Abstract
Immunogenicity and safety of ACWY-TT compared with licensed ACWY polysaccharide vaccine (MenPS) in healthy adults, and lot-to-lot consistency of three ACWY-TT lots were evaluated in a phase 3, open, controlled study. Adults aged 18–55 y were randomized to receive ACWY-TT (one of three lots) or MenPS. Serum bactericidal antibodies (rSBA) were measured pre- and 1 mo post-vaccination. Adverse events (AEs) were assessed 4 d (solicited symptoms) and 31 d (unsolicited symptoms) post-vaccination. Serious AEs were reported up to 6 mo after vaccination. The number of vaccinated subjects was 1247 (ACWY-TT, n = 935; MenPS, n = 312). ACWY-TT lot-to-lot consistency and non-inferiority of ACWY-TT as compared with MenPS groups were demonstrated according to pre-specified criteria. The percentages of subjects with a vaccine response (VR = rSBA titer ≥ 1:32 in initially seronegative; ≥ 4-fold increase in initially seropositive) to ACWY-TT vs. MenPS were 80.1%/69.8% (serogroup A), 91.5%/ 92.0% (C), 90.2%/85.5% (W-135), 87.0%/78.8% (Y). Exploratory analyses showed that for serogroups A, W-135 and Y, VR rates and GMTs were significantly higher for ACWY-TT compared with MenPS. For each serogroup, ≥ 98.0% of subjects had rSBA titers ≥ 1:128. Grade 3 solicited AEs were reported in ≤ 1.6% of subjects in any group. The immunogenicity of ACWY-TT vaccine was non-inferior to MenPS for all four serogroups in adults, with significantly higher VR rates to serogroups A, W-135 and Y and an acceptable safety profile. Consistency of 3 ACWY-TT production lots was demonstrated. These data suggest that, if licensed, ACWY-TT conjugate vaccine may be used for protection against invasive meningococcal disease in healthy adults.
This study is registered at clinicaltrials.gov NCT00453986
Keywords: ACWY-TT vaccine, Neisseria meningitidis, adult, bactericidal activity, conjugate vaccine, immunogenicity, safety, tetravalent meningococcal vaccine, vaccine
Introduction
Invasive meningococcal disease (IMD) remains a global public health concern, with 0.5 million cases estimated to occur annually, of which at least 10% result in death.1 Neisseria meningitidis serogroups A, B, C, W-135, and Y cause the majority of IMD globally, although the distribution of each serogroup varies. Epidemic IMD is most commonly due to serogroup A, whereas serogroups B, C, Y and W-135 are more frequently implicated in endemic disease and sporadic outbreaks.2
The epidemiology of IMD in much of South East Asia and the Middle East is incompletely described. In the Philippines, the distribution of serogroups causing IMD is not known, but a serogroup A outbreak was reported in Baguio City, Mt. Province and Ifugao between 2004 and 2005, with 33% mortality.3 Serogroups A and W-135 currently predominate in the Middle East, and serogroup W-135 outbreaks have been reported in Hajj pilgrims and their contacts.4-6 Serogroups B and C predominate in most of Europe, whereas in the US serogroup Y is also an important cause of disease.2,7 In Africa, serogroup A is responsible for most major epidemics.1,2 However, serogroups W-135 and serogroup X are important emerging causes of outbreaks within the African meningitis belt.8-11
IMD affects all age groups, and while the incidence of IMD is highest in infants, the burden of disease due to IMD in adults is substantial. Between 1998 and 2007 almost 43% of all IMD cases in the US were reported in adults 25 y of age and older.7 Groups particularly at risk for IMD are travelers, notably Hajj pilgrims.12
Meningococcal polysaccharide vaccines have been available for use in adults for many years and are most frequently used for travelers to regions of high IMD incidence. Meningococcal polysaccharide vaccines are efficacious in preventing IMD in adults but do not elicit long-lasting immunological memory.13 Antibody persistence only lasts for 3–5 y, but immune hyporesponsiveness may occur when polysaccharide vaccines are given more than once—this is particularly the case for serogroup C.14 The induction of hyporesponsiveness is a key limitation for the use of polysaccharide vaccines for individuals who need to retain longer term immunity. Conjugation of the polysaccharides to carrier proteins overcomes many of the limitations associated with polysaccharide vaccines by inducing a T-cell dependent response, with resulting immune memory and boostability. Importantly, immune tolerance has not been demonstrated after repeated MenC conjugate vaccination, which has been in place in the UK for the past decade.15,16
The investigational tetravalent polysaccharide conjugate vaccine against N. meningitidis serogroups A, C, W-135 and Y, using tetanus toxoid as the carrier protein [ACWY-TT, GlaxoSmithKline Biologicals (GSK) Belgium] is immunogenic in toddlers, children and adolescents.17-23 This partially double-blinded, controlled, non-inferiority study assessed the immunogenicity and safety of ACWY-TT in healthy adults between 18 and 55 y of age. Manufacturing consistency using three different manufacturing lots was established and pooled serological results were compared against the tetravalent polysaccharide vaccine control (MenPS: Mencevax™ ACWY, GSK).
