine-rich repeats (TRIL), and in inflammation, such as EPHB1, was apparent post-therapy.
TABLE 3.
Up- and downregulated genes in blood samples of patients who underwent MARS therapy
| Gene ID | Gene symbol | Gene name | Mean fold-change |
Individual fold change
|
||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | ||||
| 1960 | EGR3 | Early growth response 3 | 3.73 | 5.10 | 1.70 | 4.39 |
| 9865 | TRIL | TLR4 interactor with leucine-rich repeats | −2.58 | −1.51 | −4.52 | −1.70 |
| 375010 | LOC375010 | Ankyrin repeat domain 20 family, member A pseudogene | −2.54 | −3.03 | −2.65 | −1.94 |
| 27023 | FOXB1 | Forkhead box B1 | 2.50 | 1.62 | 3.64 | 2.23 |
| 81127 | OR4K15 | Olfactory receptor, family 4, subfamily K, member 15 | −2.50 | −1.77 | −1.71 | −4.01 |
| 219493 | OR5AR1 | Olfactory receptor, family 5, subfamily AR, member 1 | −2.49 | −1.64 | −3.36 | −2.46 |
| 153579 | BTNL9 | Butyrophilin-like 9 | −2.46 | −3.05 | −1.58 | −2.75 |
| 136371 | ASB10 | Ankyrin repeat and SOCS box-containing 10 | 2.38 | 1.84 | 3.15 | 2.16 |
| 6288 | SAA1 | Serum amyloid A1 | 2.35 | 1.74 | 3.58 | 1.74 |
| 25790 | CCDC19 | Coiled-coil domain containing 19 | −2.37 | −2.54 | −2.43 | −2.13 |
| 1943 | EFNA2 | Ephrin-A2 | 2.27 | 2.14 | 1.83 | 2.83 |
| 2047 | EPHB1 | Ephrin receptor B1 | −2.26 | −2.44 | −1.51 | −2.84 |
| 4108 | MAGEA9 | Melanoma antigen family A, 9 | −2.20 | −2.24 | −1.64 | −2.71 |
| 79933 | SYNPO2L | Synaptopodin 2-like | −2.16 | −1.82 | −3.14 | −1.52 |
| 643444 | LOC643444 | Similar to WAS protein homology region 2 domain containing 1-like 1 | 2.12 | 2.03 | 2.44 | 1.89 |
| 2128 | EVX1 | Even-skipped homeobox 1 | 2.09 | 2.69 | 1.85 | 1.73 |
| 392583 | LOC392583 | Hypothetical LOC392583 | 2.07 | 1.68 | 3.00 | 1.53 |
| 2232 | FDXR | Ferredoxin reductase | 2.01 | 1.53 | 2.90 | 1.61 |
| 22854 | NTNG1 | Netrin G1 | 2.01 | 1.58 | 2.76 | 1.68 |
| 55701 | ARHGEF40 | Rho guanine nucleotide exchange factor (GEF) 40 | −2.01 | −2.42 | −1.98 | −1.64 |
Values represent fold-change increase (normalized expression post/normalized expression pre) or decrease (–1/[normalized expression post/normalized expression pre]) for each individual patient and genes are sorted according to the absolute mean fold-change observed in the group. ID Identification; MARS Molecular adsorbent recirculating system; TLR Toll-like receptor; WAS Wiskott-Aldrich syndrome
DISCUSSION
In the present case series, we described the first Canadian experience using MARS for the treatment of cholestasis patients with intractable pruritus. Our preliminary data confirm the clinical benefit of MARS, in addition to its safety and tolerability. Symptomatic relief and reduced bile acid levels were achieved with MARS therapy in patients with intractable pruritus (Tables 1 and 4). Moreover, aside from its previously characterized filtration capacity of potential pruritogens, the clinical benefit of MARS may also be explained by associated changes in cytokine/chemokine profiles and in gene expression in blood cells.
TABLE 4.
