Abstract
Trichome development is dependent on gibberellin (GA) signaling in Arabidopsis thaliana. Using the GA-deficient mutant ga1–3, the GA-response mutant spy-5, and uniconazol (a GA-biosynthesis inhibitor), we show that the GA level response correlates positively with both trichome number and trichome branch number. Two genes, GL1 and TTG, are required for trichome initiation. In ga1–3, coexpression of GL1 and R, the maize TTG functional homolog, under control of the constitutive 35S promoter, restored trichome development, whereas overexpression of neither GL1 nor R alone was sufficient to significantly suppress the glabrous phenotype. We next focused on GL1 regulation by GAs. In the double mutant the gl1–1 glabrous phenotype is epistatic to the spy-5 phenotype, suggesting that GL1 acts downstream of the GA signal transduction pathway. The activity of a β-glucuronidase reporter gene driven by the GL1 promoter was decreased in the wild type grown on uniconazol and showed a clear GA-dependent activation in ga1–3. Finally, quantification of GL1 transcript levels by reverse transcriptase-polymerase chain reaction demonstrated that relative to wild type, ga1–3 plants contained less transcript. These data support the hypothesis that GAs induce trichome development through up-regulation of GL1 and possibly TTG genes.
GA hormones are involved in a number of growth and developmental processes in plants. Mutations in both GA biosynthesis and the GA signal transduction pathway have been isolated in several species, such as maize (Phinney et al., 1986), tomato (Jones, 1987), and Arabidopsis (Hooley, 1994; Swain and Olszewski, 1996). In Arabidopsis severe GA-deficient mutants show reduced germination rate, dwarfism, and aberrant flower development. Arabidopsis GA-response mutants have identified several genes encoding the negative regulators of GA signal transduction, including ga-insensitive (GAI; Koornneef et al., 1985; Peng et al., 1997), spindly (SPY; Jacobsen et al., 1996), and repressor of ga1–3 (RGA; Silverstone et al., 1997, 1998). The gain-of-function gai mutant resembles mutants partially deficient in GA biosynthesis but exhibits a reduced sensitivity to exogenous GAs, whereas the spy mutants show an enhanced elongation or slender phenotype together with pale green foliage, early flowering, partial male sterility, and parthenogenic fruit development. At the cellular level GAs influence a full spectrum of processes ranging from microtubule arrangement in mesocotyl epidermal cells, cell wall growth, lipid metabolism, calcium transport (Hooley, 1994), and regulation of cyclin genes (Sauter et al., 1995). Until now, only a limited number of genes involved in these processes have been characterized, mainly encoding hydrolytic enzymes required during monocotyledon germination (Ni and Bradford, 1993). Gubler et al. (1995) identified a Myb-transcription factor in barley aleurone cells in which transcription is up-regulated by GAs. In Arabidopsis, several GA-regulated cDNAs have been identified, including a water-channel cDNA (Phillips and Huttly, 1994) and GAST1 homologs (Shi et al., 1992), the functions of which are unknown (Herzog et al., 1995).
During the course of our studies we observed that extreme GA-deficient mutants, such as the ga1–3 null allele of the locus encoding ent-kaurene synthase, have almost completely glabrous leaves. Application of exogenous GAs to ga1–3 plants induces trichome formation. Similar observations were published recently (Chien and Sussex, 1996), including the demonstration that there is a differential, GA-dependent regulation of trichome development on the abaxial (lower) and adaxial (upper) surfaces of leaves (Telfer et al., 1997). Trichomes are large, single cells that differentiate from individual protodermal cells in the developing epidermis of leaves, stem, and sepals. They begin to form on the adaxial epidermis very early in leaf development (Hülskamp et al., 1994; Larkin et al., 1996). By this stage the earliest morphological sign of trichome initiation within the protodermal layer is an increase in both cell and nuclear size (Hülskamp et al., 1994) as the nucleus undergoes a set of three rounds of endoreduplication, thus leading to a DNA content of 16C. These enlarging cells then exhibit an extension growth out of the epidermis surface. Following branch primordium formation and another increase in nuclear size and DNA content (32C), the trichome cell expands further by growth of the stalk and formation of a third branch.
Trichome formation has been studied extensively in Arabidopsis and requires many genes (Hülskamp et al., 1994; Larkin et al., 1996; Wada et al., 1997), two of which, GLABROUS1 (GL1) and TRANSPARENT TESTA GLABRA (TTG), are required for trichome initiation. GL1 and TTG are necessary to initiate the set of three successive endoreduplications in trichome precursor cells. gl1–1 and ttg-1 mutants, like the ga1–3 mutant, cannot proceed to trichome initiation and show glabrous leaves. The GL1 gene has been isolated (Oppenheimer et al., 1991) and encodes a protein that contains a Myb domain, suggesting that the GL1 protein is a transcription factor. Although a characterization of the TTG gene has not yet been published, it is known that the R gene from maize is able to rescue the phenotype of the ttg-1 mutant of Arabidopsis (Lloyd et al., 1992). The R gene encodes a protein with a basic helix-loop-helix (bHLH) transcription factor motif that interacts with the GL1 protein in vitro (Larkin et al., 1997). Nevertheless, a possible candidate TTG gene has been identified recently and its sequence indicates that the TTG protein does not encode an R homolog (Walker et al., 1997). Thus, the TTG protein might regulate R homologs the product of which could interact with the GL1 protein in trichome cells. The fact that the requirement for either TTG or GL1 cannot be bypassed by constitutive expression of the other gene in ttg-1 and gl1–1 mutants suggests that GL1 and TTG act at the same point in trichome initiation (Larkin et al., 1994).