Results
Study subjects
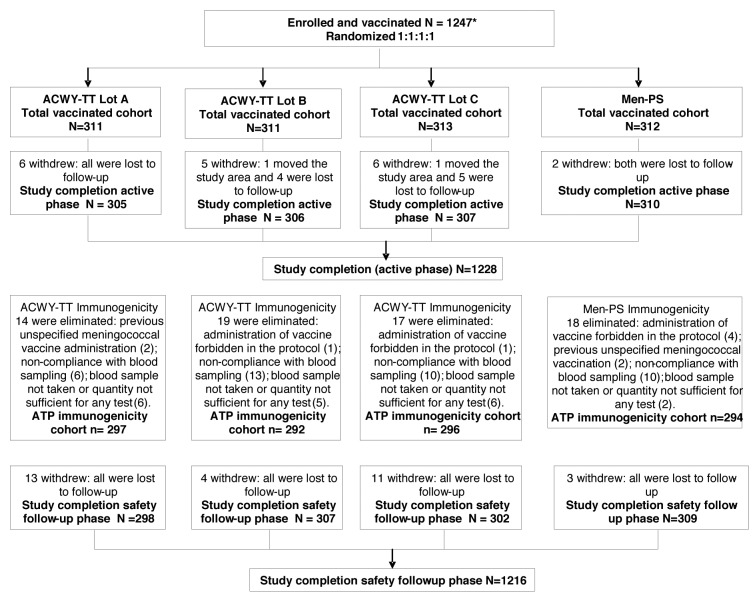
In total, 1247 subjects were vaccinated with ACWY-TT (one of three lots) or MenPS, of which 1228 subjects completed the active phase. No subject withdrew from the study due to an adverse event (AE). There were 1179 subjects included in the according to protocol (ATP) immunogenicity cohort (Fig. 1). More males were enrolled than females (Table 1). The demographic profiles of subjects in each group were comparable with respect to mean age, sex and race (Table 1).
Figure 1. Subject flow through the study. *An additional 105 subjects were enrolled in the cohort evaluated for the co-administration of influenza vaccine (the analysis of co-administration with seasonal influenza vaccine will be presented in a separate publication).
Table 1. Demographic characteristics of enrolled and vaccinated subjects (total vaccinated cohort).
| |
ACWY lot A N = 311 |
ACWY lot B N = 311 |
ACWY lot C N = 313 |
ACWY-TT Pooled lots N = 935 |
MenPS N = 312 |
|
|---|---|---|---|---|---|---|
| Characteristics | Categories | Value/n (%) | Value/n (%) | Value/n (%) | Value/n (%) | Value/n (%) |
| Age (years) |
Mean |
35.2 |
35.1 |
35.7 |
35.3 |
34.9 |
| |
SD |
10.48 |
10.50 |
10.75 |
10.57 |
10.73 |
| |
Range |
18–55 |
18–55 |
18–55 |
18–55 |
18–55 |
| Gender |
Female |
135 (43.4) |
139 (44.7) |
133 (42.5) |
407 (43.5) |
151 (48.4) |
| |
Male |
176 (56.6) |
172 (55.3) |
180 (57.5) |
528 (56.5) |
161 (51.6) |
| Race |
Southeast Asian |
223 (71.7) |
223 (71.7) |
224 (71.6) |
670 (71.7) |
224 (71.8) |
| |
Arabic/North African |
88 (28.3) |
88 (28.3) |
87 (27.8) |
263 (28.1) |
88 (28.2) |
| Other* | 0 (0.0) | 0 (0.0) | 2 (0.6) | 2 (0.2) | 0 (0.0) | |
N, total number of subjects; Value, value of the considered parameter; n/%, number/percentage of subjects in a given category; SD, standard deviation; Other, Native Hawaiian/Pacific Islander or Caucasian/European heritage
Primary study objectives
The presence of serogroup-specific serum bactericidal activity (SBA; which is measured with a functional assay which measures the capability of test serum to kill a meningococcal strain when exogenous complement is added) above threshold levels derived from effectiveness studies is widely accepted as a surrogate for protection against IMD.24,25 Lot-to-lot consistency of three ACWY-TT lots with respect to SBA geometric mean titers (using rabbit complement as the exogenous complement source: rSBA GMTs) was demonstrated. For all 12 pairwise comparisons, the 2-sided 95% confidence interval (CI) on the GMT ratio between lots was within the pre-specified interval for non-inferiority of [0.5; 2.0] for each pair of lots and for each serogroup (Table 2), justifying pooling of data in the ACWY-TT groups for evaluation of the other objectives.