Summary of publications* on cytokines and/or chemokines in MARS therapy
|
Outcomes post-MARS therapy
|
|||||
|---|---|---|---|---|---|
| Author (reference), year | Country | n | Etiology | Biochemical | Pruritus |
| Lisboa et al (present), 2012 | Canada | 3 | PBC (n=2), BRIC (n=1) | ↓Bile acids, bilirubin, urea | Improvement in 3/3 |
| Leckie et al (30), 2012 | UK | 15 | PBC (n=11), PSC (n=3), Other (n=1) | ↓Bilirubin, ALT, ALP, creatinine | Improvement in 13/15 |
| Pares et al (31), 2010 | Spain | 20 | PBC (n=10), PSC (n=1), Alagille syndrome (n=1), OLT graft rejection (n=8) | ↓Bile acid, bilirubin, GGT, cholesterol | Improvement in 19/20 |
| Lemoine et al (32), 2008 | France | 1 | PFIC3 (n=1) | ↓Bile acids | Improvement in 1/1 |
| Silvagni et al (33), 2008 | Italy | 1 | Drug-induced + Turner syndrome (n=1) | ↓Bile acid | Improvement in 1/1 |
| Novelli et al (34), 2006 | Italy | 9 | HCV (n=9) | ↓Bile acid, bilirubin, creatinine | Improvement in 9/9 |
| Montero et al (35), 2006 | Spain | 3 | PBC/AIH (n=1), post-OLT cholestasis (n=1), post-OLT HCV recurrence (n=1) | ↓Bilirubin | Improvement 4/4 |
| Saich et al (36), 2005 | UK | 1 | BRIC (n=1) | ↓Bile acids | Improvement in 1/1 |
| Ribo et al (37), 2005 | Spain | 2 | post-OLT cholestasis (n=2) | ↓Bilirubin | Improvement in 2/2 |
| Bellmann et al (38), 2004 | Austria | 2 | Drug-induced (n=2) | ↓Bile acids, bilirubin | Improvement in 2/2 |
| Pares et al (39), 2004 | Spain | 4 | PBC (n=4) | ↓Bile acids | Improvement in 4/4 |
| Bellmann et al (40), 2004 | Austria | 7 | post-OLT cholestasis (n=7) | ↓Bile acid, AST, GGT | Improvement in 6/7 |
| Macia et al (41), 2003 | Spain | 3 | post-OLT cholestasis (n=1), PBC (n=2) | ↓Bilirubin, urea, creatinine | Improvement in 3/3 |
| Doria et al (42), 2003 | Italy | 3 | HCV (n=3) | ↓Bile acid | Improvement in 3/3 |
| Sturm et al (43), 2002 | France | 1 | BRIC (n=1) | ↓Bile salts, bilirubin | Improvement in 1/1 |
Only English language, nonduplicated publications measuring cytokines or chemokines in the blood of patients receiving MARS therapy were included.
Increased;
Decreased; ACLF Acute-on-chronic liver failure; AIH Autoimmune hepatitis; ALF Acute liver failure; ALT Alanine aminotransferase; ALP Alkaline phosphatase; AST Aspartate aminotransferase; BRIC Benign recurrent intrahepatic cholestasis; GGT Gamma-glutamyl transpeptidase; HBV Hepatitis B virus; HCV Hepatitis C virus; HEV Hepatitis E virus; HRS Hepatorenal syndrome; MARS Molecular adsorbent recirculating system; NASH Nonalcoholic steatohepatitis; OLT Orthotopic liver transplantation; PBC Primary biliary cirrhosis; PFIC Progressive familial intrahepatic cholestasis; PSC Primary sclerosing cholangitis; UK United Kingdom
Nonresident leukocytes infiltrating the skin, including eosinophils and T cells, are believed to play a role in the pathogenesis of chronic pruritus (19). We postulate that changes in gene expression, cytokines and chemokines seen in the blood in association with MARS modulate the cell types with causative roles in pruritus before their migration to the skin.
Cytokine and chemokine changes associated with MARS therapy have not yet been described in the serum of stable cholestatic liver disease patients with intractable pruritus. To the best of our knowledge, the present pilot study was the first to assess this. We found that proinflammatory cytokines and chemokines comprised the bulk of the proteins concordantly decreased in all patients in this series, some of which are known to be cleared by this filtration method. The comparison between the experience with MARS in the present and other clinical scenarios suggests a similar effect of the therapy over cytokine and chemokine levels (Table 5). Interestingly, cytokines, such as IL-6 and TNF-alpha, have been previously implicated in pruritis associated with uremia or HIV infection (20, 21). The lack of a clear correlation between cytokine level changes and their corresponding mRNA in blood cells may suggest their predominant extravascular compartment origin.