Trichome cells provide a new, powerful model to dissect the GA-signaling pathway in Arabidopsis. In the present work we analyze the relationships between GAs and trichome development. Using the GA-deficient ga1–3 mutant, the GA-response spy-5 mutant, and uniconazol, a GA biosynthesis inhibitor, we investigated the influence of the endogenous GA signal on trichome number and trichome branch number on rosette leaves. We hypothesized that GAs regulate the expression of the GL1 gene at some point and tested this hypothesis using molecular and genetic tools. The regulation of TTG by GAs is also tackled, although indirectly, with the use of the R gene in genetic studies. A new role for GAs in the control of endoreduplication via GL1 in trichomes cells is discussed.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of wild-type and mutants of Arabidopsis thaliana were planted on soil or surface sterilized and grown in Petri dishes on MSAR medium (Koncz et al., 1990). Plants were grown at 22°C under a photoperiod of 16 h of light/8 h of dark. The GA-biosynthesis inhibitor uniconazol-P, provided by Sumitomo Chemical Co. (Hyogo-Ken, Japan), was used in MSAR medium at concentrations ranging from 10−7 to 10−5 m. Experiments done in Petri dishes resulted in plants with smaller numbers of trichomes than plants grown in soil. This is possibly due to an increase in RH.
The gl1-1 and ttg-1 mutants were provided by the Nottingham Arabidopsis Stock Center (Nottingham, UK). The spy-5 mutant was provided by Dr. Nicholas Harberd (John Innes Centre, Norwich, UK). The 35SGL1 line was provided by Dr. David Marks (University of Minnesota, St. Paul). The 35SR line was provided by Dr. Alan Lloyd (University of Texas, Austin). The GL1p-GUS line carrying the pGGE4 construct (Larkin et al., 1993) was a gift from Dr. Martin Hülskamp (University of Tübingen, Germany). The brassinosteroid-insensitive (bri1) mutant was sent to us by Dr. Steve Clouse (North Carolina State University, Raleigh).
Seeds homozygous for the ga1–3 locus do not germinate unless exogenous GAs are provided or the seed coat is physically removed with forceps. Exogenous GAs are a mixture of GA4 and GA7 (Sigma).
Genetic Analysis
The bri1 mutation was initially isolated in the C24 ecotype, which is glabrous (Clouse et al., 1996). A cross with the Landsberg erecta (Ler) ecotype was performed by Dr. Steve Clouse, who provided a pool of F3 seeds that were planted in soil. Dwarf plants either were glabrous (C24 ecotype) or showed trichomes on leaves (Ler ecotype). The latter population was used to determine the number of trichomes of bri1 in the Ler background.
ga1–3 35SR or ga1–3 35SGL1 plants homozygous for the ga1–3 locus were made by crossing ga1–3/ga1–3 plants grown on exogenously supplied GAs with 35SR or 35SGL1 kanamycin-resistant lines. Nongerminating F2 seeds (ga1–3 background) were isolated on MSAR medium, and embryos were dissected out as indicated above and transferred on MSAR plus kanamycin (50 μg/mL) to select for the presence of the transgenes. Trichome numbers were determined on kanamycin-resistant plants.
ga1–3 35SGL1/+ 35SR/+ plants were obtained from a cross between ga1–3 35SR and ga1–3 35SGL1 F3 plants. The seed coat of F1 seeds was removed and ga1–3 embryos were transferred onto MSAR plus kanamycin (50 μg/mL). Trichome numbers were determined on kanamycin-resistant plants.
spy-5 gl1–1 plants were selected as follows: F2 glabrous plants (gl1–1 phenotype) were allowed to self and F3 seeds were sown on MSAR medium containing uniconazol-P concentrations ranging from 10−7 to 10−5 m to identify the spy-5 homozygous mutants.
Quantitation of GUS Activity by Fluorometry
ga1–3 GL1p-GUS plants or GL1p-GUS plants were grown in Petri dishes containing MSAR medium supplemented with kanamycin (50 μg/mL) for 2 to 3 weeks. The third pair of leaves was then isolated and the petiole was removed to avoid contamination with stipules, since GL1p-GUS lines exhibit strong GUS activity in stipules, the specificity of which is unclear (Larkin et al., 1993). About 20 leaves were frozen in liquid nitrogen and used to extract proteins in 200 μL of GUS extraction buffer (50 mm NaHPO4 [pH 7.0], 10 mm β-mercaptoethanol, 10 mm EDTA, 0.1% sodium lauryl sarcosine, and 0.1% Triton X-100). A sample of 80 μL was then mixed with 450 μL of GUS assay buffer (1 mm 4-methylumbelliferyl β-d-glucuronide in extraction buffer) and the activity was measured as previously described (Gallagher, 1992). Protein concentration was determined with the protein-assay kit (Bio-Rad) based on the Bradford (1976) assay.