Table 2. Ratios of rSBA GMTs between ACWY-TT Lot groups one month after vaccination (ATP immunogenicity cohort).
| |
|
Adjusted GMT ratio* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
95% CI |
||||||||
| Serogroup | ACWT-TT Lot | N | Adjusted GMT | ACWT-TT Lot | N | Adjusted GMT | Ratio order | Value | LL | UL |
| A |
A |
253 |
3851.3 |
B |
244 |
3607.2 |
A / B |
1.07 |
0.88 |
1.29 |
| |
A |
253 |
3851.3 |
C |
246 |
3582.6 |
A / C |
1.07 |
0.89 |
1.30 |
| |
B |
244 |
3607.2 |
C |
246 |
3582.6 |
B / C |
1.01 |
0.83 |
1.22 |
| C |
A |
283 |
9945.1 |
B |
275 |
8520.9 |
A / B |
1.17 |
0.91 |
1.50 |
| |
A |
283 |
9945.1 |
C |
291 |
9393.5 |
A / C |
1.06 |
0.83 |
1.36 |
| |
B |
275 |
8520.9 |
C |
291 |
9393.5 |
B / C |
0.91 |
0.71 |
1.16 |
| W-135 |
A |
287 |
5380.8 |
B |
286 |
5020.8 |
A / B |
1.07 |
0.87 |
1.33 |
| |
A |
287 |
5380.8 |
C |
287 |
5534.7 |
A / C |
0.97 |
0.79 |
1.20 |
| |
B |
286 |
5020.8 |
C |
287 |
5534.7 |
B / C |
0.91 |
0.73 |
1.12 |
| Y |
A |
294 |
7863.7 |
B |
284 |
7204.0 |
A / B |
1.09 |
0.90 |
1.33 |
| |
A |
294 |
7863.7 |
C |
284 |
7747.9 |
A / C |
1.01 |
0.83 |
1.24 |
| B | 284 | 7204.0 | C | 284 | 7747.9 | B / C | 0.93 | 0.76 | 1.13 | |
Adjusted GMT, geometric mean antibody titer adjusted for age strata, baseline titer, and whether or not influenza vaccine was co-administered; N, Number of subjects with both pre- and post-vaccination results available. 95% CI = 95% confidence interval for the adjusted GMT ratio (ANCOVA model: adjustment for age strata, baseline titer and whether or not subjects were included in the analysis of co-administration with seasonal influenza vaccine—pooled variance with more than 2 groups); LL, lower limit; UL, upper limit. *Lot-to-lot consistency was demonstrated if for each pair of lots and for each serogroup, the two-sided 95% CI on the GMT ratio between lots was within the interval of [0.5; 2.0].
Non-inferiority of ACWY-TT compared with MenPS in terms of the percentage of subjects with a vaccine response [VR, defined as an rSBA titer ≥ 1:32 in initially seronegative subjects (pre-vaccination titer < 1:8), or a ≥ 4-fold increase over the pre-vaccination titer for initially seropositive subjects (pre-vaccination titer ≥ 1:8)] after vaccination was demonstrated: the lower limit of the 95% CI for the difference between groups was above the pre-specified non-inferiority limit of -10% for all four serogroups (Table 3).
Table 3. Comparison between groups in rSBA vaccine response rate one month after vaccination (ATP immunogenicity cohort).
| |
|
% Vaccine response |
Difference in vaccine response rate* |
||
|---|---|---|---|---|---|
| Serogroup | Group | N | n | % (95% CI) | % (95%CI) |
| A |
ACWY-TT |
743 |
595 |
80.1 (77.0; 82.9) |
10.24 (4.11; 16.78) |
| |
MenPS |
252 |
176 |
69.8 (63.8; 75.4) |
|
| C |
ACWY-TT |
849 |
777 |
91.5 (89.4; 93.3) |
-0.49 (-3.85; 3.57) |
| |
MenPS |
288 |
265 |
92.0 (88.3; 94.9) |
|
| W-135 |
ACWY-TT |
860 |
776 |
90.2 (88.1; 92.1) |
4.72 (0.49; 9.65) |
| |
MenPS |
283 |
242 |
85.5 (80.9; 89.4) |
|
| Y |
ACWY-TT |
862 |
750 |
87.0 (84.6; 89.2) |
8.19 (3.24; 13.69) |
| MenPS | 288 | 227 | 78.8 (73.6; 83.4) | ||
N, number of subjects with pre and post vaccination results; n/%, number/percentage of subjects with a vaccine response (defined as an rSBA titer ≥ 1:32 in subjects with pre-vaccination titer < 1:8, or a ≥ 4-fold increase in titer for subjects with pre-vaccination titer ≥ 1:8). 95% CI = 95% confidence interval. *ACWY-TT minus MenPS. Bold: the lower limit of the standardized asymptotic 95% CI is above the pre-specified non-inferiority limit of -10% for all four serogroups.
Immunogenicity of ACWY-TT (pooled groups)
Prior to vaccination, the percentage of subjects in the ACWY-TT group with rSBA titers ≥ 1:128 was 73.7% for serogroup A, 48.8% for C, 59.8% for W-135 and 79.0% for Y. The percentage of subjects in the MenPS group with rSBA titers ≥ 1:128 was 78.7% for serogroup A, 52.6% for C, 54.8% for W-135 and 77.8% for Y. Pre-vaccination rSBA titers for each serogroup were similar in the ACWY-TT and MenPS group (data not shown).
One month after vaccination, the percentage of subjects in both groups with rSBA titers ≥ 1:8 and rSBA titers ≥ 1:128 was ≥ 99.3% and ≥ 98.0%, respectively (data not shown). For each serogroup, ≥ 80.1% of ACWY-TT vaccinees had a VR (Table 3). An exploratory analysis showed that the VR was statistically significantly higher in the ACWY-TT group as compared with the MenPS group for serogroups A, W-135, and Y.