TABLE 5.
Summary of publications on cholestatic pruritus and MARS therapy
| Author (reference), year | Country | MARS indication | n | Etiology | Cytokine outcome post-MARS |
|---|---|---|---|---|---|
| Lisboa et al (present), 2012 | Canada | Intractable pruritus | 3 | PBC (n=2), BRIC (n=1) | ↓IL-1β, IL-2, IL-6, IL-8, IL-12 (p40), RANTES, TGF-α, TNF-α, TPO |
| Gay et al (44), 2011 | Spain | Intractable pruritus | 5 | PBC (n=2), AIH (n=1), OLT graft rejection (n=1), Wilson disease (n=1) | CCL14, CCL15, PDGFA retained by MARS SAX |
| Novelli et al (45), 2011 | Italy | ACLF + positive endotoxin activity assay | 10 | Ethanol (n=5), HCV (n=3), HBV (n=1), PBC (n=1) | ↓IL-6, TNF-α |
| Wong et al (46), 2010 | Canada | Type 1 HRS | 6 | Ethanol (n=4), NASH (n=1), HCV (n=1) | =IL-6, TNF-α |
| Novelli et al (47), 2009 | Italy | ALF | 45 | Viral (n=21), other (n=19), unknown (n=5) | ↓IL-6, TNF-α |
| Novelli et al (48), 2009 | Italy | ALF | 10 | Ethanol (n=6), HBV (n=4) | ↓IL-1, IL-6, TNF-α |
| Roth et al (49), 2009 | Austria | ALF or ACLF | 21 | HCV (n=5), Wilson disease (n=3), trauma (n=2), AIH (n=1), PBC (n=1), toxic (n=1), unknown (n=3), other (n=5) | =MCP-1, IL-18 |
| Ilonen et al (50), 2006 | Finland | ALF or ACLF | 81 | ALF (n=49), ACLF (n=32) | ↓IL-10=IL-6, IL-8, TNF-α, sIL-2Rα |
| Stadlbauer et al (51), 2006 | Austria | ACLF | 8 | Ethanol (n=5), HCV (n=1), OLT graft dysfunction (n=1), other (n=1) | =IL-6, IL-8, IL-10, TNF-α despite demonstrable clearance |
| Isoniemi et al (52), 2005 | Finland | ALF | 49 | Toxic (n=26), unknown (n=19), miscellaneous (n=4) | ↓IL-10 |
| Di Campli et al (53), 2005 | Italy | ACLF | 13 | Ethanol (n=4), viral (n=5), NASH (n=1), Wilson disease (n=1), PSC (n=1), unknown (n=1) | ↓IL-1β, IL-6, TNF-α in survivors |
| Auth et al (54), 2005 | Germany | ALF | 2 | Wilson disease (n=2) | ↓IL-6, TNF-α ↑VEGF |
| Sen et al (55), 2004 | UK | ACLF | 18 | Ethanol (n=15), ethanol + HCV (n=3) | =IL-6, IL-8, TNF-α |
| Luo et al (56), 2003 | China | MODS | 1 | Severe acute respiratory syndrome (n=1) | ↓IL-6, IL-8, TNF-α |
| Ambrosino et al (57), 2003 | Italy | ACLF | 17 | (n=17) | ↑IL-6 ↓TNF-α |
| Guo et al (58), 2003 | China | ALF or ACLF | 24 | HBV (n=17), ethanol (n=3), drug-induced (n=3), HEV (n=1) | ↓IL-4, IL-6, IL-8, TNF-α, IFN-γ |
Only English language, non-duplicated publications having cholestatic pruritus as the indication for MARS therapy were included.
