Abstract
Negative-strand (NS) RNA viruses initiate infection with a unique polymerase complex that mediates both mRNA transcription and subsequent genomic RNA replication. For nearly all NS RNA viruses, distinct enzymatic domains catalyzing RNA polymerization and multiple steps of 5′ mRNA cap formation are contained within a single large polymerase protein (L). While NS RNA viruses include a variety of emerging human and agricultural pathogens, the enzymatic machinery driving viral replication and gene expression remains poorly understood. Recent insights with Machupo virus and vesicular stomatitis virus have provided the first structural information of viral L proteins, and revealed how the various enzymatic domains are arranged into a conserved architecture shared by both segmented and nonsegmented NS RNA viruses. In vitro systems reconstituting RNA synthesis from purified components provide new tools to understand the viral replicative machinery, and demonstrate the arenavirus matrix protein regulates RNA synthesis by locking a polymerase–template complex. Inhibition of gene expression by the viral matrix protein is a distinctive feature also shared with influenza A virus and nonsegmented NS RNA viruses, possibly illuminating a conserved mechanism for coordination of viral transcription and polymerase packaging
Keywords: 5′ cap formation, L protein structure, Machupo virus, Negative-strand RNA virus, arenavirus, matrix protein, polymerase, single-particle electron microscopy, template recognition, vesicular stomatitis virus
Introduction
Viruses are the only organisms known to store their genetic information solely in the form of RNA, and have thus evolved unique machinery to replicate an RNA genome and initiate viral gene expression in the infected cell. The Large polymerase protein (L) of negative-strand (NS) RNA viruses is a particularly intriguing model, where all of the enzymatic activities required for mRNA transcription, RNA modification, and genomic RNA replication are contained within a single polypeptide. Whereas the host cell and many other viruses require a suite of enzymes to accomplish these tasks, L alone is the catalytic engine driving NS RNA viral replication.
Combined with the unique biology of L proteins, NS RNA viruses include many emerging human and agricultural pathogens.1-3 Continuous transmission of NS RNA viruses—including rabies virus, measles virus, and respiratory syncytial virus—is coincident with sporadic outbreaks of hemorrhagic fever NS RNA viruses such as Machupo virus, Lassa virus, and Ebola virus. Moreover, newly discovered NS RNA viruses continue to emerge, with recent identification of severe fever with thrombocytopenia syndrome virus in Asia (2011),4 Chapare virus and Lujo virus as new agents of hemorrhagic fever in South America and Africa (2008/2009),5,6 and Schmallenberg virus infection of livestock in Europe (2011/2012).7 As NS RNA viruses continue to grow in health and economical importance, the catalytic activities of L will become increasingly critical as potential therapeutic targets.
Mechanistic studies of L structure and function require development of tractable in vitro techniques and direct analysis of RNA products. Recently, structural insights into L proteins from two distantly related NS RNA viruses have revealed the overall architecture of this multifaceted enzyme and determined how the various enzymatic domains of L are arranged into a single protein.8,9 The development of recombinant RNA synthesis systems utilizing full-length L and purified components has provided new insight into regulation of NS RNA viral replication,10-13 and offers further opportunity to interrogate NS RNA viral biology and develop rationally designed antiviral inhibitors.
L Protein Architecture and Domain Organization
The replication machinery of all NS RNA viruses is built upon an evolutionarily related RNA-dependent RNA polymerase domain that shares phylogenetic conservation with the polymerase from positive-sense and double-strand RNA viruses.14,15 All of the NS RNA viral polymerase machinery is retained within the single L protein, with the exception of influenza A virus and members of the Orthomyxoviridae family where the polymerase is broken into a tripartite complex. The corresponding smaller polymerase fragments have facilitated studies on the influenza virus replicative proteins,16-18 and enabled a greater biochemical and structural understanding of the tripartite complex (discussed in detail below). Conversely, functional characterization of NS RNA viral L proteins has been impeded by their large size (~250 kDa) and the presence of flexible domains or connecting “hinge” regions. Hinge regions are believed to separate independent enzymatic activities;19 but regulatory interactions between the distinct activities indicate the overall organization of L is critical for proper enzymatic function.20-22 In spite of this importance, the structural architecture of L proteins has remained a mystery.