After vaccination with ACWY-TT rSBA GMTs increased by at least 20-fold for serogroups A, W-135 and Y and 109-fold for serogroup C (Fig. 2). The fold increase in GMTs observed after MenPS was at least 10-fold for serogroups A, W-135 and Y and 81-fold for serogroup C.
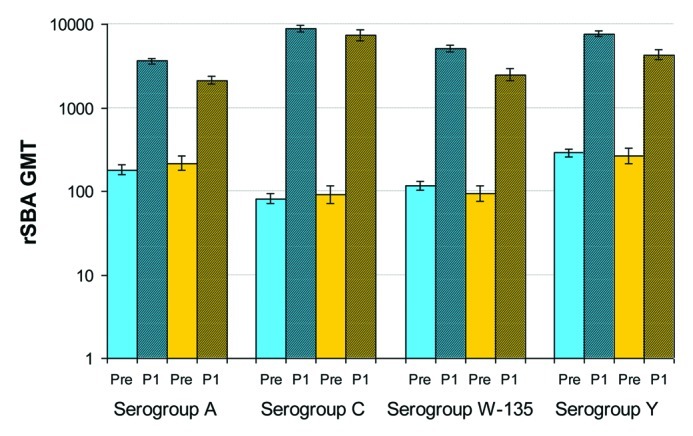
Figure 2. rSBA GMTs (with 95% CIs) before and one month after vaccination (ATP immunogenicity cohort). Pre, pre-vaccination (plain columns); P1, 1 mo post vaccination (hatched columns). Blue bars, ACWY-TT group; Orange bars, MenPS group.
Exploratory analyses showed statistically significantly higher rSBA GMTs in the ACWY-TT group compared with the MenPS group for serogroups A, W-135 and Y.
The percentage of subjects with anti-tetanus antibody concentrations ≥ 0.1 IU/mL increased from 51.5% to 79.4% in the ACWY-TT group (ACWY contains 44 μg TT), but remained unchanged in the MenPS group (52.2% to 53.2%). Similarly, the anti-tetanus antibody geometric mean concentration (GMC) increased by 14-fold in the ACWY-TT group but did not increase in the MenPS group after vaccination (data not shown).
Immunogenicity in the 18–25 and 26–55 y age strata
An exploratory analysis of the primary objective by age stratum (18–25 y and 26–55 y) showed that the lower limit of the 95% CI for the difference between ACWY-TT and MenPS groups in percentages of subjects with a VR in each age strata was above -10% for all 4 serogroups in both age strata (Table 4) .
Table 4. Comparison between groups in rSBA vaccine response rate one month after vaccination stratified by age (exploratory analysis, ATP immunogenicity cohort).
| |
|
18–25 y subgroup |
26–55 y subgroup |
||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
% Vaccine response |
Difference in vaccine response rate* |
|
% Vaccine response |
Difference in vaccine response rate* |
|||
| Serogroup | Group | N | n | % (95% CI) | % (95%CI) | N | n | % (95% CI) | % (95%CI) |
| A |
ACWY-TT |
177 |
147 |
83.1 (76.7; 88.3) |
10.47 (-0.96; 23.70) |
566 |
448 |
79.2 (75.6; 82.4) |
10.20 (3.10; 17.82) |
| |
MenPS |
62 |
45 |
72.6 (59.8; 83.1) |
|
190 |
131 |
68.9 (61.8; 75.4) |
|
| C |
ACWY-TT |
204 |
193 |
94.6 (90.6; 97.3) |
-5.39 (-9.40; -0.30) |
645 |
584 |
90.5 (88.0; 92.7) |
1.24 (-3.06; 6.49) |
| |
MenPS |
73 |
73 |
100 (95.1; 100) |
|
215 |
192 |
89.3 (84.4; 93.1) |
|
| W-135 |
ACWY-TT |
209 |
193 |
92.3 (87.9; 95.6) |
0.80 (-5.63; 10.07) |
651 |
583 |
89.6 (86.9; 91.8) |
6.06 (0.96; 12.05) |
| |
MenPS |
71 |
65 |
91.5 (82.5; 96.8) |
|
212 |
177 |
83.5 (77.8; 88.2) |
|
| Y |
ACWY-TT |
213 |
194 |
91.1 (86.4; 94.5) |
5.94 (-1.92; 16.33) |
649 |
556 |
85.7 (82.7; 88.3) |
9.03 (3.10; 15.65) |
| MenPS | 74 | 63 | 85.1 (75.0; 92.3) | 214 | 164 | 76.6 (70.4; 82.1) | |||
N, number of subjects with pre and post vaccination results; n/%, number/percentage of subjects with a vaccine response (defined as an rSBA titer ≥ 1:32 in subjects with pre-vaccination titer < 1:8, or a ≥ 4-fold increase in titer for subjects with pre-vaccination titer ≥ 1:8). 95% CI = 95% confidence interval. *ACWY-TT minus MenPS.
Safety
Pain and headache were the most frequently reported local and general solicited symptoms within 4 d of vaccination, in both groups (Table 5). The percentage of subjects who reported pain, redness and swelling at the injection site was higher in ACWY-TT recipients than in MenPS recipients (for swelling, the 95% CIs did not overlap), while the occurrence of general symptoms was similar in both groups. Notably, grade 3 local and general symptoms were infrequently reported in both groups.