Increased;
Decreased;
Unchanged; ACLF Acute-on-chronic liver failure; AIH Autoimmune hepatitis; ALF Acute liver failure; ALP Alkaline phosphatase; ALT Alanine transaminase; AST Aspartate transaminase; BRIC Benign recurrent intrahepatic cholestasis; CCL Chemokine (C-C motif) ligand; GGT Gamma glutamyl transpeptidase; HBV Hepatitis B virus; HCV Hepatitis C virus; HEV Hepatitis E virus; HRS Hepatorenal syndrome; IL Interleukin; IFN Interferon; MARS Molecular adsorbent recirculating system; MCP Monocyte chemotactic protein; MODS Multiple organ dysfunction syndrome; NASH Nonalcoholic steatohepatitis; OLT Orthotopic liver transplantation; PDGFA Platelet-derived growth factor A; PBC Primary biliary cirrhosis; PFIC3 Progressive familial intrahepatic cholestasis type 3; RANTES Regulated on activation, normal T cell expressed and secreted; SAX Strong anion exchange; TGF Transforming growth factor; TNF Tumour necrosis factor; TPO Thrombopoietin; UK United Kingdom; VEGF Vascular endothelial growth factor
MARS therapy in our patients also impacted circulating leukocytes at the transcriptional level. Among the upregulated genes and with the highest mean fold-change (3.73) were EGR3, which is a negative regulator of T cell activation (22,23), playing a role in T cell hyporesponsiveness (24). The mRNA expression of the acute-phase protein SAA1 was also increased during therapy (mean fold-change 2.35), which can induce IL-10 secretion by neutrophils (25). EFNA2 expression is augmented after MARS, also being an inhibitor of T cell chemotaxis (26) and apoptosis (27). Among the downregulated genes was TRIL, an innate immunity gene involved in the response to bacterial lipopolysaccharide. Its knockdown in human peripheral blood mononuclear cells has been shown to attenuate lipopolysaccharide signalling through TLR4 thereby affecting cytokine production (28). EPHB1 (Ephrin receptor B1 – 2.26-fold decrease in expression post-MARS) has been shown to be elevated in the blood and in exudate lymphocytes in synovial tissues from patients with rheumatoid arthritis (29). Taken together, the changes in protein mediators and gene transcripts appear to suggest a potential immunomodulatory effect associated with MARS therapy.
Patients with pruritus show a significantly higher activity of autotaxin, an enzyme that converts lysophosphatidylcholine into lysophosphatidic acid, when compared with their control counterparts (14). MARS was associated with reduction of activity of autotaxin in two of three patients in the present series, while one patient achieved comparable symptomatic improvement despite a further increase in the activity of this enzyme, albeit from much lower baseline levels.
Our study was conceived as an exploratory pilot study to test the the feasibility of detecting circulating cytokines/chemokines and potentially meaningful changes in gene expression in the blood of chol-estasis patients with intractable pruritus and it was performed before the intensification of referrals of cholestatic pruritus patients for therapy at our centre. A number of limitations, such as the number of patients, the lack of characterization of filtered substances in the dialysate and the determination of cell types responsible for the mRNA transcription patterns found, are acknowledged and remain to be addressed in future studies.
SUMMARY
We conclude that MARS therapy is associated with acute changes in cytokine/chemokine levels concurrent with changes in gene expression in blood cells. It is conceivable that the clinical benefit of such therapy in cholestasis patients with intractable pruritus may not exclusively result from filtration of pruritogens but also from systemic changes in cytokine and chemokine levels, and to those ascertained in blood cells at the transcriptome level.
Acknowledgments
The authors thank Anna Hutton, Chelsea MacDougall, Kara Allanach, Karyn Berry-Wynne and Vido Ramassar for their technical assistance. They also thank the General Systems Intensive Care Unit. Gambro provided MARS circuits for patients in this study but Gambro had no role in the study concept, design, data collection, analysis, preparation of the manuscript, and did not review the data or manuscript before submission for publication.