Detection of GL1 mRNA by RT-PCR
Seed coats of ga1–3 were removed as mentioned above and embryos were transferred on MSAR medium, whereas wild-type seeds were sown directly on MSAR medium. mRNAs were isolated from 100 mg of young, four-leaf rosettes using the Oligotex Direct mRNA kit (Qiagen, Chatsworth, CA). First, cDNA strands were synthesized using oligo(dT) and RT. In the case of GL1, PCR was performed with primers encompassing the entire GL1-coding sequence (forward primer: ATGAGAATAAG GAGAAGAG; reverse primer: CTAAAGGCAGTACT CAATATC). The amplification of GL1 cDNA would give rise to a 687-bp band, whereas amplification of the genomic DNA would give rise to a 1557-bp band. The expression of adenine phosphoribosyltransferase was chosen as a control since its GA-independent expression in the ga1–3 background has been assessed (Cowling, 1997) and primers to sites inside the coding sequence (forward primer: TCCCAGAATCGCTAAGATTGCC; reverse primer: CCTTTCCCT TAAGCTCTG) were used.
RESULTS
Regulation of Trichome and Branch Numbers by GAs
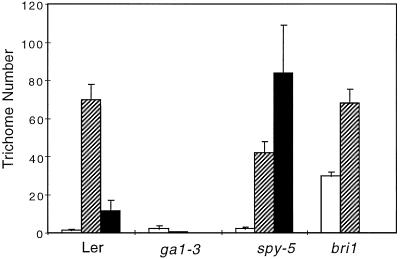
The rosette leaves of a wild-type Arabidopsis plant, Ler ecotype, carry an average of 10 (on the first leaf) to 40 trichomes (on the fourth leaf; Larkin et al., 1996). Although most of these trichomes are three-branch trichomes, a few two-branch and four-branch trichomes are also present. To determine whether the endogenous GA signal influences trichome number and the proportion of each trichome class, we compared the trichome population on the first four rosette leaves of wild-type, spy-5, and ga1–3 mutants, all of Ler background. Figure 1 shows that, on a wild-type, four-leaf rosette with 83 trichomes on average, 70 were three-branch trichomes, 1.5 were two-branch trichomes, and 11.5 were four-branch trichomes. In contrast, the spy-5 mutant showed an average number of 128 trichomes. This significantly higher number was largely due to a dramatic increase (7.3-fold) of four-branch trichomes since the number of three-branch trichomes decreased (1.7-fold) and the number of two-branch trichomes remained approximately the same. This increase in trichome number was more pronounced on leaves 3 and 4. The GA-deficient ga1–3 mutant grown in the absence of GAs showed a very low average of 2.5 trichomes, most of which were two-branch trichomes. To ensure that the glabrous phenotype of the ga1–3 mutant was specific to the GA pathway and not an indirect consequence of the dwarfism, we determined the number of trichomes on leaves of the bri1 mutant (Clouse et al., 1996). Like the ga1–3 mutant, the bri1 mutant exhibits a very reduced size and has dwarf leaves similar to the ga1–3 mutant. In contrast to ga1–3, the bri1 mutation did not prevent trichome development, although the two-branch trichome number seemed to increase in this genotype (Fig. 1).
Figure 1.
Trichome numbers on rosette leaves of wild type and mutants. The average number of trichomes on leaves 1 through 4 of 10 rosette plants grown in soil from two independent experiments is shown for trichomes with two (white columns), three (hatched columns), or four (black columns) branches. Unlike the bri1 dwarf leaves, the ga1–3 dwarf leaves harbor almost no trichomes, whereas the spy-5 mutant has about 50% more trichomes, mainly four-branch trichomes, than the wild type.
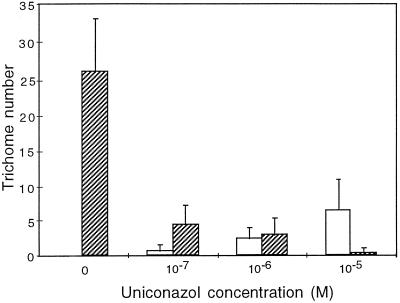
To confirm these data, we analyzed the variation in trichome number and branch number when wild-type plants were grown in MSAR medium in the presence of uniconazol (Fig. 2). When grown in Petri dishes, the overall number of trichomes on the wild-type leaves was lower than that observed when grown in soil (Fig. 1), presumably because of the greater RH. At 10−7 m, uniconazol had a dramatic effect on trichome number, which decreased to about 18% of the wild-type on the first four rosette leaves. Branch number was also reduced, since about 20% of the trichomes were two-branch trichomes in the presence of uniconazol, whereas no two-branch trichomes were observed in the control with no uniconazol. When the uniconazol concentration was increased to 10−6 m, the trichome number remained constant but the number of three-branch trichomes and two-branch trichomes was equivalent. A further increase of uniconazol to 10−5 m led to formation of mainly two-branch trichomes. This inhibition of trichome formation by uniconazol was not observed when exogenous GAs were supplied simultaneously, confirming the specificity and reversibility of the inhibitor effect (data not shown).