Table 5. Percentage of subjects with solicited local and general symptoms reported during the 4 d (Days 0–3) post-vaccination period (total vaccinated cohort).
| |
|
ACWY–TT |
MenPS |
||||
|---|---|---|---|---|---|---|---|
| Symptom | Intensity | N | n | % (95% CI) | N | n | % (95% CI) |
| Pain |
All |
927 |
180 |
19.4 (16.9–22.1) |
310 |
42 |
13.5 (9.9–17.9) |
| |
Grade 3 |
927 |
4 |
0.4 (0.1–1.1) |
310 |
1 |
0.3 (0–1.8) |
| Redness (mm) |
All |
927 |
82 |
8.8 (7.1–10.9) |
310 |
14 |
4.5 (2.5–7.5) |
| |
> 50 mm |
927 |
12 |
1.3 (0.7–2.3) |
310 |
0 |
0 (0–1.2) |
| Swelling (mm) |
All |
927 |
73 |
7.9 (6.2–9.8) |
310 |
6 |
1.9 (0.7–4.2) |
| |
> 50 mm |
927 |
10 |
1.1 (0.5–2) |
310 |
0 |
0 (0–1.2) |
| Fatigue |
All |
927 |
114 |
12.3 (10.3–14.6) |
310 |
30 |
9.7 (6.6–13.5) |
| |
Grade 3 |
927 |
8 |
0.9 (0.4–1.7) |
310 |
0 |
0 (0–1.2) |
| Fever(Axillary) |
≥ 37.5°C |
927 |
37 |
4 (2.8–5.5) |
310 |
14 |
4.5 (2.5–7.5) |
| |
> 39.5°C |
927 |
2 |
0.2 (0.0–0.8) |
310 |
2 |
0.6 (0.1–2.3) |
| GI symptoms |
All |
927 |
43 |
4.6 (3.4–6.2) |
310 |
10 |
3.2 (1.6–5.9) |
| |
Grade 3 |
927 |
2 |
0.2 (0–0.8) |
310 |
1 |
0.3 (0–1.8) |
| Headache |
All |
927 |
151 |
16.3 (14.0–18.8) |
310 |
44 |
14.2 (10.5–18.6) |
| Grade 3 | 927 | 14 | 1.5 (0.8–2.5) | 310 | 5 | 1.6 (0.5–3.7) | |
N, number of subjects with at least one documented dose; n/%, number/percentage of subjects reporting the symptom at least once, 95% CI, exact 95% confidence interval; Grade 3, Adverse events preventing normal activities; GI symptoms, Gastrointestinal symptoms
The percentage of subjects reporting unsolicited symptoms during the 31-d follow-up period was 14.4% (95% CI 12.2%; 16.9%) in the ACWY-TT group and 15.1% (95% CI 11.3%; 19.5%) in the MenPS group. The percentage of subjects reporting a grade 3 unsolicited symptom was 1.4% (95% CI 0.7%; 2.4%) in the ACWY-TT group and 1.0% (95% CI 0.2%; 2.8%) in the MenPS group. Each individual grade 3 symptom was reported by only one subject, with the exception of toothache, which was reported by two subjects in the ACWY-TT group (data not shown).
Eight subjects (ACWY-TT group n = 7, 0.7%; MenPS group n = 1, 0.3%) reported 11 serious AEs (SAEs) after vaccination (10 in the ACWY-TT group and 1 in the MenPS group). Two of these events (reported by one subject) were considered to be related to vaccination: a subject in the ACWY-TT group reported abdominal pain and gastritis beginning 5 d after vaccination and required hospitalization. All SAEs resolved without sequelae. No deaths occurred during the study.
At the conclusion of the 6-mo safety follow-up, the percentage of subjects who reported rash was 1.1% (95% CI 0.5%; 2.0%) in the ACWY-TT group and 1.0% (95% CI 0.2%; 2.8%) in the MenPS group. The percentage reporting an Emergency Room visit was 1.4% (95% CI 0.7%; 2.4%) in the ACWY-TT group and 0.3% (95% CI 0.0%; 1.8%) in the MenPS group. No subject reported new onset of chronic illness.
Discussion
Major disadvantages of meningococcal polysaccharide vaccines in adults include their short-lived protection and the induction of hyporesponsiveness on repeated exposure. These two factors combined make it difficult to maintain long-term protective antibody titers in adults in highly endemic regions using polysaccharide vaccines. Although the clinical implications of hyporesponsiveness are not well understood, there is a theoretical risk of increased disease susceptibility.15 This may be particularly important in settings where meningococcal epidemics regularly occur—for example, during the Hajj pilgrimage and in countries within the African meningitis belt; and in older age groups when the immune response to vaccination is attenuated. Conjugate vaccines induce boostable responses and do not induce hyporesponsiveness on repeated exposure,15 thereby overcoming the major limitations of polysaccharide vaccines.
This study demonstrated lot-to-lot consistency of the ACWY-TT vaccine and showed that ACWY-TT was non-inferior to commercially available MenPS in terms of VR rates. Statistically significantly higher VR rates and rSBA GMTs were observed in ACWY-TT recipients for 3 out of 4 serogroups (exploratory analysis) suggesting a more robust immune response than that following MenPS.