REFERENCES
- 1.Battezzati PM, Podda M, Bianchi FB, et al. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double-blind multicenter trial. Italian Multicenter Group For The Study Of UDCA in PBC. J Hepatol. 1993;17:332–8. doi: 10.1016/s0168-8278(05)80214-4. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzaki Y, Tanaka N, Osuga T, et al. Improvement of biliary enzyme levels and itching as a result of long-term administration of ursodeoxycholic acid in primary biliary cirrhosis. Am J Gastrenterol. 1990;85:15–23. [PubMed] [Google Scholar]
- 3.Pares A, Caballeria L, Rodes J, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: Results of a double-blind controlled multicentric trial. UDCA-Cooperative Group From The Spanish Association For The Study Of The Liver. J Hepatol. 2000;32:561–6. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 4.Poupon RE, Balkau B, Eschwege E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548–54. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 5.Hadzigiannis S, Hadzigiannis E, Makris A. A randomized controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis. Hepatology. 1989;10:580. (Abst) [Google Scholar]
- 6.Vuoristo M, Farkkila M, Karvonen AL, et al. A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology. 1995;108:1470–8. doi: 10.1016/0016-5085(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 7.Bachs L, Pares A, Elena M, Piera C, Rodes J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;1:574–6. doi: 10.1016/s0140-6736(89)91608-5. [DOI] [PubMed] [Google Scholar]
- 8.Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology. 1966;50:323–32. [PubMed] [Google Scholar]
- 9.Kuiper EM, Van Erpecum KJ, Beuers U, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: Results of a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:1334–40. doi: 10.1002/hep.23821. [DOI] [PubMed] [Google Scholar]
- 10.Bergasa NV, Alling DW, Talbot TL, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123:161–7. doi: 10.7326/0003-4819-123-3-199508010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bergasa NV, Schmitt JM, Talbot TL, et al. Open-label trial of oral nalmefene therapy for the pruritus of cholestasis. Hepatology. 1998;27:679–84. doi: 10.1002/hep.510270307. [DOI] [PubMed] [Google Scholar]
- 12.Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992;102:544–9. doi: 10.1016/0016-5085(92)90102-5. [DOI] [PubMed] [Google Scholar]
- 13.Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: A double-blind, placebo-controlled study. Gastroenterology. 1997;113:1264–9. doi: 10.1053/gast.1997.v113.pm9322521. [DOI] [PubMed] [Google Scholar]
- 14.Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–18. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Alallam A, Barth D, Heathcote E J. Role of plasmapheresis in the treatment of severe pruritus in pregnant patients with primary biliary cirrhosis: Case reports. Can J Gastroenterol. 2008;22:505–7. doi: 10.1155/2008/969826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huster D, Schubert C, Achenbach H, Caca K, Mossner J, Berr F. Successful clinical application of extracorporal albumin dialysis in a patient with benign recurrent intrahepatic cholestasis (BRIC) Z Gastroenterol. 2001;39(Suppl 2):13–4. doi: 10.1055/s-2001-919024. [DOI] [PubMed] [Google Scholar]
- 17.Quintero E, Puig L, PaRes A, Mazzara R, Castillo R, Rodes J. Utilidad del recambio plasmatico intermitente en la cirrosis biliar primaria. Gastroenterol Hepatol. 1986;9:329–33. [Google Scholar]
- 18.Nakamura K, Ohkawa R, Okubo S, et al. Measurement of lysophospholipase D/autotaxin activity in human serum samples. Clin Biochem. 2007;40:274–7. doi: 10.1016/j.clinbiochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Metz M, Stander S. Chronic pruritus – pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2010;24:1249–60. doi: 10.1111/j.1468-3083.2010.03850.x. [DOI] [PubMed] [Google Scholar]
- 20.Breuer-Mcham J N, Marshall G D, Lewis D E, Duvic M. Distinct serum cytokines in AIDS-related skin diseases. Viral Immunol. 1998;11:215–20. doi: 10.1089/vim.1998.11.215. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel M, Alscher D M, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21:749–55. doi: 10.1093/ndt/gfi204. [DOI] [PubMed] [Google Scholar]