Figure 2.
Trichome numbers of wild-type plants grown in the presence of uniconazol. The average number of trichomes on leaves 1 through 4 of 10 rosette plants from two independent experiments is shown for trichomes with two (white columns) or three (hatched columns) branches. Plants were grown in Petri dishes containing MSAR plus uniconazol at the indicated concentration for 3 weeks. Compared with Figure 1, trichomes had fewer branches on average, presumably because the growth conditions in Petri dishes involved increased RH. At 10−5 m uniconazol, only 5 to 10% of the seeds germinated.
Thus, the analysis of trichome populations of the spy-5 mutant and of uniconazol-treated wild-type plants indicated that both the number of adaxial trichomes and the number of trichome branches on these trichomes are positively regulated by the endogenous GA signal.
Overexpression of GL1 and R in the ga1–3 Background
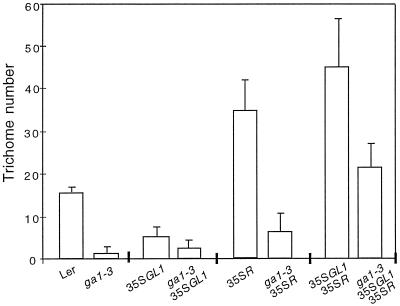
Since the ga1–3, gl1–1, and ttg-1 mutants had a glabrous phenotype, we postulated that GAs could regulate GL1 and/or TTG expression in wild-type plants. We asked whether the expression of GL1 and/or R, the maize TTG functional homolog, would be sufficient to restore trichome formation in a GA-deficient mutant background. We constructed ga1–3 35SGL1 plants and examined trichome formation on rosette leaves (Fig. 3). Constitutive expression of GL1 in the ga1–3 background resulted in the formation of one to three trichomes per leaf, depending on the leaf rank (leaves 1–3). Under the same conditions, 35SGL1 expression in wild-type plants resulted in one to six trichomes per leaf (Oppenheimer et al., 1991; Fig. 3), whereas ga1–3 leaves had zero to two trichomes. Trichomes of ga1–3 35SGL1 plants were similar to trichomes on 35SGL1 plants, i.e. mostly two-branch and three-branch trichomes. These data show that GL1 expression alone is not sufficient in a GA-deficient background to fully restore trichome formation to wild-type or 35SGL1 controls. Constitutive expression of R in the ga1–3 background also resulted in the formation of a small number of trichomes that, similar to trichomes produced when R is expressed in a wild type, had high stalks and two small branches, or were aborted. This result indicates that expression of R alone is not sufficient to fully restore ga1–3 trichome number to wild-type or to 35SR trichome numbers. Finally, coexpression of GL1 and R in the ga1–3 background led to the development of 10 to 25 trichomes per leaf, restoring trichome number to wild type and to about 40 to 50% of the number of trichomes in plants expressing both 35SGL1 and 35SR. These were mainly unbranched and two-branch trichomes. Trichomes were located mostly at the margin of the leaves and were also present on cotyledons and on the abaxial side of the leaves. The location and branching features of these trichomes were also observed on trichomes of 35SGL1 35SR in the wild-type background and were not related to GA deficiency. To illustrate these results, Figure 4 shows representative views of leaves from wild-type plants (B and C), mutants (A, F, and I), and plants overexpressing GL1 and/or R in a ga1–3 background (D, G, and J). For comparison, constitutive expression of GL1 and/or R in a wild-type background (E, H, and K) are also shown.
Figure 3.
Trichome number on plants overexpressing GL1 and/or R in the ga1–3 background. The numbers represent average adaxial trichome numbers per leaf on the first three leaves of 10 rosettes from three independent experiments. Plants were grown in Petri dishes containing MSAR medium plus kanamycin. Compared with Figure 1, trichomes had fewer branches on average, presumably because the growth conditions in Petri dishes involved increased RH. Leaf 4 of these plants was not included, since ga1–3 35SGL1 35SR plants did not fully develop.
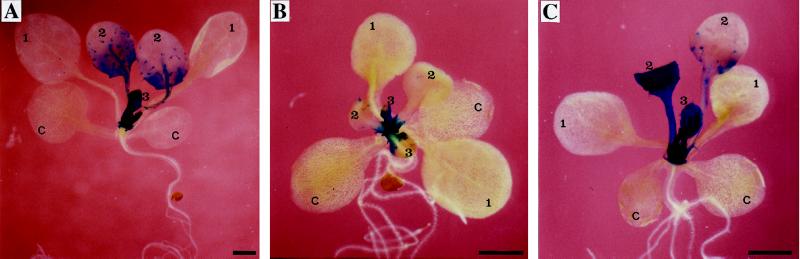
Figure 4.
Effect of GL1 and R overexpression on trichome formation in wild-type and ga1–3 backgrounds. All plants were grown in Petri dishes containing MSAR medium for 20 d except G and J, which were grown for 34 d because of delayed growth. Leaves are third rosette leaves. Bars represent 1 mm. wt, Wild type.