Blocks of phylogenetically conserved domains have been identified within the amino acid sequence of L genes.19 The hallmark presence of fingers, palm and thumb subdomains shared by all known polymerases allowed identification of the RNA-dependent RNA polymerase domain,14 which was later verified by mutations to a catalytically essential motif predicted to form a divalent cation coordination site.23-25 Further genetic and biochemical studies implicated additional conserved regions involved in template recognition, cofactor association, and formation of the 5′ mRNA cap structure during viral transcription.11,26-29
While all L proteins share a phylogenetically conserved RNA-dependent RNA polymerase domain, the L proteins of segmented and nonsegmented NS RNA viruses differ in the conserved domains associated with cap formation (Fig. 1). Segmented viruses, including the tripartite influenza virus polymerase, recognize host mRNAs30 and cleave off the 5′ mRNA cap with a conserved endonuclease activity.31-34 The host mRNA cap is then used to directly prime viral mRNA transcription such that the viral mRNAs are capped with stolen host 5′ sequence in a process termed “cap-snatching.”35 In contrast, most nonsegmented viruses directly synthesize their 5′ cap structure using a mechanistically unparalleled 5′ polyribonucleotidyl-transferase activity (PRNTase)11,36 and a dual-specificity guanine-N7 and 2’-O methyltransferase (MTase).37-39
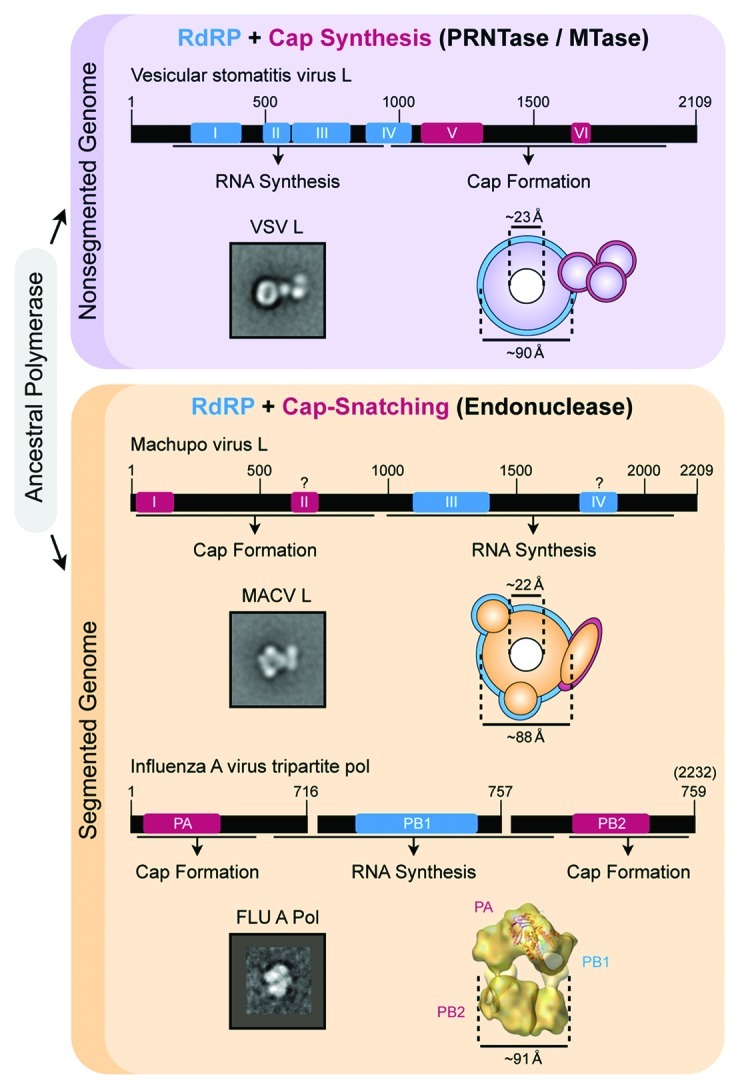
Figure 1. Structural architecture and organization of NS RNA viral polymerases. Conserved architecture and domain organization found in nonsegmented (top, purple) and segmented (bottom, orange) polymerases. The linear amino acid sequence of L and the tripartite influenza virus polymerase contain highly conserved regions dedicated to RNA synthesis (blue boxes). L and the influenza virus polymerase also contain blocks of conservation dedicated to 5′ cap formation (maroon boxes), including an endonuclease domain for cap-snatching (domain I or PA, segmented NS RNA viruses) or PRNTase / MTase domains for de novo cap synthesis (domains V and VI, nonsegmented NS RNA viruses). The regions containing cap formation enzymatic activities are also required for RNA synthesis, and the exact function of domains II and IV in the L protein of segmented NS RNA viruses remains unknown. EM of purified L from Machupo virus and vesicular stomatitis virus reveals a shared structural architecture conserved within NS RNA viral L proteins. L consists of a central ring-like RNA polymerase domain (blue highlight in cartoon) and a large appendage dedicated to 5′ cap formation (maroon highlight in cartoon) attached with a flexible linkage. As described in the text, comparison with EM analysis and 3D reconstruction of the influenza virus polymerase complex provides further insight into the architecture of L. Influenza A virus polymerase images reproduced with permission from refs. 47 and 49
The structural organization of each type of L protein was investigated using single-particle electron microscopy (EM) and recombinant L that maintains functional RNA synthesis activity in vitro.8,9 The architecture of L was compared between Machupo virus (MACV), a cap-snatching segmented virus of the Arenaviridae family, and vesicular stomatitis virus (VSV), a nonsegmented virus of the Rhabdoviridae family that performs de novo cap-synthesis. The corresponding EM class averages revealed a shared architecture where L consists of a central ring-like RNA-dependent RNA polymerase domain upon which divergent appendages dedicated to the distinct 5′ mRNA capping activities are attached (Fig. 1).8,9 The EM maps confirmed that L consists of discrete domains, and demonstrated that the shared conserved function (polymerase) relates to a shared architecturally conserved domain (central ring). Correspondingly, the divergent 5′ capping functions (MACV cap-endonuclease vs. VSV PRNTase/MTase) relate to divergent, architecturally distinct domains (MACV large arm-like appendage vs. VSV large globular appendage) (Fig. 1).
Structural insight into MACV and VSV L also revealed a large degree of conformational flexibility between the ring-like polymerase and cap formation appendages.8,9 Observed alternative orientations of the capping domains agrees with the previous prediction of flexible hinge regions linking distinct enzymatic activities.19 Hinge regions are remarkably tolerant to domain insertion, and fluorescent reporter sequences have been successfully introduced within several L proteins without compromising catalytic function or virus viability.40-42 Therefore, flexible hinge regions not only maintain the modular architecture of L, but may also play an additional role in preserving functional plasticity of the NS RNA viral polymerase complex and facilitating viral evolution.
