Abstract
This study assessed antibody persistence and immune memory to hepatitis B vaccine 20 y after priming with a recombinant hepatitis B virus (HBV) vaccine during infancy. Infants were vaccinated according to a 0, 1, 6 mo schedule with or without simultaneous administration of hepatitis B immunoglobulin (HBIg). Half of the subjects enrolled received an interim booster dose at year 5 (boosted) group, whereas the other half of the subjects enrolled did not (unboosted group). Antibody persistence was assessed until year 20. Immune memory was assessed by administration of a final HBV vaccine challenge dose at year 20 in a second study.
At year 20, anti-HBs antibody concentration ≥ 10 mIU/ml rates and GMCs were higher among subjects in the boosted group (84.2% [16/19]; 95%CI: 60.4–96.6) when compared with those in the unboosted group [44.0% (11/25)]; 95% CI: 24.4–65.1). After the HBV vaccine challenge dose at year 20, anti-HBs anamnestic response for subjects in the unboosted and boosted groups was observed in 93.1% (95% CI: 77.2–99.2) and 100% (95% CI: 76.8–100) of subjects, respectively. The mean anti-HBs antibody concentration (GMC) was 562.0 mIU/ml (292.5–1079.7 mIU/ml) post administration of the challenge dose; this is a 28.5 fold increase from the pre- to post-challenge dose administration at year 20.
This study demonstrates persistence of anti-HBs antibodies and presence of immune memory following hepatitis B vaccination for up to at least 20 y in Thailand. Immune memory was demonstrated for virtually all subjects, regardless whether they received they had received the additional HBV dose or not. The challenge dose at year 20 was well tolerated and a robust response was demonstrated. ClinicalTrials.gov Identifier: NCT00240526, NCT00774995.
Keywords: Thailand, anamnestic response, efficacy, hepatitis B, persistence, vaccine
Introduction
Despite the availability of effective hepatitis B vaccines, infections with the hepatitis B virus (HBV) remains a global public health concern.1 It has been estimated that each year, approximately 4.5 million new HBV infections occur worldwide.2 At least two billion people are known to be infected with HBV, and approximately 360 million people (~6% of the world population) are chronically infected,1 which are a result of infection acquired mainly in childhood via vertical (maternal) or possible horizontal (child-to-child) transmission.2,3
It is well known that countries in South East Asia are highly endemic for hepatitis B.4-6 Perinatal infection is common in South East Asia with a high prevalence of hepatitis B carriers.6 The risk of perinatal infection is higher at the time of delivery if the mother is positive for both, the hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg).7 If the mother is a carrier of both HBsAg and HBeAg, the risk of chronic infection in the child is estimated to be as high as 70–90%.7 Children who are infected by their mothers are suggested to be a source of lateral transmission for other younger children.2,3
Infant vaccination against HBV for all countries was recommended by the World Health Organization in 1991 to control HBV infection on a global scale and subsequently the mortality and morbidity associated with HBV.8 A substantial decline in the HBV-related disease burden and prevalence of chronic HBV infection has been observed among children following introduction of universal infant vaccination against hepatitis B.2,4,9-11 Some studies suggest that infant vaccination might not be sufficient to provide a lasting protection against HBV infection when children are exposed later in their lives.12,13 Other studies indicate that primary immunization with hepatitis B vaccine during childhood and adulthood offers protection against HBV at least 20 y without the need for booster dose.7,14
Universal hepatitis B vaccination of newborns was integrated into the national immunisation program of Thailand in 1992. Countries like Taiwan15 and Malaysia,16 along with Thailand11 have registered a marked decrease in HBsAg seroprevalence in children up to 18 y of age following introduction of universal infant hepatitis B vaccination in the respective countries.11,15,16 In Thailand, three prospective studies were initiated in 1986 to investigate the immunogenicity, reactogenicity and efficacy of a recombinant hepatitis B vaccine (Engerix™-B, GlaxoSmithKline Biologicals) in high-risk subjects.7,17,18 The studies assessed different vaccination scheduled and the results of these trials have been published previously.7,17,18 The success of infant vaccination in preventing vertical transmission of HBV during early childhood is well established.4,11 However, there is an ongoing debate whether infant vaccination is sufficient to protect against infection when exposed to HBV later in life.
We describe the results of the long-term follow-up study in Thailand that assessed the persistence of anti-HBs antibodies and the results of the hepatitis B challenge study that assessed the immune memory 20 y after primary neonatal vaccination with the hepatitis B vaccine (Engerix™-B) with or without booster vaccination at year 5, among children whose mothers were positive for HBsAg and HBeAg.