Constitutive expression of GL1 or R had additional effects on overall plant development in addition to trichome formation. For example, ga1–3 35SGL1 rosettes always developed earlier than ga1–3 rosettes and leaves were slightly more elongated and paler than leaves of ga1–3. The growth of ga1–3 35SR rosettes, in contrast, was much slower than the growth of ga1–3 or ga1–3 35SGL1 rosettes and required about 10 to 15 additional days to be completed. By then, ga1–3 35SR plants were still smaller compared with ga1–3 plants. Coexpression of GL1 and R in the ga1–3 background impaired plant growth, since only 10 to 30% of the embryos germinated and plants stopped their development prior to full expansion of the third or fourth rosette leaf. These phenotypes were observed on all plants and were reproducible.
These results show that expression of both GL1 and R is required to allow trichome formation in a GA-deficient background.
GA-Dependent Expression of GL1
We initiated a series of experiments to determine whether GAs could regulate the GL1 gene. To determine whether SPY and GL1 genes were acting along the same pathway, we made spy-5 gl1–1 double mutants and looked at trichome formation. No trichomes were observed on spy-5 gl1–1 rosette leaves (data not shown). Thus, the gl1–1 glabrous phenotype is epistatic to the spy-5 four-branch trichome phenotype, suggesting that GAs could regulate the GL1 gene via the SPY locus. To determine whether the GL1 gene is transcriptionally regulated by GAs, we first analyzed the expression level of the GUS reporter gene under the control of the GL1 promoter/enhancer when plants were grown in the presence of uniconazol. The promoter and 3′ enhancer of GL1 drive the high expression of the GUS reporter gene in nascent trichome cells and to a lesser extent in epidermal cells of GL1p-GUS transgenic lines (Larkin et al., 1993).
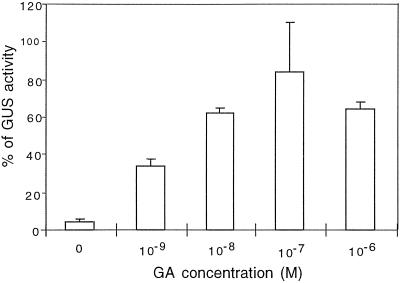
In our conditions young leaves of GL1p-GUS plants were stained with a clearly decreasing gradient from the base to the tip of the leaf, whereas nearly all trichomes exhibited a strong GUS staining (Fig. 5A). Leaf primordia at the center of the rosette also exhibited a strong GUS activity. Staining in older leaves was restricted to trichome cells. These observations are in agreement with previously published data (Larkin et al., 1993) showing that the GUS activity persists longer in trichomes than in the rest of the leaf. When GL1p-GUS plants were grown on 10−6 m uniconazol, very little or no staining was visible either in young leaves or in trichomes of older leaves (Fig. 5B). Staining at the center of the rosette remained visible and could be due to incomplete shutoff of endogenous GA biosynthesis. When exogenous GAs were supplied to uniconazol-treated plants (Fig. 5C), the GUS activity was similar to untreated wild-type plants. To analyze the regulation of the GL1 promoter/enhancer in a GA-deficient background, the GL1p-GUS construct was introduced in the ga1–3 background. We quantified the GUS activity in young leaf blades of ga1–3 GL1p-GUS plants grown with increasing concentrations of exogenous GAs (Fig. 6). When no exogenous GAs were provided, a basal GUS activity was detected corresponding to about 3 to 5% of the GL1p-GUS activity in the wild-type background. Addition of 10−9 m GAs led to a 10- to 12-fold increase of GUS activity. When plants were grown on 10−8 to 10−6 m GAs, the specific GUS activity reached a maximum level, corresponding to 60 to 80% of the GL1p-GUS activity in the wild-type background. Higher concentrations of GAs did not lead to higher GUS activity. These data suggest that GL1 transcription is up-regulated by GAs. This result predicts that GL1 transcription is strongly down-regulated in ga1–3. To confirm this hypothesis, we performed RT-PCR experiments to reveal the presence or absence of GL1 transcript in wild-type and ga1–3 backgrounds. Primers were chosen to encompass the entire GL1-coding region (see Methods) and should give rise to a 687-bp band. The expected GL1 amplification product was observed in the wild type after 35 cycles of PCR. Under the same conditions the presence of the GL1 band remained undetectable in the ga1–3 background (data not shown). However, a new PCR reaction with 10 cycles from an aliquot of the previous reaction allowed the detection of the amplified GL1 band. The adenine phosphoribosyltransferase gene gave rise to a 479-bp band and was chosen as a control since it is expressed at similar levels in both wild type and ga1–3 (Cowling, 1997). This result shows that the GL1 transcripts are present at very low levels in ga1–3 compared with the wild type.
Figure 5.