The RNA-dependent RNA polymerase domain of MACV and VSV L appears as a closed ring with a hollow stain-collecting center,8,9 and this architectural organization parallels the caged appearance to the reo- and rotavirus dsRNA viral polymerases.43,44 Enclosure of the reovirus RNA-dependent RNA polymerase active site results from an N-terminal bridging domain and a carboxyl-terminal “bracelet” domain. These bracketing domains have been implicated in enhancing polymerase processivity and forming a unique secondary RNA exit tunnel to facilitate positive-sense transcript release.45 L and the reovirus polymerase complex have dual functions as an mRNA transcriptase and an RNA replicase, and a similar four-tunnel architecture may be required by negative-sense RNA viral polymerases to separate template and replication RNAs for independent nucleoprotein encapsidation.
Further insight into the low-resolution maps of L can be gained through comparison with structural analyses of the influenza virus polymerase complex. Although the influenza virus polymerase is a heterotrimeric complex of three subunits (PA, PB1 and PB2), the tripartite polymerase is also ~250 kDa and is closely related to NS RNA viral L proteins (Fig. 1). Pioneering EM studies with influenza virus provided the first structural information for a recombinant NS RNA viral polymerase complex,46 and considerable progress has since resulted in an ~18Å resolution 3D reconstruction suitable for initial quasi-atomic modeling of fragments of the individual polymerase subunits.47 Compared with the core structure of MACV and VSV L, the influenza virus polymerase exhibits a similar overall structural shape and central hollow architecture.47,48 However, the influenza virus polymerase adopts a more compact structure that lacks the large flexible appendage observed in 2D EM analysis of MACV and VSV L (Fig. 1). The compact architecture of the influenza virus polymerase can be partially explained by conformational changes induced upon interaction with the viral genomic RNA termini. The soluble RNA-free form of the influenza virus polymerase adopts a more open architecture compared with the RNA-bound or N–RNA associated isoforms, and conformational rearrangements have been proposed to involve movement of the N-terminal domain of PB2.47-49 The large appendage of VSV L undergoes a dramatic rearrangement upon association with the viral phosphoprotein, indicating that similar conformation changes can occur within the capping domains of L proteins.9 These changes may represent different functional states within NS RNA viral polymerases, and support the hypothesis that flexibility between domains dedicated to RNA synthesis and cap formation is required to facilitate alternate functionality in mRNA transcription and genomic RNA replication.
3D reconstruction and subunit localization revealed that in addition to their functional roles during cap formation, the influenza virus PA and PB2 subunits are structurally important to bracket the core PB1 polymerase domain structure.47,50,51 Similarly, N-terminal portions of the VSV L capping domains are required to complete the central ring structure.9 Therefore, the domains primarily thought to be involved in cap formation are likely additionally required to enclose the RNA-dependent RNA polymerase active site and may explain the importance of intramolecular L–L interactions evolutionarily conserved in segmented and nonsegmented L proteins.52,53
Increased EM resolution and improved three-dimensional reconstructions will facilitate ongoing efforts to determine the high-resolution structure of L and individual enzymatic domains. Currently, the only high-resolution structures available are the L endonuclease domain from segmented viruses of the Arenaviridae and Bunyaviridae families33,34 and the L ovarian-tumor protease domain unique to nairoviruses within the Bunyaviridae family.54-56 Deletion mapping confirmed that the globular domains within the VSV L appendage contain the PRNTase and MTase activities,9 and by architectural comparison it is likewise predicted that the endonuclease structures relate to the end of the MACV L large arm-like domain.8 However, exact placement of these structures will require considerably improved EM map resolution and additional future high-resolution structures.
Comparison of MACV and VSV L EM structures reveals a potential evolutionary path whereby an ancestral ring-like RNA-dependent RNA polymerase was appended with various cap formation activities to suit the specific requirements of different NS RNA viral replication mechanisms. Discrete domains dedicated to cap formation are further confirmed by the ability to express and independently fold a functional endonuclease domain from segmented L proteins33,34 and the MTase domain from a nonsegmented L protein.37 Similarly, it is possible to split the RNA-dependent RNA polymerase from the cap formation machinery and maintain an L protein capable of cellular-based replicon expression for both segmented L52 and nonsegmented L proteins.53 These genetic and biochemical experiments further support the EM maps of MACV and VSV L, and attest to the evolutionary addition of capping domains to a conserved RNA polymerase. Intriguingly, the L proteins of some segmented NS RNA viruses contain additional discrete domains dedicated to antagonism of the host antiviral machinery,57-59 and indicate that the evolutionary process of domain addition is continually ongoing.
Regulation of L and Viral RNA Synthesis
Recombinant L has led to the reconstitution of RNA synthesis from purified components within both segmented and nonsegmented systems.8,10-13 In combination with viral genetics and cellular-based RNA synthesis systems using genomic replicons, these in vitro assays allow the mechanistic study of template interactions and regulation of NS RNA viral gene expression. During infection, the genomic template for RNA synthesis is single-stranded RNA tightly encapsidated within a protein sheath formed by the viral nucleocapsid (N) protein (reviewed in ref. 60). In spite of high-resolution structures, how L remodels this N–RNA template during mRNA transcription and genome replication remains a mystery. However, N is not required for RNA synthesis using short naked RNA templates in vitro8,12,13 and is likely necessary for integrity of the full-length genome and reduction of RNA secondary structure.60 Additionally, the N-encapsidated template is suggested to increase processivity of VSV L, indicating a further role for N in forming a fully elongation-competent polymerase complex.13 The sequence and character of the RNA template itself also directs the viral polymerase machinery and can control L protein function. DsRNA intertermini interactions regulate the ability of arenavirus L to recognize a conserved 3′ CGUG 5′ motif essential for polymerase recruitment and allows fine-tuning of viral gene expression.8,61 Sequence-specific promoter interactions are also critical to activate cap-recognition and endonuclease activity within the influenza virus polymerase.62,63 Although an exact sequence-specific recognition motif has not been identified for recruitment of nonsegmented viral L proteins, sequence-specific initiation events occur for VSV13 and have been implicated with a variety of cellular-based replicon systems.