Results
Demographic characteristics
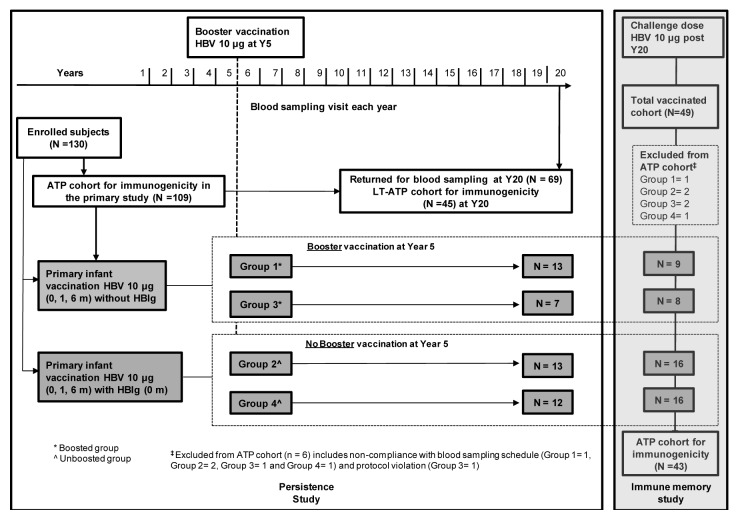
In the primary study, a total of 130 subjects were enrolled; among these 109 subjects were included in the ATP immunogenicity cohort. At year 20, 69 subjects returned for blood sampling of which 45 subjects were included in the LT-ATP cohort of immunogenicity (19 were eliminated at previous time points and five subjects were eliminated due to an abnormal increase in anti-HBs antibody concentrations at year 19). To assess immune memory by administration of a challenge dose (at year 20), 49 subjects were enrolled and 48 subjects completed the study. Among these, 43 were included in the ATP cohort for immunogenicity (challenge dose at year 20) (Fig. 1).
Figure 1. Study design.
The mean age of subjects who were administered the challenge dose was 19.5 y [standard deviation (SD): 0.51 y]. The percentage of male subjects was 58.1%. All subjects were of South East Asian origin.
Twenty year anti-HBs antibody persistence
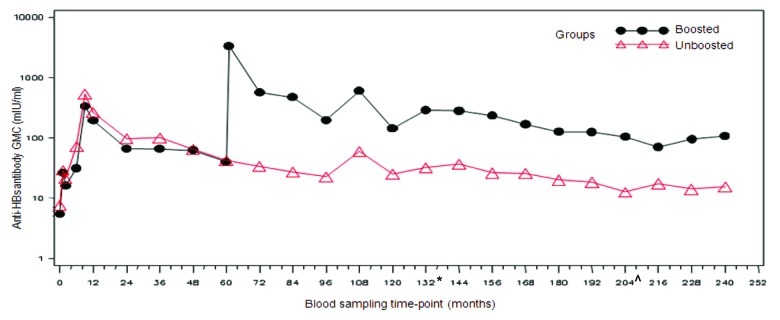
The percentage of subjects with anti-HBs antibody concentrations ≥ 10 mIU/ml in the boosted and unboosted groups at 20 y time point was 84.2% (16/19; 95% CI: 60.4–96.6) and 44.0% (11/25; 95% CI: 24.4–65.1), respectively. The percentage of subjects with antibody concentrations ≥ 10 mIU/ml in the boosted and unboosted groups for each of the time points is detailed in Table 1 and Figure 2. In the boosted group, anti-HBs antibody GMCs was 108.7 mIU/ml (95% CI: 46.2–255.9) while in the unboosted group, the antibody GMCs was 15.3 mIU/ml (95% CI: 9.3–25.4) (Table 1 and Fig. 2). A peak in the anti-HBs antibody concentrations was first observed at Month 9, two months after the completion of primary vaccination course in all groups. Over the subsequent time points, the antibody concentrations in both the groups plateaued. Anti-HBs antibody GMCs at year 20 in the four individual groups were—group 1: 73.9 mIU/ml (95% CI: 26.6–205.4); group 2: 14.3 mIU/ml (95% CI: 6.7–30.6); group 3: 297.1 mIU/ml (95% CI: 46.0–1919.5); and group 4: 16.3 mIU/ml (95% CI: 7.2–36.9). Subjects who were administered HBIg at birth showed a similar pattern of antibody decline and had similar GMTs during the persistence timepoints when compared with those subjects that had not received the HBIg at birth, indicating that the HBIg did not contribute to the persistence of immune response (data not shown).
Table 1. Anti-HBs antibody seropositivity rates and geometric mean concentrations from primary vaccination at infancy until year 20 for pooled groups with boosted and unboosted at year 5 (Long-term ATP cohort for immunogenicity).