GUS staining of GL1p-GUS plants grown on MSAR medium in the presence of uniconazol. A, Plant grown on MSAR medium for 17 d showing a strong GUS staining throughout the third pair of leaves and younger leaf primordia at the center of the rosette. The second leaf pair shows staining mainly in trichomes. Note the preferential trichome staining in the second leaf pair. The first pair of leaves bear trichomes that are weakly or no longer stained. B, Plant grown on MSAR medium plus 10−6 m uniconazol for 17 d showing some staining in the third pair of leaves. Note the absence of staining in trichomes of the second leaf pair. Staining of young leaf primordia at the center of the rosette is still visible. The development of the plant was slowed by uniconazol, hence the smaller size of the plant compared with A. C, Plant grown on MSAR medium plus 10−6 m uniconazol for 13 d and transferred on MSAR medium plus 10−6 m uniconazol plus 10−5 m GAs for 4 d showing that the absence of staining in B can be specifically reversed when exogenous GAs are supplied. The third leaf pair and a second leaf pair are strongly stained, together with young primordia, at the center of the rosette. The numbers indicate the position of leaf pairs. c, Cotyledons. Bars represent 10 mm.
Figure 6.
GUS activity of ga1–3 GL1p-GUS plants grown in the presence of exogenous GAs. The specific GUS activity was measured by fluorometry as described in Methods. The background GUS activity measured from wild-type plant without the transgene has been subtracted from the GUS activities measured from transgenic lines. The 100% value corresponds to the specific activity of GL1p-GUS plants. Concentrations as low as 10−9 m GAs induced GUS expression. When exogenous GAs were increased to a 10−8 m or higher concentration, the GUS activity reached a plateau corresponding to about 80% of the GL1p-GUS activity. Higher concentrations of GAs are toxic to the plants and were not tested.
These data demonstrate that GL1 transcription is up-regulated by GAs in leaves and that the glabrous phenotype of GA-deficient plants is due at least in part to a lack of GL1 transcription.
DISCUSSION
Trichome Formation in a GA-Deficient Background
Trichome development is impaired in ga1–3, gl1–1, and ttg-1 mutants. Previous work demonstrated that GL1 and TTG genes act at the same point in trichome initiation (Larkin et al., 1994). What is the relationship between GAs and trichome initiation? Two hypotheses can be proposed: (a) GAs regulate trichome formation through regulation of GL1 and/or TTG genes, or (b) GAs regulate an additional, unknown pathway. The latter hypothesis can be ruled out since we show here that trichome formation in ga1–3 is restored when GL1 and R, the maize gene that rescues the ttg-1 mutant, are coexpressed. This result implies that, other than GL1 and possibly TTG pathways, no unknown pathway for trichome development is missing in ga1–3. This is in accordance with the first hypothesis proposed above.
GL1 and Possibly TTG Regulation by GAs
The epistasis of gl1–1 to spy-5 suggests that the GL1 gene acts downstream of the GA signal transduction pathway. The GA-dependent expression of the GUS gene under control of the GL1 promoter/enhancer, together with the absence of GL1 transcript in ga1–3, supports this conclusion and strongly suggests that the GL1 gene is transcriptionally activated by GAs. The lack of trichome development on GA-deficient leaves is therefore due, at least in part, to the lack of GL1 expression.
Is either GL1 alone or both GL1 and TTG regulated by GAs? This question was addressed by expressing either GL1 or R in ga1–3. Because both GL1 and TTG are required for trichome formation, it is important to note here that the absence of trichome restoration in ga1–3 when expressing one of these two genes reveals the absence of the other gene product. For instance, the fact that constitutive expression of R in the ga1–3 background showed no significant trichome restoration indicates that GL1 activity is not present in the absence of GAs. In the case of the constitutive expression of GL1 alone in the ga1–3 background, two possibilities could account for the absence of trichome development. First, TTG activity could also be missing in ga1–3. Second, because the 35SGL1 construct poorly complements the gl1–1 mutant (Fig. 4L; Oppenheimer et al., 1991), it is possible that this construct is also not able to complement the ga1–3 mutant even in the presence of the TTG protein. The 35SGL1 construct also led, for unknown reasons, to a decrease in trichome number in a wild-type background (Fig. 4E) (Oppenheimer et al., 1991). The GA regulation of TTG will be best assessed once the TTG gene is available. Nevertheless, unlike the gl1–1 mutation, the ttg-1 mutation causes not only a glabrous phenotype but also additional phenotypes, such as a defect in seed coat pigmentation (Koornneef, 1981). No such phenotype is observed in ga1–3. This suggests that TTG regulation by GAs would not take place in all cell types in which TTG is expressed but might be restricted to trichome precursor cells. The relative absence of restoration when either GL1 or R are expressed in ga1–3 is in favor of a coregulation of GL1 and TTG by GAs, since trichome formation is obtained when both are expressed in ga1–3.
Although neither GL1 nor R were sufficient in ga1–3 to provide full trichome restoration, a few more trichomes were produced compared with ga1–3. The formation of such small numbers of trichomes could be attributed either to a basal level of both GL1 and TTG expression in ga1–3 or to some cross-regulation of these two genes. The latter hypothesis is unlikely, however, since the level of GL1 transcripts is not affected in the ttg-1 background (Di Cristina et al., 1996). The former hypothesis is supported by the basal GUS activity measured in ga1–3 GL1p-GUS plants when no exogenous GAs were provided and by the detection of a GL1 amplification product in ga1–3 when additional amplification cycles were applied.
In conclusion, these data strongly indicate that GL1 expression is strongly reduced in a GA-deficient mutant and therefore is positively regulated by GAs in the wild type.