L from nonsegmented viruses is stimulated by the polymerase phosphoprotein cofactor (P).64 No functional analog to P is required for segmented viruses, possibly due to differences in template interactions or primed12,65,66 and de novo13,67 RNA synthesis initiation. Additional viral cofactors have been implicated in modifying mRNA synthesis by nonsegmented NS RNA viral L proteins. The accessory proteins M2–1 of respiratory syncytial virus and VP30 of Ebola virus impact proper regulation of viral mRNA transcription,68-70 but do not appear to be critical for genomic RNA replication. Studies with the influenza virus NS2 protein indicate that accessory factors may also control relative mRNA transcriptase and RNA replicase activities of segmented NS RNA viruses.71 In contrast to the core conserved enzymatic machinery, the divergent occurrence and function of accessory proteins reveals that regulation of NS RNA viral polymerase activity is complex and varies between viral systems.
In addition to accessory proteins, the major structural components of the virion have also been reported to serve regulatory roles influencing L for both segmented and nonsegmented viruses. This regulation has long been hypothesized to be important to ensure polymerase packaging into mature virions, and recent work has now revealed a possible mechanism for this connection.12 All NS RNA viruses, dsRNA viruses, and reverse-transcribing viruses initiate infection by first copying or transcribing the RNA genome inside an infected cell, and must therefore package their polymerase machinery in order to generate an infectious particle. Many viruses solve this problem by including the viral polymerase as an integral structural component of the virion, thereby rendering mature viral particle formation impossible in the absence of the polymerase. However, NS RNA viruses do not invoke this strategy, and it is possible to bud virus-like particles in the absence of the polymerase.72-74 It has thus remained unclear how NS RNA viruses ensure the polymerase is packaged along with the N–RNA template during virion egress.
A possible safeguard to ensure accurate packaging of all necessary virion components resides within the diverse roles of the NS RNA viral matrix protein—the viral protein primarily responsible for mediating virion egress. In addition to recruitment of cellular machinery to promoter budding, overexpression of the matrix or matrix-like protein typically results in potent downregulation of viral gene expression in both segmented and nonsegmented systems (Fig. 2). Recently, the molecular basis for gene expression inhibition was determined for the arenavirus matrix protein (Z).12 In addition to the known role in virion budding, Z was demonstrated to specifically interact with L and directly inhibit viral RNA synthesis independent of host cellular factors. Z–L complex formation catalytically inactivates L polymerase activity but does not impact promoter engagement, resulting in a frozen Z–L–RNA complex trapped on the 3′ RNA promoter sequence. It is therefore hypothesized that this trapped complex not only regulates viral gene expression, but also serves as an intermediate to ensure a functional polymerase remains locked on the template and is packaged into mature virions. The intravirion concentration of the arenavirus matrix protein is sufficient to maintain L in a quiescent state within the viral particle, and once released into a newly infected cell the reduced local concentration of matrix will liberate the complex and free the polymerase to initiate a new round of infection (Fig. 3).12
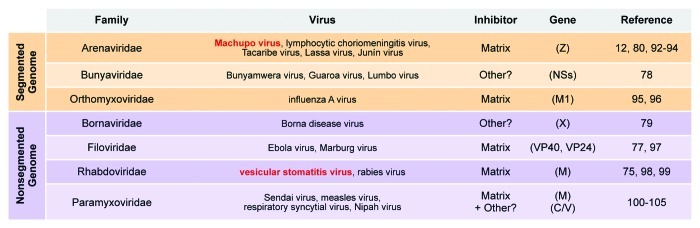
Figure 2. Inhibitory NS RNA viral proteins. Inhibition of gene expression by the viral matrix protein is a conserved feature of both segmented (top, orange) and nonsegmented (bottom, purple) NS RNA viruses. The table indicates the NS RNA virus families and main individual viruses for which inhibition by matrix or other accessory factors has been observed.
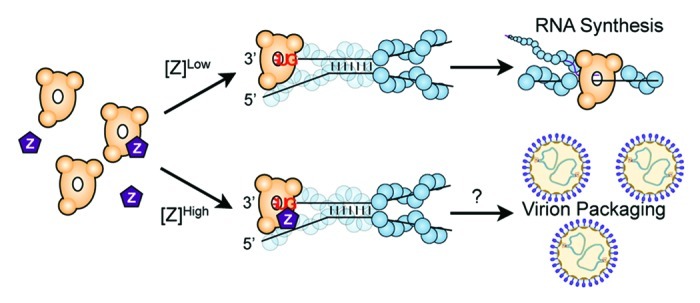
Figure 3. Model of arenavirus RNA synthesis regulation by the viral matrix protein. Low concentrations of matrix (Z) permit ongoing RNA synthesis, while high concentrations of Z result in an inhibited Z–L–RNA complex bound to the viral promoter. As described in the text, the Z–L–RNA complex may serve as an important intermediate ensuring L is packaged into mature virions. Other NS RNA viral matrix proteins or accessory inhibitory factors may play a similar role in viral replication, indicating a potentially conserved link between regulation of viral transcription and virion formation. (Fig. adapted from ref. 12).