| |
N |
S+ |
≥ 10 mIU/ml |
GMC* mIU/ml |
|||
|---|---|---|---|---|---|---|---|
| Time Point (Year) |
|
% |
95% CI |
% |
95% CI |
mIU/ml |
95% CI |
| Unboosted group | |||||||
| 1 |
60 |
100 |
(94.0,100) |
100 |
(94.0,100) |
265.3 |
(179.9,391.3) |
| 2 |
55 |
100 |
(93.5,100) |
96.4 |
(87.5,99.6) |
96.6 |
(65.7,142.2) |
| 3 |
51 |
98.0 |
(89.6,100) |
92.2 |
(81.1,97.8) |
99.4 |
(69.8,141.7) |
| 4 |
46 |
97.8 |
(88.5,99.9) |
91.3 |
(79.2,97.6) |
64.3 |
(44.9,91.9) |
| 5 |
44 |
97.7 |
(88.0,99.9) |
86.4 |
(72.6,94.8) |
42.0 |
(27.9,63.2) |
| 6 |
40 |
97.5 |
(86.8,99.9) |
75.0 |
(58.8,87.3) |
33.3 |
(20.6,53.7) |
| 7 |
41 |
97.6 |
(87.1,99.9) |
70.7 |
(54.5,83.9) |
27.0 |
(17.5,41.8) |
| 8 |
34 |
97.1 |
(84.7,99.9) |
67.6 |
(49.5,82.6) |
22.5 |
(14.1,35.7) |
| 9^ |
4 |
100 |
(39.8,100) |
100 |
(39.8,100) |
59.3 |
(46.9,74.8) |
| 10 |
27 |
96.3 |
(81.0,99.9) |
66.7 |
(46.0,83.5) |
25.0 |
(13.9,45.0) |
| 11 |
33 |
72.7 |
(54.5,86.7) |
57.6 |
(39.2,74.5) |
32.2 |
(18.6,55.7) |
| 12 |
31 |
67.7 |
(48.6,83.3) |
54.8 |
(36.0,72.7) |
36.8 |
(18.4,73.6) |
| 13 |
33 |
66.7 |
(48.2,82.0) |
45.5 |
(28.1,63.6) |
26.2 |
(14.1,48.5) |
| 14 |
33 |
63.6 |
(45.1,79.6) |
45.5 |
(28.1,63.6) |
25.5 |
(13.4,48.6) |
| 15 |
32 |
56.3 |
(37.7,73.6) |
34.4 |
(18.6,53.2) |
20.0 |
(11.3,35.5) |
| 16 |
29 |
69.0 |
(49.2,84.7) |
48.3 |
(29.4,67.5) |
18.4 |
(10.8,31.5) |
| 17 |
26 |
65.4 |
(44.3,82.8) |
26.9 |
(11.6,47.8) |
12.6 |
(6.9,22.8) |
| 18 |
27 |
55.6 |
(35.3,74.5) |
37.0 |
(19.4,57.6) |
17.3 |
(10.2,29.2) |
| 19 |
23 |
65.2 |
(42.7,83.6) |
39.1 |
(19.7,61.5) |
14.1 |
(7.6,26.1) |
| 20 | 25 | 76.0 | (54.9,90.6) | 44.0 | (24.4,65.1) | 15.3 | (9.3,25.4) |
| Boosted group | |||||||
|---|---|---|---|---|---|---|---|
| 1 |
43 |
100 |
(91.8,100) |
100 |
(91.8,100) |
195.8 |
(120.2,319.0) |
| 2 |
43 |
100 |
(91.8,100) |
93.0 |
(80.9,98.5) |
67.2 |
(42.7,106.0) |
| 3 |
43 |
100 |
(91.8,100) |
86.0 |
(72.1,94.7) |
65.8 |
(40.4,107.3) |
| 4 |
41 |
97.6 |
(87.1,99.9) |
82.9 |
(67.9,92.8) |
62.3 |
(37.0,104.8) |
| 5 |
43 |
97.7 |
(87.7,99.9) |
79.1 |
(64.0,90.0) |
40.0 |
(24.6,65.1) |
| 5* |
41 |
100 |
(91.4,100) |
100 |
(91.4,100) |
3375.9 |
(1722.0,6618.5) |
| 6 |
38 |
100 |
(90.7,100) |
92.1 |
(78.6,98.3) |
568.3 |
(269.6,1198.1) |
| 7 |
37 |
97.3 |
(85.8,99.9) |
89.2 |
(74.6,97.0) |
471.5 |
(219.1,1014.8) |
| 8 |
32 |
100 |
(89.1,100) |
93.8 |
(79.2,99.2) |
198.7 |
(99.5,396.6) |
| 9^ |
6 |
100 |
(54.1,100) |
83.3 |
(35.9,99.6) |
603.1 |
(29.9,12171.4) |
| 10 |
27 |
100 |
(87.2,100) |
92.6 |
(75.7,99.1) |
144.3 |
(66.3,313.8) |
| 11 |
34 |
97.1 |
(84.7,99.9) |
94.1 |
(80.3,99.3) |
288.2 |
(146.0,568.8) |
| 12 |
31 |
96.8 |
(83.3,99.9) |
96.8 |
(83.3,99.9) |
282.1 |
(145.9,545.6) |
| 13 |
32 |
93.8 |
(79.2,99.2) |
87.5 |
(71.0,96.5) |
234.1 |
(122.6,447.0) |
| 14 |
32 |
96.9 |
(83.8,99.9) |
87.5 |
(71.0,96.5) |
167.5 |
(83.4,336.6) |
| 15 |
29 |
93.1 |
(77.2,99.2) |
86.2 |
(68.3,96.1) |
127.0 |
(63.3,254.9) |
| 16 |
27 |
100 |
(87.2,100) |
88.9 |
(70.8,97.6) |
125.7 |
(58.9,268.5) |
| 17 |
28 |
92.9 |
(76.5,99.1) |
85.7 |
(67.3,96.0) |
104.6 |
(50.0,218.9) |
| 18 |
24 |
100 |
(85.8,100) |
83.3 |
(62.6,95.3) |
70.4 |
(32.2,153.8) |
| 19 |
22 |
95.5 |
(77.2,99.9) |
81.8 |
(59.7,94.8) |
95.9 |
(40.9,224.5) |
| 20 | 19 | 94.7 | (74.0,99.9) | 84.2 | (60.4,96.6) | 108.7 | (46.2,255.9) |
Boosted: subjects who received booster dose at year 5 (month 60). Unboosted: subjects who did not receive booster dose at year 5 (month 60)
, year 5 (one month post-administration of booster dose); ^, At year 9, the sample size was low since fewer subjects were loaded onto the database in both boosted and unboosted groups; N, number of subjects with available results; n/%, number/percentage of seropositive subjects or subjects with anti-HBs antibody concentrations ≥ 10 mIU/ml; GMC*, Geometric mean concentrations calculated on seropositive subjects; S+, Seropositivity defined as anti-HBs antibody concentrations ≥ 1.0 mIU/ml up to year 10 and ≥ 3.3 mIU/ml from year 11 onwards
Figure 2. Evolution of anti-HBs geometric mean concentrations in boosted and unboosted groups (LT-ATP immunogenicity cohort). Boosted group: Subjects who received booster dose at year 5 (month 60). Unboosted group: Subjects who did not receive the booster dose at year 5 (month 60). Note: At year 9 the results of only 10 subjects were included in the analysis- hence this time point should not be taken into account to assess the kinetic of the immune response. This limited number of subjects was due to logistic problems during the conduct of the study which resulted in fewer subjets being loaded into the database. From the commencement of primary vaccination until follow-up year 10, anti-HBs antibodies were measured using AUSAB RIA (Abbott Laboratories). *Assay change from year 11 until year 16: Antibodies were measured using AUSAB EIA (Abbott Laboratories, IL, USA). ^Assay change from year 17 until year 20: Antibodies were measured using an in-house validated ELISA.