Prior to vaccination the majority of adults were seropositive for rSBA against all 4 vaccine serogroups, despite only 4 individuals reporting a prior history of meningococcal polysaccharide vaccination (> 5 y previously). High pre-existing rSBA levels ≥ 1:8 have also been reported in other studies in adults conducted in the US (between 30–100% initially seropositive for each serogroup26) and in India (between 88–92% initially seropositive27). Circulating meningococcal or cross-reacting strains causing asymptomatic nasopharyngeal carriage and/or cross-reactivity of antibodies with other bacteria may have contributed to the high seropositivity rate observed. The observation that IMD incidence decreases with age7 suggests that the observed rSBA provides protection against meningococcal invasion. Notably, the percentage of subjects in the ACWY-TT group with rSBA titers ≥ 1:128 increased from between 48.8% and 79% for each serogroup pre-vaccination, to between 98.9% and 99.5% post vaccination, suggesting a benefit of vaccination in conferring immunity across all 4 serogroups. The high pre-vaccination seropositivity supports use of defined VRs as a key endpoint of the study, since it measures the percentage of participants that had a response to vaccination, rather than simply seropositivity.
Comparisons between studies that differ in design, population studied and in serological methods should be made cautiously. However, the results of this study showing robust immunogenicity of ACWY-TT in adults are broadly consistent with those of other ACWY-conjugate vaccines in the US and South America and a monovalent MenA-TT vaccine that has been developed for use in the African Meningitis Belt for which immunogenicity in adults was also demonstrated.26-30
The safety profile of ACWY-TT was acceptable, with a low reported incidence of grade 3 symptoms. Increased reactogenicity following vaccination with meningococcal conjugate vaccines conjugated to diphtheria toxoid and diphtheria toxoid variant (CRM197) as compared with a polysaccharide vaccine has been observed in adolescents and children,31,32and the monovalent MenA-TT conjugate was shown to have higher local reactogenicity than a quadrivalent MenPS vaccine.30 Thus the higher incidence of local symptoms in ACWY-TT recipients as compared with MenPS recipients is not unexpected, and may be due to the TT component, for which local reactogenicity has been well described.33
Potential limitations of the study include the lack of a licensed conjugate ACWY vaccine as a control. This is because at the time of the study, none was licensed in the countries where the study took place. However, a head-to-head study of ACWY-TT and another ACWY-conjugate vaccine showed that the immune responses induced by the two vaccines and their respective safety profiles were comparable.19 Additionally, the current study was conducted as open-blind with respect to receipt of ACWY-TT vs. MenPS control, primarily because the routes of vaccine administration were different (intramuscular route for the ACWY-TT vaccine and subcutaneous route for the MenPS control). However, the risk of bias in the analysis of immunogenicity was reduced since laboratory personnel were blinded as to age and group. Attribution of the relationship of AEs to vaccination could have been influenced by the open design, but reporting bias would more likely be against the investigational product. A final limitation was that numerous statistical comparisons were made without adjustment for multiplicity, increasing the risk that a significant difference may have arisen by chance alone.
ACWY-TT was immunogenic against all four meningococcal serogroups (A, C, W-135 and Y) in healthy adults 18–55 y of age, with VRs that were non-inferior to MenPS, and rSBA GMTs that were significantly higher than MenPS for serogroups A, W-135 and Y (exploratory analysis). These data suggest potential benefits of ACWY-TT conjugate vaccination over MenPS in this age-group, although this would need to be confirmed with antibody persistence studies. ACWY-TT had an acceptable safety profile in healthy adults, and lot-to-lot consistency of 3 ACWY-TT lots was demonstrated. These data suggest that, if licensed, ACWY-TT could provide enhanced protection against IMD in healthy adults.
Materials and Methods
Study design
This was a Phase III, randomized, partially double-blinded, controlled, non-inferiority study conducted at one study center in Lebanon and in three centers in the Philippines (109067/NCT00453986) between April 2007 and May 2008. The study was conducted according to Good Clinical Practice and in accordance with the Declaration of Helsinki (1996). The protocol and associated documents were reviewed and approved by ethics committees at each study center. Written informed consent was obtained from subjects before study entry.
Enrolled adults received a single dose of one of 3 manufacturing lots of ACWY-TT (ACWY-TT group, lots A, B and C), or Mencevax™ ACWY (MenPS group), or ACWY-TT (lot A) co-administered with the seasonal influenza vaccine, (Fluarix™: GSK Biologicals, Coad group), respectively. Subjects in the main study cohort were randomized 1:1:1:1 to the ACWY-TT (3 lots) and MenPS groups for the analysis of lot-to-lot consistency and immunogenicity and safety of ACWY-TT vs. MenPS. Safety and immunogenicity when ACWY-TT and seasonal influenza vaccine were co-administered (as assessed in the ‘Influenza' cohort) is reported elsewhere.
Vaccines were numbered using a randomization list generated at GSK Biologicals and a blocking scheme ensured that balance between treatments was maintained. Randomization was performed using a central, web-based system. The randomization algorithm included a minimization procedure that ensured a balanced allocation between groups at individual centers and between age strata (18–25 y, 26–35 y, 36–45 y, 46–55 y).