Inhibition by overexpression of the viral matrix protein is a highly conserved feature of NS RNA viral replication (Fig. 2), and it is possible that arenavirus Z–L–RNA complex formation serves as an important paradigm for the link between transcription regulation and polymerase packaging of NS RNA viruses. In further support of this hypothesis, previous results with nonsegmented NS RNA viruses have demonstrated that matrix inhibits purified ribonucleoprotein complexes75 and this inhibitory activity can be genetically separated from other functions of matrix during virion assembly.76,77 Moreover, the two families of viruses that do not express inhibitory matrix proteins (segmented viruses of the Bunyaviridae family and nonsegmented viruses of the Bornaviridae family), each encode alternative accessory proteins that downregulate polymerase activity and could maintain the conserved functional link between polymerase regulation and virion maturation (Fig. 2).78,79
If matrix has a conserved role in polymerase packaging, a key remaining question is how are levels of inhibitory matrix protein controlled such that they remain low enough to permit viral RNA synthesis and gene expression early during infection yet peak late when virion egress is favored over RNA production? Levels of Z vary throughout the arenavirus infection cycle,80 and differential promoter strength and transcription from distinct genomic segments and individual promoters can explain altered levels of inhibitory products for bunyaviruses and influenza A virus. However, the linear transcription mechanism of monopartite nonsegmented NS RNA viral genomes results in significant quantities of matrix produced throughout the entire replication cycle (reviewed in ref. 81).81 Interestingly, phosphorylation of matrix has been observed for representative members of all nonsegmented NS RNA viral families encoding an inhibitory matrix protein,82-86 and hyperphosphorylation of matrix has been associated with inhibition of VSV RNA synthesis,87 indicating alternative mechanisms such as control of a post-translational switch could determine when nonsegmented L proteins become template-locked and virion egress is favored. However, successful rearrangement of gene order88 and segment number89 indicate the exact mechanisms regulating polymerase inhibitory proteins are likely more complex, and understanding the individual mechanisms at play for each virus will require significant future research. Reconstituted RNA synthesis systems available for influenza A virus and nonsegmented NS RNA viruses can now be used to directly assess polymerase inhibition by recombinant matrix protein and determine if the arenavirus packaging mechanism extends to other NS RNA viruses.
Perspectives
The future of NS RNA polymerase biology is inexorably tied to a further structural understanding of L and various cognate protein and N–RNA complexes. Low-resolution EM provides a tantalizing first glimpse of the basic machine,8,9 but high-resolution studies are essential to decipher the working components of NS RNA replication. Continued development of in vitro systems should focus on reconstitution of template encapsidation for authentic viral replication studies and single-particle measurements capable of analyzing transition states and replication intermediates. Recently developed minimal RNA synthesis assays using naked RNA template hold great promise for high-throughput screening and discovery of small molecule inhibitors.12,13 Nucleoside analogs like ribavirin and T-70590 demonstrate the potential of broad-spectrum NS RNA polymerase antagonists, but many more steps of the replication cycle should also be prone to inhibition by small molecules.91 Critically, as experiments become increasingly sophisticated and specialized to individual NS RNA viral systems, the instructive value of comparisons between segmented and nonsegmented viruses must not be overlooked.
Acknowledgments
We thank Amal Rahmeh and Benjamin Morin for comments and extensive discussions. We are grateful to Juan Ortín for incorporation of influenza virus polymerase structural data and for insightful comments and discussion. The work in the laboratory of SPJW is supported by NIH grants AI057159 and AI059371. SPJW is a recipient of a Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/20345
References
- 1.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(Suppl):S110–21. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 2.Whitfield AE, Ullman DE, German TL. Tospovirus-thrips interactions. Annu Rev Phytopathol. 2005;43:459–89. doi: 10.1146/annurev.phyto.43.040204.140017. [DOI] [PubMed] [Google Scholar]
- 3.Pappu HR, Jones RA, Jain RK. Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Res. 2009;141:219–36. doi: 10.1016/j.virusres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–32. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado S, Erickson BR, Agudo R, Blair PJ, Vallejo E, Albariño CG, et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008;4:e1000047. doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5:e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann B, Scheuch M, Höper D, Jungblut R, Holsteg M, Schirrmeier H, et al. Novel orthobunyavirus in cattle, europe, 2011. Emerg Infect Dis. 2012;18:469–72. doi: 10.3201/eid1803.111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranzusch PJ, Schenk AD, Rahmeh AA, Radoshitzky SR, Bavari S, Walz T, et al. Assembly of a functional Machupo virus polymerase complex. Proc Natl Acad Sci U S A. 2010;107:20069–74. doi: 10.1073/pnas.1007152107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmeh AA, Schenk AD, Danek EI, Kranzusch PJ, Liang B, Walz T, et al. Molecular architecture of the vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A. 2010;107:20075–80. doi: 10.1073/pnas.1013559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur M, Das T, Banerjee AK. Expression of L protein of vesicular stomatitis virus Indiana serotype from recombinant baculovirus in insect cells: requirement of a host factor(s) for its biological activity in vitro. J Virol. 