Immune response to the challenge dose
One month post administration of the challenge dose (year 20), all subjects were seropositive for anti-HBs antibodies in the ATP cohort for immunogenicity (Table 2). For subjects in the unboosted and boosted groups, anamnestic response was observed in 93.1% (95% CI: 77.2–99.2) and 100% (95% CI: 76.8–100) of subjects, respectively. The anti-HBs antibody GMCs among the subjects boosted at year 5 (boosted group) and those not given the booster dose at year 5 (unboosted group) was 2115.4 mIU/ml (1053.6–4247.3 mIU/ml) and 296.3 mIU/ml (95% CI: 129.1–680.4) post-administration of the challenge dose at year 20, respectively (Table 2). Serum antibody concentrations relative to the pre and post challenge dose is represented as a box plot (Fig. 3) for both the boosted and unboosted groups.
Table 2. Anti-HBs seropositivity rates, percentage of subjects with antibody concentrations ≥ 10 mIU/ml and GMCs at the pre- and post-challenge dose time-point (long-term ATP cohort for immunogenicity) in the groups boosted and unboosted at year 5.
| Group |
Time point |
N |
S+ |
≥ 10 mIU/ml |
GMC* mIU/ml |
|||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | mIU/ml | 95% CI | |||
| Boosted at year 5 |
Pre |
14 |
92.9 |
(66.1,99.8) |
71.4 |
(41.9,91.6) |
33.1 |
(15.2,72.0) |
| |
Post |
14 |
100 |
(76.8,100) |
100 |
(76.8,100) |
2115.4 |
(1053.6,4247.3) |
| Unboosted at year 5 |
Pre |
29 |
75.9 |
(56.5,89.7) |
44.8 |
(26.4,64.3) |
14.5 |
(10.0,21.2) |
| Post | 29 | 100 | (88.1,100) | 96.6 | (82.2,99.9) | 296.3 | (129.1,680.4) | |
N, number of subjects with available results; %, percentage of subjects with antibody concentrations ≥ the specified cut-off; GMC*, geometric mean antibody concentration calculated on subjects with antibody concentrations ≥ 3.3 mIU/ml; 95% CI: 95% confidence interval; PRE, blood sampling at the last available time-point; POST, blood sampling post-challenge dose
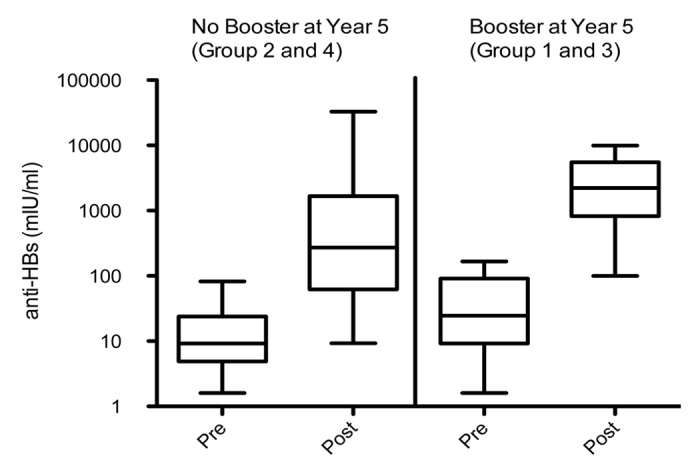
Figure 3. Serum antibody concentrations at the pre- and post-challenge dose time-point (Long-term ATP cohort for immunogenicity) in the groups boosted and unboosted at year 5. Each box shows the median (horizontal line), quartile range (the box itself), and high (97.5%) and low (2.5%) (whiskers).