- 22.Safford M, Collins S, Lutz MA, et al. Egr-2 And Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–80. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Martin D, Diaz-Zamudio M, Galindo-Campos M, Alcocer-Varela J. Early growth response transcription factors and the modulation of immune response: Implications towards autoimmunity. Autoimmun Rev. 2010;9:454–8. doi: 10.1016/j.autrev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Collins S, Lutz MA, Zarek PE, Anders RA, Kersh GJ, Powell JD. Opposing regulation of T cell function by Egr-1/Nab2 And Egr-2/Egr-3. Eur J Immunol. 2008;38:528–36. doi: 10.1002/eji.200737157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of Il-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Luo H. Recent Advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol. 2005;12:292–7. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- 27.Holen HL, Shadidi M, Narvhus K, Kjosnes O, Tierens A, Aasheim HC. Signaling through ephrin-A ligand leads to activation of Src-family kinases, Akt phosphorylation, and inhibition of antigen receptor-induced apoptosis. J Leukoc Biol. 2008;84:1183–91. doi: 10.1189/jlb.1207829. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter S, Carlson T, Dellacasagrande J, et al. TRIL, a functional component of the TLR4 signaling complex, highly expressed in brain. J Immunol. 2009;183:3989–95. doi: 10.4049/jimmunol.0901518. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura T, Kabuyama Y, Kamataki A, et al. Enhancement of lymphocyte migration and cytokine production by EphrinB1 system in rheumatoid arthritis. Am J Physiol Cell Physiol. 2008;294:C189–96. doi: 10.1152/ajpcell.00314.2007. [DOI] [PubMed] [Google Scholar]
- 30.Leckie P, Tritto G, Mookerjee R, Davies N, Jones D, Jalan R. ‘Out-patient’ albumin dialysis for cholestatic patients with intractable pruritus. Aliment Pharmacol Ther. 2012;35:696–704. doi: 10.1111/j.1365-2036.2012.04994.x. [DOI] [PubMed] [Google Scholar]
- 31.Pares A, Herrera M, Aviles J, Sanz M, Mas A. Treatment of resistant pruritus from cholestasis with albumin dialysis: Combined analysis of patients from three centers. J Hepatol. 2010;53:307–12. doi: 10.1016/j.jhep.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Lemoine M, Revaux A, Francoz C, et al. Albumin liver dialysis as pregnancy-saving procedure in cholestatic liver disease and intractable pruritus. World J Gastroenterol. 2008;14:6572–4. doi: 10.3748/wjg.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvagni E, Coli L, Stagni B, Stefoni S, Bolondi L. A case of intractable pruritus in Turner’s syndrome successfully treated with molecular adsorbent recirculating system. Intern Emerg Med. 2008;3:65–7. doi: 10.1007/s11739-008-0094-6. [DOI] [PubMed] [Google Scholar]
- 34.Novelli G, Rossi M, Poli L, et al. Intractable pruritus in patients with hepatitis C virus. Transplant Proc. 2006;38:1089–91. doi: 10.1016/j.transproceed.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Montero JL, Pozo JC, Barrera P, et al. Treatment of refractory cholestatic pruritus with molecular adsorbent recirculating system (MARS) Transplant Proc. 2006;38:2511–3. doi: 10.1016/j.transproceed.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 36.Saich R, Collins P, Ala A, Standish R, Hodgson H. Benign recurrent intrahepatic cholestasis with secondary renal impairment treated with extracorporeal albumin dialysis. Eur J Gastroenterol Hepatol. 2005;17:585–8. doi: 10.1097/00042737-200505000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Acevedo Ribo M, Moreno Planas J M, Sanz Moreno C, et al. Therapy of intractable pruritus with MARS. Transplant Proc. 2005;37:1480–1. doi: 10.1016/j.transproceed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Bellmann R, Feistritzer C, Zoller H, et al. Treatment of intractable pruritus in drug induced cholestasis with albumin dialysis: A report of two cases. Asaio J. 2004;50:387–91. doi: 10.1097/01.mat.0000132552.58214.00. [DOI] [PubMed] [Google Scholar]
- 39.Pares A, Cisneros L, Salmeron JM, et al. Extracorporeal albumin dialysis: A procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:1105–10. doi: 10.1111/j.1572-0241.2004.30204.x. [DOI] [PubMed] [Google Scholar]
- 40.Bellmann R, GraziaDei I W, Feistritzer C, et al. Treatment of refractory cholestatic pruritus after liver transplantation with albumin dialysis. Liver Transpl. 2004;10:107–14. doi: 10.1002/lt.20001. [DOI] [PubMed] [Google Scholar]
- 41.Macia M, Aviles J, Navarro J, Morales S, Garcia J. Efficacy of molecular adsorbent recirculating system for the treatment of intractable pruritus in cholestasis. Am J Med. 2003;114:62–4. doi: 10.1016/s0002-9343(02)01354-2. [DOI] [PubMed] [Google Scholar]