1996;70:2252–9. doi: 10.1128/jvi.70.4.2252-2259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Rahmeh A, Morelli M, Whelan SP. A conserved motif in region v of the large polymerase proteins of nonsegmented negative-sense RNA viruses that is essential for mRNA capping. J Virol. 2008;82:775–84. doi: 10.1128/JVI.02107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzusch PJ, Whelan SP. Arenavirus Z protein controls viral RNA synthesis by locking a polymerase-promoter complex. Proc Natl Acad Sci U S A. 2011;108:19743–8. doi: 10.1073/pnas.1112742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin B, Rahmeh AA, Whelan SPJ. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. EMBO J. 2012;31:1320–9. doi: 10.1038/emboj.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989;8:3867–74. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hass M, Lelke M, Busch C, Becker-Ziaja B, Günther S. Mutational evidence for a structural model of the Lassa virus RNA polymerase domain and identification of two residues, Gly1394 and Asp1395, that are critical for transcription but not replication of the genome. J Virol. 2008;82:10207–17. doi: 10.1128/JVI.00220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das K, Aramini JM, Ma LC, Krug RM, Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol. 2010;17:530–8. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boivin S, Cusack S, Ruigrok RW, Hart DJ. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. 2010;285:28411–7. doi: 10.1074/jbc.R110.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resa-Infante P, Jorba N, Coloma R, Ortin J. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 2011;8:207–15. doi: 10.4161/rna.8.2.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poch O, Blumberg BM, Bougueleret L, Tordo N. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990;71:1153–62. doi: 10.1099/0022-1317-71-5-1153. [DOI] [PubMed] [Google Scholar]
- 20.Rose JK, Lodish HF, Brock ML. Giant heterogeneous polyadenylic acid on vesicular stomatitis virus mRNA synthesized in vitro in the presence of S-adenosylhomocysteine. J Virol. 1977;21:683–93. doi: 10.1128/jvi.21.2.683-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galloway SE, Wertz GW. S-adenosyl homocysteine-induced hyperpolyadenylation of vesicular stomatitis virus mRNA requires the methyltransferase activity of L protein. J Virol. 2008;82:12280–90. doi: 10.1128/JVI.01225-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Rahmeh A, Brusic V, Whelan SP. Opposing effects of inhibiting cap addition and cap methylation on polyadenylation during vesicular stomatitis virus mRNA synthesis. J Virol. 2009;83:1930–40. doi: 10.1128/JVI.02162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Elliott RM. Mutagenesis of the L protein encoded by Bunyamwera virus and production of monospecific antibodies. J Gen Virol. 1992;73:2235–44. doi: 10.1099/0022-1317-73-9-2235. [DOI] [PubMed] [Google Scholar]
- 24.Sleat DE, Banerjee AK. Transcriptional activity and mutational analysis of recombinant vesicular stomatitis virus RNA polymerase. J Virol. 1993;67:1334–9. doi: 10.1128/jvi.67.3.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez AB, de la Torre JC. Genetic and biochemical evidence for an oligomeric structure of the functional L polymerase of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2005;79:7262–8. doi: 10.1128/JVI.79.11.7262-7268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrika R, Horikami SM, Smallwood S, Moyer SA. Mutations in conserved domain I of the Sendai virus L polymerase protein uncouple transcription and replication. Virology. 1995;213:352–63. doi: 10.1006/viro.1995.0008. [DOI] [PubMed] [Google Scholar]
- 27.Smallwood S, Easson CD, Feller JA, Horikami SM, Moyer SA. Mutations in conserved domain II of the large (L) subunit of the Sendai virus RNA polymerase abolish RNA synthesis. Virology. 1999;262:375–83. doi: 10.1006/viro.1999.9933. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Fontaine-Rodriguez EC, Whelan SP. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J Virol. 2005;79:13373–84. doi: 10.1128/JVI.79.21.13373-13384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino T, Yadav SP, Banerjee AK. Histidine-mediated RNA transfer to GDP for unique mRNA capping by vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A. 2010;107:3463–8. doi: 10.1073/pnas.0913083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilligay D, Tarendeau F, Resa-Infante P, Coloma R, Crepin T, Sehr P, et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol. 2008;15:500–6. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- 31.Dias A, Bouvier D, Crépin T, McCarthy AA, Hart DJ, Baudin F, et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–8. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 32.Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–13. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 33.Morin B, Coutard B, Lelke M, Ferron F, Kerber R, Jamal S, et al. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog. 2010;6:e1001038. doi: 10.1371/journal.ppat.1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reguera J, Weber F, Cusack S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 2010;6:e1001101. doi: 10.1371/journal.ppat.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plotch SJ, Bouloy M, Ulmanen I, Krug RM. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–58. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 36.Ogino T, Banerjee AK. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol Cell. 2007;25:85–97. doi: 10.1016/j.molcel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Ogino T, Kobayashi M, Iwama M, Mizumoto K. Sendai virus RNA-dependent RNA polymerase L protein catalyzes cap methylation of virus-specific mRNA. J Biol Chem. 2005;280:4429–35. doi: 10.1074/jbc.M411167200. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Wang JT, Whelan SP. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2006;103:8493–8. doi: 10.1073/pnas.0509821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahmeh AA, Li J, Kranzusch PJ, Whelan SP. Ribose 2′-O methylation of the vesicular stomatitis virus mRNA cap precedes and facilitates subsequent guanine-N-7 methylation by the large polymerase protein. J Virol. 