Incidence and prevalence of hepatitis markers
For the 130 subjects enrolled, a total of 4956 read-outs were performed, of which 48 test results in 30 subjects had a weak positive read-out signal (most cases showed an isolated HBsAg+ result). Subclinical infection was observed in 32 subjects out of the 130 subjects enrolled. Four subjects were diagnosed with new HBV infection. These subjects were non-responders to the primary vaccination and maintained their chronically infected status till year 20. One subject acquired new chronic infection from year 18 onwards. Therefore a total of 5 chronic infections were observed. None of them reported clinical symptoms of HBV disease during the follow-up period.
Safety
No AEs/SAEs were reported during the study period (challenge dose). Of the 49 subjects who received the challenge dose, pyrexia (grade 1) was reported by one subject (2.0%) during the 31-d follow-up period post-vaccination. This was considered causally related to vaccination by the investigator and resolved within two days post-vaccination. No SAEs and pregnancies were reported during the study period.
Discussion
In Thailand, a highly endemic region for HBV infection with a high risk of transmission from mother to child,6 it is recommended to vaccinate subjects against hepatitis B preferably at birth to help reduce the HBV associated mortality and morbidity.8 The study aimed at assessing the long-term antibody persistence and immune memory against HBsAg, 20 y after primary infant vaccination in Thailand with a recombinant hepatitis B vaccine in neonates born of HBsAg+ and HBeAg+ mothers. Previous follow-up studies with hepatitis B vaccine in Thailand have established that this vaccine when administered alone or together with HBIg is immunogenic and effective in preventing hepatitis B infection in high-risk neonates born to carrier mothers.7,17,18
In this study, at year 5, it was shown that subjects with or without HBIg at birth had the same persistence of anti-HBs antibodies, indicating that the HBIg did not interfere with the immune response of vaccination. HBIg administration is an important medical intervention to help prevent transmission and disease in early childhood. However, HBIg is not expected to have long-term persistence and therefore the data are evaluated for two groups: those who received vaccine based on 0, 1, 6 mo schedule (unboosted group) and those who received vaccines based on 0, 1, 6, 60 mo schedule (boosted group) regardless of whether HBIg was administered at month 0. Twenty years after primary vaccination, seroprotection rates and anti-HBS antibody GMCs were generally higher in the boosted group.
Persistence of anti-HBs antibodies and immune memory to mount a response to exposure of HBV later in life is necessary for the long-term protection against hepatitis B.19 Several studies have confirmed persistence of antibodies and immune memory following priming with recombinant vaccine.20-22 while others confirm waning antibody concentrations 13–15 y after primary vaccination among those vaccinated at birth.21,22 However, the adaptive immune response consists of two major effector arms, the cell-mediated and humoral immunity. While the results from this study provide data that suggests that the anti-HBs antibodies (humoral response) persist for at least up to 20 y after primary vaccination, a recently published study shows that cell-mediated immune response also display long-term persistence after HBV vaccination.23
It has been demonstrated that the peak response for anti-HBs are expected to be around 14 d post-vaccination. Although the immune responses decline following 14 d, sufficiently high measures of antibody response is observed at day 30 to allow for the assment of anamnestic response. In addition, day 30 was chosen to assess the safety of the vaccine for adverse reactions.24
Further, the results from the challenge study show that the anti-HBs GMCs observed for the boosted group were approximately 64-fold higher at the post-challenge dose time-point when compared with the pre-challenge dose time-point, whereas the increase for the unboosted group was approximately 20-fold. The antibody titers distribution is also in favor of the boosted group as shown in Figure 3. This could indicate that the additional booster dose at year 5 allowed for the availability of a larger pool of memory B cells.25 Despite a significant difference in circulating anti-HBs antibody concentration reported between boosted and unboosted groups until year 20, anamnestic responses to an additional vaccine challenge dose at year 20 were observed for virtually all subjects in both groups, irrespective of their residual anti-HBs serostatus before the challenge dose, indicating the presence of immune memory. A previously published study suggests that if the primary series of vaccinations have been complied with, there is no need for booster vaccination.26 However additional long-term follow-up studies extending to the third decade after vaccine administration and surveillance of hepatitis B vaccine recipients are warranted to establish whether a primary vaccination course in subjects can offer long-term protection, without the need for an additional booster dose at year 5.
Protection against hepatitis B is critical due to the associated serious sequelae (e.g., hepatocellular carcinoma, cirrhosis) that are attributed to chronic HBV infection. Studies have consistently demonstrated that hepatitis B vaccination is the most efficient method of preventing chronic HBV infections years after primary vaccination. Few follow-up studies have indicated the prevalence of chronic infection in ≤ 1% of vaccinated children.27,28 To optimally reduce the risk of acquiring chronic infection during infancy or early childhood, the Advisory Committee on Immunization Practices (ACIP) recommends giving the first dose of hepatitis B vaccine at birth, and completing the vaccination series by 18 mo of age.29 Without concomitant administration of HBIg at birth, the second dose of the vaccine is recommended at 4–6 weeks of age.29 However, in this study it was observed that some vaccinees were chronically infected without clinical sequelae. This is an important aspect which could be an indication that if the subjects have anti-HBs antibodies they are most likely to be clinically protected against disease and chronicity. The four cases who did not respond to the primary vaccination course in this study, maintained a chronic HBV infection status. However, they did not report any clinical symptoms of HBV disease during the follow-up period. Although the exact reason for this chronic carriage status is not clear, these subjects were born to HBsAg+ and HBeAg+ mothers and their chronic infection status could be due to natural history.