The study was double-blind with respect to ACWY-TT lot, and open with respect to whether ACWY-TT or MenPS was administered. This is because ACWY-TT is administered intramuscularly, whereas MenPS is administered subcutaneously.
Study objectives
The co-primary objectives for the main study cohort were to demonstrate lot-to-lot consistency of the 3 ACWY-TT lots with respect to rSBA GMTs for meningococcal serogroups A, C, W-135, and Y, and to demonstrate non-inferiority of the rSBA VR induced by ACWY-TT compared with MenPS 1 mo post-vaccination.
The assessment of the reactogenicity and safety of the study vaccines was a secondary objective.
Study subjects
Subjects were not eligible if they were immunosuppressed from any cause, had previously been vaccinated with a meningococcal polysaccharide vaccine within the past 5 y or meningococcal conjugate vaccine at any time previously, had received tetanus toxoid within the last month, or had a history of meningococcal disease. Pregnant or lactating females were also excluded.
Vaccines
One 0.5 mL dose of ACWY–TT contained 5 μg of each meningococcal serogroups A, C, W–135 and Y polysaccharide conjugated to a total of approximately 44 μg TT. One 0.5 mL dose of Mencevax™ACWY contained 50 μg of each meningococcal serogroups A, C, W–135 and Y polysaccharide.
Immunogenicity assessment
Blood samples were collected from all subjects prior to and one month (21–48 d) after vaccination. Pre and post-vaccination sera were tested for rSBA for each meningococcal serogroup as previously described,34 and for antibodies against tetanus toxoid with an enzyme-linked immunosorbent assay (ELISA).35 The cut–off of the rSBA assay was a 1:8 dilution and was considered indicative of seroprotection.24,25
Rabbit complement source (rather than human complement) was used because of its wider availability, and because a cut-off for a population based protective titer (≥ 1:8) has been estimated post-surveillance data in the UK after licensure of meningococcal serogroup C conjugate.25
Safety and reactogenicity assessment
Diary cards were used to record the occurrence of local and general solicited AEs for 4 d after vaccination, and other (unsolicited) AEs for 31 d after vaccination. Symptom intensity of redness, swelling and fever was graded by millimeter of reaction and degrees Celsius of fever, respectively, and all other symptoms were graded by the subject using a pre-defined scale. SAEs were recorded throughout the study. A scripted phone call at 6 mo recorded the occurrence of any SAEs and other significant AEs (rash, new onset of chronic disease and adverse events resulting in an emergency room visit) that had occurred since the last study visit.
Statistical analyses
The analysis of immunogenicity was conducted on the ATP immunogenicity cohort that included all vaccinated subjects who complied with protocol-defined procedures.
In addition to the primary objectives, exploratory analyses were conducted. The 95% CIs of the rSBA GMT ratios between vaccine groups were calculated using an ANCOVA model on the log10 transformation of the titers, using the pre-vaccination log10 transformation of the titers, age strata, and whether or not subjects participated in the analysis of co-administration with seasonal influenza vaccine as covariates. Antibody titers below the cut-off of the assay were given an arbitrary value of half the cut-off for the purpose of GMT calculation. Vaccine groups were considered significantly different if the 95% CI for the GMT ratio between groups did not contain the value 1, or, if the asymptotic standardized 95% CI for the difference in threshold rates or VR rates between groups did not contain the value 0. Due to the multiplicity of endpoints, statistically significant findings from the exploratory analyses should be interpreted with caution.
The analysis of safety was performed on the total vaccinated cohort that included all vaccinated subjects. The incidence and intensity of symptoms were calculated with exact 95% CI for each group. A key safety objective was to demonstrate non-inferiority of ACWY-TT compared with MenPS in terms of grade 3 systemic symptoms based on a pooled analysis of safety with another similarly designed study in adolescents. The results of the pooled safety analysis have been reported elsewhere.23
With 285 subjects who received each vaccine lot, the overall power to meet the primary immunogenicity consistency objective was at least 98.8% if all lots elicited similar immune responses. With a sample size of 1140 evaluable subjects overall, the study had 86.5% power to achieve the non-inferiority primary objective, assuming both vaccines induced identical VRs.
Analyses were performed using SAS® software version 9.1 (SAS Institute Inc., Cary, NC, United States) and Proc StatXact 7.0.
FLUARIX and MENCEVAX are trademarks of the GlaxoSmithKline group of companies.
Acknowledgments
The authors thank the families and children who participated in the study. The authors also thank all study investigators involved in conducting the study, including Dr. Major Nabil Nassif (Lebanese Army Medical Healthcare Center), Dr. Elie Abu Jawdeh, and Dr. George Karam (American University of Beirut) for technical assistance. The authors also thank Sally Gatchalian, Sameh Anis, Nada Riachi, Aline Stukkens and Jane Nappa for their assistance in coordination of the study; Dr. Fotios Vikas (GSK) for assistance in preparation of study reports; Laurence Fissette (GSK) for performing the statistical analysis and Dr. Brigitte Cheuvart (GSK) for statistical input in the protocol and study set-up; Dr. Dominique Boutriau and Dr. Peter Vink for input into protocol development; Dr. Pascal Lestrate and Koen Maleux for conducting the laboratory assays; Dr. Joanne Wolter (on behalf of GSK) for preparation of the first draft of the manuscript and Virginie Durbecq and Juliette Gray (Xpe Pharma and Science) for editorial assistance.