Do GAs Regulate Endoreduplication in Trichome Cells through GL1 Gene Activity?
Analysis of ga1–3 and spy-5 mutants and of wild-type plants grown on uniconazol demonstrated that the endogenous GA level and/or activity of the signal transduction pathway positively modulates both the number of trichomes and trichome branching. This result suggests that GAs are involved not only in trichome cell initiation but also in trichome cell morphogenesis. The involvement of GAs in these aspects of trichome formation could be explained in two ways. First, GAs could regulate two independent components in trichome development: in this case, a first component would have to act early in trichome specification (since the ga1–3 mutant is glabrous), and the second component would have to act late in trichome morphogenesis (since uniconazol-treated plants make two-branch trichomes and the spy-5 mutant makes overbranched trichomes). Alternatively, GAs could simply regulate a unique component essential for trichome specification, which in turn would also play a role in trichome morphogenesis. As suggested by Hülskamp et al. (1994) and Esch et al. (1994), GL1 is likely to play a role not only in the initiation process but also later in trichome development. This involvement of GL1 in both initiation and morphogenesis is also illustrated by the phenotype of the weak gl1–2 allele, which leads to formation of fewer trichomes, most of which are two-branch trichomes (Esch et al., 1994). The fact that the GL1 promoter is positively regulated by GAs and that constitutive expression of GL1 and R in ga1–3 restores not only trichome initiation and a degree of development that, although incomplete, is similar to what is observed when GL1 and R are ectopically expressed in a wild-type background, reinforces the latter alternative and supports the idea that the GL1 gene is the essential component, the activity of which influences both trichome initiation and morphogenesis. Indeed, if GAs were acting independently through one early (e.g. GL1) and one late target gene, constitutive expression of the early-acting gene in the absence of GAs could not lead to trichome formation (unbranched and two-branch trichomes in both ga1–3 and wild-type backgrounds).
During trichome development, endoreduplication level and branch number are closely related (Hülskamp et al., 1994). For example, mutations in the KAKTUS or TRIPTYCHON genes lead to formation of trichomes having extrabranches and an additional round of endoreduplication (Hülskamp et al., 1994). On the other hand, the number of endoreduplications in trichome cells is positively controlled by GL1, TTG, and, later in development, GLABROUS3. The high frequency of four-branch trichomes on spy-5 leaves could be explained by an overactivation of the endogenous GA-regulated GL1 gene, which in turn could induce extra round(s) of endoreduplication leading to extra branch formation (or vice versa). DNA content analysis of isolated trichomes indicate that spy-5 overbranched trichomes indeed have twice as much DNA as wild-type trichomes on average (D. Perazza, unpublished data). The role of GAs in cell division has been suggested since GAs induce a rapid rise in the abundance of a CDK1 homolog transcript in rice (Sauter et al., 1995; Jacobs, 1997). Endoreduplication can be seen as an alternative to cell division. Are ga1–3 plants deficient in endoreduplication? Flow cytometry from entire leaves reveals that ga1–3, like gl1–1 and ttg-1, exhibits 2C- to 16C-ploidy levels similar to wild-type leaves (data not shown), indicating that if GAs have a role in endoreduplication, it is restricted essentially to trichome or epidermal cells.
The present study has shown that GAs positively regulate GL1 Myb-gene expression in trichome cells. The reporter gene construct used in this study harbors a promoter fragment and a small enhancer region located downstream of the GUS gene, which reproduces the GL1 expression pattern (Larkin et al., 1993). It will be of interest to determine whether GA regulation requires the presence of the 3′ enhancer or only upstream sequences. The regulation of GL1 by GAs constitutes the first molecular evidence for the involvement of these hormones in the commitment of plant cells to a specific developmental fate. The continued analysis of the control of GL1 expression should give us insights into the GA signal transduction pathway in Arabidopsis.
ACKNOWLEDGMENTS
We thank Dr. David Marks for providing the 35SGL1 line, Dr. Martin Hülskamp for the GL1p-GUS line, Dr. Alan Lloyd for the 35SR line, Dr. Nicholas Harberd for the spy-5 mutant, Dr. Steve Clouse for the bri1 mutant, the Nottingham Arabidopsis Stock Center for the gl1–1 and ttg-1 mutants, and the Sumitomo Chemical Co. for the gift of uniconazol-P. We are grateful to Dr. Christelle Perrin (Institut Albert Bonniot, Grenoble, France) for her advice in fluorometry analysis, and Dr. Spencer Brown (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) for his contribution to flow cytometry experiments. We would like to thank Dr. Jean-Marc Bonneville for useful discussions of results and critical reading of the manuscript, Dr. Pierre Carol for critical reading of the manuscript and for providing APT primers, Dr. Jean-Gabriel Valay for critical discussions, and Dr. Malcolm Campbell and Dr. Campbell Wyndham for reading the manuscript. We thank Mireille Rocipon for her technical assistance.