The tests for HBV markers were reliable, and only a limited number of tests were weakly positive (< 1%). Due to the lack of clinical signs and other supportive serological data, these values were considered as false-positive. Over the 20 y of reporting period, subclinical infections were observed in 32 subjects belonging to both boosted and unboosted groups. It is therefore worth noting that Thai children are at high risk of exposure to HBV due to the HBV endemicity but none of the subjects with sublclinical HBV infection had clinical symptoms of HBV disease.
Although this is one of the fewer studies with a 20 y follow-up in subjects vaccinated at birth with recombinant hepatitis B vaccine, the findings should be interpreted with caution, in the light of several limitations. The present study was a long-term follow-up study where subjects were followed-up for 20 y from the start of the study. Hence, subject attrition and dropouts were expected over this long follow-up period and hence the low sample size at year 20. Furthermore, the number of subjects who were eligible to receive the challenge dose at year 20 was smaller than the subjects who were enrolled at the study start. This small number might have affected the representativeness of the study. The study was not designed to evaluate the effect of a booster dose at year 5; hence, a selection bias in the cohort of subjects who received booster dose at year 5 cannot be excluded. Also, there is an unequal distribution of subjects from different primary groups in boosted and unboosted groups which only suggests that the results of booster dose in study subjects is to be construed carefully. Blood sampling for assessment of anti-HBs antibody concentration was not done prior to the administration of challenge-dose at year 20 but at year 18, which was much in advance as compared with administration of challenge-dose. Presence of anti-HBc in small number of subjects indicated the effect of natural boosting which might have affected the study results of the challenge-dose phase. The ATP cohort excluded therefore subjects with abnormal rise in antibody titers, that would have been reflective of natural booster. A bias to the kinetic curve at year 9 needs to be considered, since only a limited number of subjects were evaluated for that time point (Fig. 2). This limited number of subjects was due to logistic problems during the conduct of the study where fewer subjets were loaded onto the database, hence the year 9 results should not be considered for the study results interpretation and conclusion.
In conclusion, this study demonstrates the persistence of anti-HBs antibodies and presence of immune memory following hepatitis B vaccination for up to at least 20 y in Thailand. Although immune memory to challenge dose was observed in all subjects, the response was higher in children who received a booster dose at year 5 of the study. The challenge dose at year 20 was well tolerated and a robust response was demonstrated in the majority of subjects.
Methods
Study design
The primary vaccination study was conducted from May 1988 to February 1991 at Chulalongkorn University Hospital, Bangkok, Thailand. Neonates of HBsAg+ and HBeAg+ mothers were enrolled in this open-label long-term follow-up study. After 20 y of follow-up, eligible subjects were enrolled in the immune memory study and received an HBV challenge dose. The study designs are summarized in Figure 1.
All enrolled subjects received the hepatitis B vaccine (10 μg recombinant HBsAg; Engerix™-B) according to a 0,1,6 mo schedule with or without concomitant administration of HBIg. An booster dose of hepatitis B vaccine was given to a subset of subjects at year 5 (groups 1 and 3) to form the boosted group and the subjects who did not receive this booster dose at year 5 (groups 2 and 4) were included in the unboosted group (Fig. 1).
Subjects who complied with the study protocol requirements (e.g., completion of the diary cards, return for follow-up visits) to assess the presence of immune response after vaccination were included in the following studies.
Study on antibody persistence (NCT00240526)
Blood samples were collected at yearly intervals up to year 20 to test for the presence of anti-HBs antibodies and serological markers of hepatitis B infection (anti-HBc, HBsAg, HBV DNA). Details on vaccination history and development of clinical signs possibly related to hepatitis B were obtained from each of the subjects through an interview at each sampling visit by the investigator.7
Study of immune memory (NCT00774995)
The year 18 results (or previous timepoint if no year 18 timepoint was available) of the persistence study, was used to determine eligibility criteria into the immune memory study. Any subjects with anti-HBs antibody concentrations < 100 mIU/ml were invited to receive a single dose challenge dose at year 20 of the hepatitis B vaccine (10 μg recombinant HBsAg; Engerix™-B). Blood samples were collected one month post-administration of this challenge dose.
Response to the challenge dose was evaluated by comparing the same anti-HBs antibody concentrations prior to vaccination i.e the last available time point and one month post-vaccination. Blood samples (5 ml) were collected from each subject one month post-administration of the challenge dose.
Written informed consent was obtained from each subject before initiation of study procedures. The study protocol was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. The study was conducted according to Good Clinical Practice, the Declaration of Helsinki and all applicable local rules and regulations of Thailand.
Assessment of immunogenicity
Blood samples were collected at each annual time point up to year 20 to evaluate persistence of anti-HBs antibodies. From the commencement of primary vaccination until follow-up year 10, anti-HBs antibodies were measured using a radioimmunoassay (AUSAB RIA, Abbott Laboratories) with an assay cut-off of 1.0 mIU/ml. From year 11 until year 16, antibodies were measured using Enzyme-Linked Immunosorbent Assay (ELISA) (AUSAB EIA, Abbott Laboratories). Samples collected from year 17 until year 20 were tested using an in-house validated ELISA. The cut-off for the commercial and in-house ELISA was 3.3 mIU/ml. Seropositivity was defined as anti-HBs antibody concentrations ≥ assay cut-off. The anti-HBs seropositivity rates, subjects with anti-HBs antibody concentrations ≥ 10 mIU/ml, anti-HBs geometric mean concentrations (GMCs) calculated on seropositive subjects at each time point and percentage of subjects with an anamnestic response to hepatitis B vaccine challenge dose at year 20 were reported with 95% confidence interval (CI). The same anti-HBs assay was used to assess immune responses and challenge response.