- 42.Doria C, Mandala L, Smith J, et al. Effect of molecular adsorbent recirculating system in hepatitis C virus-related intractable pruritus. Liver Transpl. 2003;9:437–43. doi: 10.1053/jlts.2003.50055. [DOI] [PubMed] [Google Scholar]
- 43.Sturm E, Franssen CF, Gouw A, et al. Extracorporal albumin dialysis (MARS) improves cholestasis and normalizes low Apo A-I levels in a patient with benign recurrent intrahepatic cholestasis (BRIC) Liver. 2002;22(Suppl 2):72–5. doi: 10.1034/j.1600-0676.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 44.Gay M, Pares A, Carrascal M, et al. Proteomic analysis of polypeptides captured from blood during extracorporeal albumin dialysis in patients with cholestasis and resistant pruritus. Plos One. 2011;6:E21850. doi: 10.1371/journal.pone.0021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novelli G, Morabito V, Pugliese F, et al. Management of sepsis during MARS treatment in acute on chronic liver failure. Transplant Proc. 2011;43:1085–90. doi: 10.1016/j.transproceed.2011.01.150. [DOI] [PubMed] [Google Scholar]
- 46.Wong F, Raina N, Richardson R. Molecular adsorbent recirculating system is ineffective in the management of type 1 hepatorenal syndrome in patients with cirrhosis with ascites who have failed vasoconstrictor treAtment. Gut. 2010;59:381–6. doi: 10.1136/gut.2008.174615. [DOI] [PubMed] [Google Scholar]
- 47.Novelli G, Rossi M, Ferretti G, et al. Predictive criteria for the outcome of patients with acute liver failure treated with the albumin dialysis molecular adsorbent recirculating system. Ther Apher Dial. 2009;13:404–12. doi: 10.1111/j.1744-9987.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 48.Novelli G, Annesini M C, Morabito V, et al. Cytokine level modifications: Molecular adsorbent recirculating system versus standard medical therapy. Transplant Proc. 2009;41:1243–8. doi: 10.1016/j.transproceed.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 49.Roth GA, Faybik P, Hetz H, et al. MCP-1 And MIP3-alpha serum levels in acute liver failure and molecular adsorbent recirculating system (MARS) treatment: A pilot study. Scand J Gastroenterol. 2009;44:745–51. doi: 10.1080/00365520902770086. [DOI] [PubMed] [Google Scholar]
- 50.Ilonen I, Koivusalo AM, Hockerstedt K, Isoniemi H. Albumin dialysis has no clear effect on cytokine levels in patients with life-threatening liver insufficiency. Transplant Proc. 2006;38:3540–3. doi: 10.1016/j.transproceed.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 51.Stadlbauer V, Krisper P, Aigner R, et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care. 2006;10:R169. doi: 10.1186/cc5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isoniemi H, Koivusalo A M, Repo H, Ilonen I, Hockerstedt K. The effect of albumin dialysis on cytokine levels in acute liver failure and need for liver transplantation. Transplant Proc. 2005;37:1088–90. doi: 10.1016/j.transproceed.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 53.Di Campli C, Zocco MA, Gaspari R, et al. The decrease in cytokine concentration during albumin dialysis correlates with the prognosis of patients with acute on chronic liver failure. Transplant Proc. 2005;37:2551–3. doi: 10.1016/j.transproceed.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 54.Auth MK, Kim HS, Beste M, et al. Removal of metabolites, cytokines and hepatic growth factors by extracorporeal liver support in children. J Pediatr Gastroenterol Nutr. 2005;40:54–9. doi: 10.1097/00005176-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Sen S, Davies NA, Mookerjee RP, et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: A randomized controlled study. Liver Transpl. 2004;10:1109–19. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 56.Luo HT, Wu M, Wang MM. Case report of the first severe acute respiratory syndrome patient in China: Successful application of extracorporeal liver support MARS therapy in multiorgan failure possibly induced by severe acute respiratory syndrome. Artif Organs. 2003;27:847–9. doi: 10.1046/j.1525-1594.2003.02003.x. [DOI] [PubMed] [Google Scholar]
- 57.Ambrosino G, Naso A, Feltracco P, et al. Cytokines and liver failure: Modification of Tnf- And Il-6 in patients with acute on chronic liver decompensation treated with molecular adsorbent recycling system (MARS) Acta Biomed. 2003;74(Suppl 2):7–9. [PubMed] [Google Scholar]
- 58.Guo LM, Liu JY, Xu DZ, et al. Application of molecular adsorbents recirculating system to remove no and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 2003;23(Suppl 3):16–20. doi: 10.1034/j.1478-3231.23.s.3.7.x. [DOI] [PubMed] [Google Scholar]