2009;83:11043–50. doi: 10.1128/JVI.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duprex WP, Collins FM, Rima BK. Modulating the function of the measles virus RNA-dependent RNA polymerase by insertion of green fluorescent protein into the open reading frame. J Virol. 2002;76:7322–8. doi: 10.1128/JVI.76.14.7322-7328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruedas JB, Perrault J. Insertion of enhanced green fluorescent protein in a hinge region of vesicular stomatitis virus L polymerase protein creates a temperature-sensitive virus that displays no virion-associated polymerase activity in vitro. J Virol. 2009;83:12241–52. doi: 10.1128/JVI.01273-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fix J, Galloux M, Blondot ML, Eléouët JF. The insertion of fluorescent proteins in a variable region of respiratory syncytial virus L polymerase results in fluorescent and functional enzymes but with reduced activities. Open Virol J. 2011;5:103–8. doi: 10.2174/1874357901105010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage--structural studies of reovirus polymerase lambda3. Cell. 2002;111:733–45. doi: 10.1016/S0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- 44.Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, Nibert ML, et al. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure. 2008;16:1678–88. doi: 10.1016/j.str.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald SM, Tao YJ, Patton JT. The ins and outs of four-tunneled Reoviridae RNA-dependent RNA polymerases. Curr Opin Struct Biol. 2009;19:775–82. doi: 10.1016/j.sbi.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega J, Martín-Benito J, Zürcher T, Valpuesta JM, Carrascosa JL, Ortín J. Ultrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J Virol. 2000;74:156–63. doi: 10.1128/JVI.74.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coloma R, Valpuesta JM, Arranz R, Carrascosa JL, Ortín J, Martín-Benito J. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torreira E, Schoehn G, Fernández Y, Jorba N, Ruigrok RW, Cusack S, et al. Three-dimensional model for the isolated recombinant influenza virus polymerase heterotrimer. Nucleic Acids Res. 2007;35:3774–83. doi: 10.1093/nar/gkm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resa-Infante P, Recuero-Checa MA, Zamarreño N, Llorca O, Ortín J. Structural and functional characterization of an influenza virus RNA polymerase-genomic RNA complex. J Virol. 2010;84:10477–87. doi: 10.1128/JVI.01115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martín-Benito J, Area E, Ortega J, Llorca O, Valpuesta JM, Carrascosa JL, et al. Three-dimensional reconstruction of a recombinant influenza virus ribonucleoprotein particle. EMBO Rep. 2001;2:313–7. doi: 10.1093/embo-reports/kve063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Area E, Martín-Benito J, Gastaminza P, Torreira E, Valpuesta JM, Carrascosa JL, et al. 3D structure of the influenza virus polymerase complex: localization of subunit domains. Proc Natl Acad Sci U S A. 2004;101:308–13. doi: 10.1073/pnas.0307127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunotte L, Lelke M, Hass M, Kleinsteuber K, Becker-Ziaja B, Günther S. Domain structure of Lassa virus L protein. J Virol. 2011;85:324–33. doi: 10.1128/JVI.00721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dochow M, Krumm SA, Crowe JE, Jr., Moore ML, Plemper RK. Independent structural domains in paramyxovirus polymerase protein. J Biol Chem. 2012;287:6878–91. doi: 10.1074/jbc.M111.325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capodagli GC, McKercher MA, Baker EA, Masters EM, Brunzelle JS, Pegan SD. Structural analysis of a viral ovarian tumor domain protease from the Crimean-Congo hemorrhagic fever virus in complex with covalently bonded ubiquitin. J Virol. 2011;85:3621–30. doi: 10.1128/JVI.02496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James TW, Frias-Staheli N, Bacik JP, Levingston Macleod JM, Khajehpour M, García-Sastre A, et al. Structural basis for the removal of ubiquitin and interferon-stimulated gene 15 by a viral ovarian tumor domain-containing protease. Proc Natl Acad Sci U S A. 2011;108:2222–7. doi: 10.1073/pnas.1013388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akutsu M, Ye Y, Virdee S, Chin JW, Komander D. Molecular basis for ubiquitin and ISG15 cross-reactivity in viral ovarian tumor domains. Proc Natl Acad Sci U S A. 2011;108:2228–33. doi: 10.1073/pnas.1015287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honig JE, Osborne JC, Nichol ST. Crimean-Congo hemorrhagic fever virus genome L RNA segment and encoded protein. Virology. 2004;321:29–35. doi: 10.1016/j.virol.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 58.Kinsella E, Martin SG, Grolla A, Czub M, Feldmann H, Flick R. Sequence determination of the Crimean-Congo hemorrhagic fever virus L segment. Virology. 2004;321:23–8. doi: 10.1016/j.virol.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 59.Frias-Staheli N, Giannakopoulos NV, Kikkert M, Taylor SL, Bridgen A, Paragas J, et al. Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe. 2007;2:404–16. doi: 10.1016/j.chom.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruigrok RW, Crépin T, Kolakofsky D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr Opin Microbiol. 2011;14:504–10. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Hass M, Westerkofsky M, Müller S, Becker-Ziaja B, Busch C, Günther S. Mutational analysis of the lassa virus promoter. J Virol. 2006;80:12414–9. doi: 10.1128/JVI.01374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagen M, Chung TD, Butcher JA, Krystal M. Recombinant influenza virus polymerase: requirement of both 5′ and 3′ viral ends for endonuclease activity. J Virol. 1994;68:1509–15. doi: 10.1128/jvi.68.3.1509-1515.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li ML, Ramirez BC, Krug RM. RNA-dependent activation of primer RNA production by influenza virus polymerase: different regions of the same protein subunit constitute the two required RNA-binding sites. EMBO J. 1998;17:5844–52. doi: 10.1093/emboj/17.19.