A pooled analysis of anti-HBs antibody persistence was performed for the subjects who received the booster dose at month 60 (groups 1 and 3) to form the boosted group and subjects who did not receive the booster dose at month 60 (groups 2 and 4) to form the unboosted group (Fig. 1). Subjects from both groups were administered the challenge dose at year 20, if their anti-HBs concentration at year 18 or before < 100 mIU/ml.
Assessment of hepatitis markers
Anti-HBc and anti-HBe antibody concentrations were measured using an ELISA method (AxSYM/CORE/HBe, Abbott Laboratories). HBsAg concentration was determined using an ELISA method (AxSYM/HBsAg, Abbott Laboratories). The presence of HBV DNA was detected using in-house validated Polymerase Chain Reaction (PCR) and Cobas® Monitor methodologies. The clinical significance of HBsAg positive and/or anti-HBc positive blood samples was assessed for each subject by considering the following: presence of HBV markers (anti-HBs, HBeAg, anti-HBe), HBV DNA (pre-core/core and surface antigen), and alanine aminotransferase (ALT)/ aspartate aminotransferase (AST) serum concentrations.
False positive was defined as single marker of HBV infection (HBsAg, PCR, HBeAg, antiHBc), positive in one sample and negative for all other markers in this same sample and lack of clinical signs and other supportive serological data indicative of HBV infection
Subclinical infection was defined as one or more HBV marker positive in one or more consecutive samples, excluding conditions for chronic HBV infection and false-positive result, without any reported clinical symptoms of hepatitis
New HBV infections was defined as detection of two or more positive markers in the same sample with no other positive markers at consecutive time points.
Chronic infections was defined as HBsAg positive and anti-HBc positive at more than two consecutive time points.
Assessment of safety
Adverse events (AEs) and pregnancies that occurred within the 31-d follow-up period after the hepatitis B vaccine challenge dose (year 20) were reported. The length of the follow-up period was pre-defined in the study protocol and as per the company norms. The conservative follow-up period of 31 d was to ensure that subjects were followed for at least four weeks after the vaccine administration. Relationship to vaccination of unsolicited symptoms was assessed by the investigator. Serious adverse events (SAEs) were reported during the entire study period (year 20).
Statistical analyses
Primary immunogenicity analysis was performed on the long-term according-to-protocol cohort (LT-ATP) for immunogenicity at all long-term follow-up time points and on the ATP cohort for immunogenicity for assessment of immune memory by administration of a challenge dose. The LT-ATP cohort for immunogenicity included all subjects who returned for the particular follow-up study and were not eliminated at the time points due to the pre-defined protocol violations. The ATP cohort for immunogenicity included all evaluable subjects included in the ATP cohort for safety (i.e., those meeting all eligibility criteria, complying with the study procedures and intervals defined in the protocol, with no elimination criteria during the study) who had received the challenge dose of hepatitis B vaccine and for whom data concerning immunogenicity endpoint measures were available at the post-hepatitis B vaccine challenge dose time-point.
Anamnestic response was defined as anti-HBs antibody concentration ≥ 10 mIU/ml one month post-challenge dose as compared with pre-challenge anti-HBs concentration for seronegative subjects (i.e., < 3.3 mIU/ml), and at least a 4-fold increase in anti-HBs concentration post-challenge dose as compared with pre-challenge anti-HBs concentration ≥ 3.3 mIU/ml for seropositive subjects.
The study was not powered to draw any statistical conclusion. The statistical analysis was performed using statistical analysis system (SAS) version 9.2.
Acknowledgments
We thank the infants and their families for participating in this trial; all investigators, the study nurses, and other staff members of the Center of Excellence in Clinical Virology, the Distinguished Research Professor Fund (Thailand Research Fund) and National Research University (HR1155A) for contributing in many ways to this study. The authors thank the following from GlaxoSmithKline Biologicals: Amrita Ostawal and Manjula K (both employees) for providing writing and editorial support in preparing this manuscript.
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- Anti-HBs antibody
Anti-hepatitis B surface antibody
- AST
aspartate aminotransferase
- ATP
according to protocol
- CI
confidence interval
- GMC
geometric mean concentration
- ELISA
enzyme linked immunosorbent assay
- HBcAg
hepatitis B core antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBIg
hepatitis B immunoglobulin
- HBV
hepatitis B virus
- LT-ATP
long-term according to protocol
- PCR
polymerase chain reaction
- SD
standard deviation
Disclosure of Potential Conflicts of Interest
The investigator YP declares that his center received grants (Thailand Research Fund) to conduct the trial. Outside the scope of this submitted work, YP also has declared to have received payments by various pharmaceutical companies to present lectures; VC and AT declare to have no conflict of interest; GLR acts as principal investigator for clinical trials conducted on behalf of the Ghent University and Hospital, for which these institutions obtain compensation from the sponsoring vaccine manufacturers. GLR received no personal remuneration for this work. PC is employed at GSK Biologicals; KH is employed at GSK Biologicals and also has stock ownership at GSK Biologicals.