Sources of Support
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
Glossary
Abbreviations:
- ACWY-TT
Investigational tetravalent serogroups A, C, W-135 and Y conjugate vaccine with all serogroups conjugated to the tetanus toxoid carrier protein
- (S)AE
(serious) adverse event
- ATP cohort
according-to-protocol cohort
- CI
confidence interval
- GMC
geometric mean concentration
- GMT
geometric mean antibody titer
- GSK
GlaxoSmithKline Biologicals
- IMD
Invasive meningococcal disease
- MenPS
tetravalent meningococcal polysaccharide vaccine
- rSBA
meningococcal bactericidal titers using rabbit complement as exogenous complement source
- VR
vaccine response
Disclosure of Potential Conflicts of Interest
MRALR, GD and ED have received consulting fees and honoraria from GSK within the past 3 y. NM declares no conflict of interest. VB, YB and JM are employees of GSK Biologicals. YB and JM report ownership of GSK Biologicals stocks and stock options.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/20211
References
- 1.Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(Suppl):S274–9. [PubMed] [Google Scholar]
- 2.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Epidemic and pandemic alert and response (EPR). Meningococcal disease in the Philippines – update 2 2005 [Internet]. [cited 2009 Dec 30]; Available from: http: //www.who.int/csr/don/2005_01_28a/en/index.html
- 4.Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis. 2003;36:679–83. doi: 10.1086/367858. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff WP, Hajjeh R, Al-Mazrou A, Shibl A, Soriano-Gabarro M, Middle East & North Africa Vaccine-Preventable Diseases Regional Advisory Group The epidemiology of pneumococcal, meningococcal, and Haemophilus disease in the Middle East and North Africa (MENA) region--current status and needs. Vaccine. 2007;25:1935–44. doi: 10.1016/j.vaccine.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–71. doi: 10.3201/eid0906.020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 8.Gagneux SP, Hodgson A, Smith TA, Wirth T, Ehrhard I, Morelli G, et al. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002;185:618–26. doi: 10.1086/339010. [DOI] [PubMed] [Google Scholar]
- 9.Boisier P, Nicolas P, Djibo S, Taha M-K, Jeanne I, Maïnassara HB, et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 10.Decosas J, Koama J-BT. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect Dis. 2002;2:763–5. doi: 10.1016/S1473-3099(02)00455-3. [DOI] [PubMed] [Google Scholar]
- 11.Collard JM, Maman Z, Yacouba H, Djibo S, Nicolas P, Jusot JF, et al. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis. 2010;16:1496–8. doi: 10.3201/eid1609.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memish ZA, Goubeaud A, Bröker M, Malerczyk C, Shibl AM. Invasive meningococcal disease and travel. J Infect Public Health. 2010;3:143–51. doi: 10.1016/j.jiph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Meningococcal vaccines: polysaccharide and polysaccharide conjugate vaccines. Wkly Epidemiol Rec. 2002;77:331–9. [PubMed] [Google Scholar]
- 14.Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–4. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 15.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10:307–22. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 16.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–67. doi: 10.1016/S0264-410X(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 17.Ostergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine. 2009;27:161–8. doi: 10.1016/j.vaccine.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 18.Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine. 2010;28:744–53. doi: 10.1016/j.vaccine.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 19.Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J. 2011;30:e41–8. doi: 10.1097/INF.0b013e3182054ab9. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine. 2011;29:4274–84. doi: 10.1016/j.vaccine.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J. 2011;30:e56–62. doi: 10.1097/INF.0b013e31820e6e02. [DOI] [PubMed] [Google Scholar]
- 22.Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine. 2011;29:4264–73. doi: 10.1016/j.vaccine.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Bermal N, Huang L-M, Dubey AP, Jain H, Bavdekar A, Lin T-Y, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7:239–47. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 24.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine. 2005;23:2222–7. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10:780–6. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JD, Edelman R, King JC, Jr., Papa T, Ryall R, Rennels MB. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis. 2002;186:1848–51. doi: 10.1086/345763. [DOI] [PubMed] [Google Scholar]
- 27.Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Préziosi M-P, et al. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine. 2007;25(Suppl 1):A101–7. doi: 10.1016/j.vaccine.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009;16:1810–5. doi: 10.1128/CVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, et al. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM(197) conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. 2010;14:e868–75. doi: 10.1016/j.ijid.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 31.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 32.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2-10 years of age. Vaccine. 2010;28:657–63. doi: 10.1016/j.vaccine.2009.10.104. [DOI] [PubMed] [Google Scholar]
- 33.Wassilak S, Murphy TV, Roper M, Orenstein W. Tetanus toxoid. In: Vaccines. Philadelphia: Saunders Elsevier; 2004. p. 745-824. [Google Scholar]
- 34.Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al. The Multilaboratory Study Group Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–67. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melville-Smith ME, Seagroatt VA, Watkins JT. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1983;11:137–44. doi: 10.1016/S0092-1157(83)80038-9. [DOI] [PubMed] [Google Scholar]