Abbreviation:
- RT-PCR
reverse transcriptase-PCR
Footnotes
This work was supported by the Centre National de la Recherche Scientifique ACC-SV1 program (1995–1997) and the Ministère de l'Education Nationale de la Recherche et de la Technologie.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chien JC, Sussex IM. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996;111:1321–1328. doi: 10.1104/pp.111.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling R (1997) Characterization of gibberellin responses in Arabidopsis thaliana seedlings. PhD thesis. University of East Anglia, UK
- Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 1996;10:393–402. doi: 10.1046/j.1365-313x.1996.10030393.x. [DOI] [PubMed] [Google Scholar]
- Esch JJ, Oppenheimer DG, Marks MD. Characterization of a weak allele of the GL1 gene of Arabidopsis thaliana. Plant Mol Biol. 1994;24:203–207. doi: 10.1007/BF00040586. [DOI] [PubMed] [Google Scholar]
- Gallagher SR (1992) Quantitation of GUS activity by fluorometry. In SR Gallagher, ed, GUS Protocols using the GUS Gene as a Reporter of Gene Expression. Academic Press, Inc., San Diego, CA, pp 47–59
- Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactivation of a high-pI α-amylase gene promoter. Plant Cell. 1995;7:1879–1891. doi: 10.1105/tpc.7.11.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Dorne AM, Grellet F. GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST 1 gene. Plant Mol Biol. 1995;27:743–752. doi: 10.1007/BF00020227. [DOI] [PubMed] [Google Scholar]
- Hooley R. Gibberellins: perception, transduction and responses. Plant Mol Biol. 1994;26:1529–1555. doi: 10.1007/BF00016489. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Misera S, Jurgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76:555–566. doi: 10.1016/0092-8674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Jacobs T. Why do plant cells divide? Plant Cell. 1997;9:1021–1029. doi: 10.1105/tpc.9.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MG. Gibberellins and the procera mutant of tomato. Planta. 1987;172:280–284. doi: 10.1007/BF00394598. [DOI] [PubMed] [Google Scholar]
- Koncz C, Mayerhofer R, Koncz-Kalman Z, Nawrath C, Reiss B, Rédei GP, Schell J. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 1990;9:1337–1346. doi: 10.1002/j.1460-2075.1990.tb08248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M. The complex syndrome of ttg mutants. Arabidopsis Inform Serv. 1981;18:45–51. [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant. 1985;65:33–39. [Google Scholar]
- Larkin JC, Marks MD, Nadeau J, Sack F. Epidermal cell fate and patterning in leaves. Plant Cell. 1997;9:1109–1120. doi: 10.1105/tpc.9.7.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Lloyd AM, Paparozzi ET, Marks MD. Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell. 1994;6:1065–1076. doi: 10.1105/tpc.6.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Oppenheimer DG, Pollock S, Marks MD. Arabidopsis GLABROUS1 gene requires downstream sequence for function. Plant Cell. 1993;5:1739–1748. doi: 10.1105/tpc.5.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks DM. The control of trichome spacing and number in Arabidopsis. Development. 1996;122:991–1005. doi: 10.1242/dev.122.3.997. [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Walbot V, Davis RW. Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science. 1992;258:1773–1775. doi: 10.1126/science.1465611. [DOI] [PubMed] [Google Scholar]
- Ni B-R, Bradford KJ. Germination and dormancy of abscisic acid- and gibberellin-deficient mutant tomato (Lycopersicon esculentum) seeds. Sensitivity of germination to abscisic acid, gibberellin, and water potential. Plant Physiol. 1993;101:607–617. doi: 10.1104/pp.101.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD. A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell. 1991;67:483–493. doi: 10.1016/0092-8674(91)90523-2. [DOI] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Huttly AK. Cloning of two gibberellin-regulated cDNAs from A. thaliana by subtractive hybridization: expression of the tonoplast water channel γ-TIP is increased by GA3. Plant Mol Biol. 1994;24:603–615. doi: 10.1007/BF00023557. [DOI] [PubMed] [Google Scholar]
- Phinney BO, Freeling M, Robertson DS, Spray CR, Silverthorne J. Dwarf mutants in maize—the gibberellin biosynthetic pathway and its molecular future. In: Bopp M, editor. Plant Growth Substances. New York: Springer-Verlag; 1986. pp. 55–64. [Google Scholar]
- Sauter M, Mekhedov SL, Kende H. Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J. 1995;7:623–632. doi: 10.1046/j.1365-313x.1995.7040623.x. [DOI] [PubMed] [Google Scholar]
- Shi L, Gast RT, Gopalraj M, Olszewski NE. Characterization of a shoot-specific, GA3- and ABA-regulated gene from tomato. Plant J. 1992;2:153–159. [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T-p. The Arabidopsis RGA gene encodes a transcriptional regulator expressing the gibberellin signal transduction pathway. Plant Cell. 1998;10:155–169. doi: 10.1105/tpc.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Mak PY, Martinez EC, Sun TP. Genetics. 1997;146:1087–1099. doi: 10.1093/genetics/146.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Olszewski NE. Genetic analysis of gibberellin signal transduction. Plant Physiol. 1996;112:11–17. doi: 10.1104/pp.112.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development. 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Walker A, Davison PA, James CJ, Esch J, Marks DM, Gray JC (1997) The TTG1 gene does not encode a myc transcription factor. 8th International Conference on Arabidopsis Research (abstract no. 3–79). June 25–29, Madison, WI