Role of the Funding Source
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study conduct and analyses. GlaxoSmithKline Biologicals also took in charge of all costs associated with the development and the publishing of the present manuscript. We would like to acknowledge the partial support by the Center of Excellence in Clinical Research Fund and the Thailand Research Fund.
Trademark
Engerix-B is a trademark of GlaxoSmithKline group of companies.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/19989
References
- 1.World Health Organization Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–19. [PubMed] [Google Scholar]
- 2.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–73. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 4.Poovorawan Y, Chongsrisawat V, Tangkijvanich P. Problems and prevention of viral hepatitis in Thailand. J Med Assoc Thai. 2001;84(Suppl 1):S18–25. [PubMed] [Google Scholar]
- 5.Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356–61. doi: 10.1046/j.1440-1746.2000.0150121356.x. [DOI] [PubMed] [Google Scholar]
- 6.Lolekha S, Warachit B, Hirunyachote A, Bowonkiratikachorn P, West DJ, Poerschke G. Protective efficacy of hepatitis B vaccine without HBIG in infants of HBeAg-positive carrier mothers in Thailand. Vaccine. 2002;20:3739–43. doi: 10.1016/S0264-410X(02)00358-4. [DOI] [PubMed] [Google Scholar]
- 7.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28:730–6. doi: 10.1016/j.vaccine.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization WHO expanded programme on immunization. Global advisory group. Wkly Epidemiol Rec. 1992;3:11–6. [Google Scholar]
- 9.Heron L, Selnikova O, Moiseieva A, Van Damme P, van der Wielen M, Levie K, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11-15 years: a randomised controlled trial. Vaccine. 2007;25:2817–22. doi: 10.1016/j.vaccine.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:868–70. [PubMed] [Google Scholar]
- 11.Chongsrisawat V, Yoocharoen P, Theamboonlers A, Tharmaphornpilas P, Warinsathien P, Sinlaparatsamee S, et al. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop Med Int Health. 2006;11:1496–502. doi: 10.1111/j.1365-3156.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 12.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, et al. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat. 2011;18:369–75. doi: 10.1111/j.1365-2893.2010.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 14.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–6. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 15.Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–35. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 16.Ng KP, Saw TL, Baki A, Rozainah K, Pang KW, Ramanathan M. Impact of the Expanded Program of Immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194:163–8. doi: 10.1007/s00430-004-0231-4. [DOI] [PubMed] [Google Scholar]
- 17.Poovorawan Y, Sanpavat S, Pongpunglert W, Chumdermpadetsuk S, Sentrakul P, Vandepapelière P, et al. Long term efficacy of hepatitis B vaccine in infants born to hepatitis B e antigen-positive mothers. Pediatr Infect Dis J. 1992;11:816–21. doi: 10.1097/00006454-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Poovorawan Y, Sanpavat S, Chumdermpadetsuk S, Safary A. Long-term hepatitis B vaccine in infants born to hepatitis B e antigen positive mothers. Arch Dis Child Fetal Neonatal Ed. 1997;77:F47–51. doi: 10.1136/fn.77.1.F47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banatvala JE, Van Damme P. Hepatitis B vaccine—do we need boosters? J Viral Hepat. 2003;10:1–6. doi: 10.1046/j.1365-2893.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 20.Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, et al. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J. 2008;27:881–5. doi: 10.1097/INF.0b013e31817702ba. [DOI] [PubMed] [Google Scholar]
- 21.Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25:6958–64. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 22.Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, et al. Differences in response to a hepatitis B vaccine booster dose among Alaskan children and adolescents vaccinated during infancy. Pediatrics. 2007;120:e373–81. doi: 10.1542/peds.2007-0131. [DOI] [PubMed] [Google Scholar]
- 23.Chinchai T, Chirathaworn C, Praianantathavorn K, Theamboonlers A, Hutagalung Y, Bock PH, et al. Long-term humoral and cellular immune response to hepatitis B vaccine in high-risk children 18-20 years after neonatal immunization. Viral Immunol. 2009;22:125–30. doi: 10.1089/vim.2008.0087. [DOI] [PubMed] [Google Scholar]
- 24.Gesemann M, Scheiermann N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13:443–7. doi: 10.1016/0264-410X(94)00010-K. [DOI] [PubMed] [Google Scholar]
- 25.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGS, Dörner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 26.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. doi: 10.1093/cid/cir270. [DOI] [PubMed] [Google Scholar]
- 27.Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, et al. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004;40:1415–20. doi: 10.1002/hep.20490. [DOI] [PubMed] [Google Scholar]
- 28.Mele A, Tancredi F, Romanò L, Giuseppone A, Colucci M, Sangiuolo A, et al. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J Infect Dis. 2001;184:905–8. doi: 10.1086/323396. [DOI] [PubMed] [Google Scholar]
- 29.Tharmaphornpilas P, Rasdjarmrearnsook AO, Plianpanich S, Sa-nguanmoo P, Poovorawan Y. Increased risk of developing chronic HBV infection in infants born to chronically HBV infected mothers as a result of delayed second dose of hepatitis B vaccination. Vaccine. 2009;27:6110–5. doi: 10.1016/j.vaccine.2009.08.034. [DOI] [PubMed] [Google Scholar]