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emerson SU, Wagner RR. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973;12:1325–35. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcin D, Kolakofsky D. Tacaribe arenavirus RNA synthesis in vitro is primer dependent and suggests an unusual model for the initiation of genome replication. J Virol. 1992;66:1370–6. doi: 10.1128/jvi.66.3.1370-1376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcin D, Lezzi M, Dobbs M, Elliott RM, Schmaljohn C, Kang CY, et al. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J Virol. 1995;69:5754–62. doi: 10.1128/jvi.69.9.5754-5762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noton SL, Cowton VM, Zack CR, McGivern DR, Fearns R. Evidence that the polymerase of respiratory syncytial virus initiates RNA replication in a nontemplated fashion. Proc Natl Acad Sci U S A. 2010;107:10226–31. doi: 10.1073/pnas.0913065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collins PL, Hill MG, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci U S A. 1996;93:81–5. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fearns R, Collins PL. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol. 1999;73:5852–64. doi: 10.1128/jvi.73.7.5852-5864.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mühlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol. 1999;73:2333–42. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robb NC, Smith M, Vreede FT, Fodor E. NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J Gen Virol. 2009;90:1398–407. doi: 10.1099/vir.0.009639-0. [DOI] [PubMed] [Google Scholar]
- 72.Justice PA, Sun W, Li Y, Ye Z, Grigera PR, Wagner RR. Membrane vesiculation function and exocytosis of wild-type and mutant matrix proteins of vesicular stomatitis virus. J Virol. 1995;69:3156–60. doi: 10.1128/jvi.69.5.3156-3160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A. 2000;97:13871–6. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A. 2003;100:12978–83. doi: 10.1073/pnas.2133782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll AR, Wagner RR. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979;29:134–42. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finke S, Conzelmann KK. Dissociation of rabies virus matrix protein functions in regulation of viral RNA synthesis and virus assembly. J Virol. 2003;77:12074–82. doi: 10.1128/JVI.77.22.12074-12082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoenen T, Jung S, Herwig A, Groseth A, Becker S. Both matrix proteins of Ebola virus contribute to the regulation of viral genome replication and transcription. Virology. 2010;403:56–66. doi: 10.1016/j.virol.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Weber F, Dunn EF, Bridgen A, Elliott RM. The Bunyamwera virus nonstructural protein NSs inhibits viral RNA synthesis in a minireplicon system. Virology. 2001;281:67–74. doi: 10.1006/viro.2000.0774. [DOI] [PubMed] [Google Scholar]
- 79.Perez M, Sanchez A, Cubitt B, Rosario D, de la Torre JC. A reverse genetics system for Borna disease virus. J Gen Virol. 2003;84:3099–104. doi: 10.1099/vir.0.19467-0. [DOI] [PubMed] [Google Scholar]
- 80.Cornu TI, de la Torre JC. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J Virol. 2001;75:9415–26. doi: 10.1128/JVI.75.19.9415-9426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whelan SP, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 82.Lamb RA, Choppin PW. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977;81:382–97. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- 83.Wechsler SL, Lambert DM, Galinski MS, Pons MW. Intracellular synthesis of human parainfluenza type 3 virus-specified polypeptides. J Virol. 1985;54:661–4. doi: 10.1128/jvi.54.3.661-664.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambert DM, Hambor J, Diebold M, Galinski B. Kinetics of synthesis and phosphorylation of respiratory syncytial virus polypeptides. J Gen Virol. 1988;69:313–23. doi: 10.1099/0022-1317-69-2-313. [DOI] [PubMed] [Google Scholar]
- 85.Kaptur PE, McCreedy BJ, Jr., Lyles DS. Sites of in vivo phosphorylation of vesicular stomatitis virus matrix protein. J Virol. 1992;66:5384–92. doi: 10.1128/jvi.66.9.5384-5392.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolesnikova L, Mittler E, Schudt G, Shams-Eldin H, Becker S. Phosphorylation of Marburg virus matrix protein VP40 triggers assembly of nucleocapsids with the viral envelope at the plasma membrane. Cell Microbiol. 2012;14:182–97. doi: 10.1111/j.1462-5822.2011.01709.x. [DOI] [PubMed] [Google Scholar]
- 87.Chang TL, Reiss CS, Huang AS. Inhibition of vesicular stomatitis virus RNA synthesis by protein hyperphosphorylation. J Virol. 1994;68:4980–7. doi: 10.1128/jvi.68.8.4980-4987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wertz GW, Perepelitsa VP, Ball LA. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc Natl Acad Sci U S A. 1998;95:3501–6. doi: 10.1073/pnas.95.7.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takeda M, Nakatsu Y, Ohno S, Seki F, Tahara M, Hashiguchi T, et al. Generation of measles virus with a segmented RNA genome. J Virol. 2006;80:4242–8. doi: 10.1128/JVI.80.9.4242-4248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, et al. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de la Torre JC. Reverse genetics approaches to combat pathogenic arenaviruses. Antiviral Res. 2008;80:239–50. doi: 10.1016/j.antiviral.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.López N, Jácamo R, Franze-Fernández MT. Transcription and RNA replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J Virol. 2001;75:12241–51. doi: 10.1128/JVI.75.24.12241-12251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hass M, Gölnitz U, Müller S, Becker-Ziaja B, Günther S. Replicon system for Lassa virus. J Virol. 2004;78:13793–803. doi: 10.1128/JVI.78.24.13793-13803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loureiro ME, Wilda M, Levingston Macleod JM, D’Antuono A, Foscaldi S, Marino Buslje C, et al. Molecular determinants of arenavirus Z protein homo-oligomerization and L polymerase binding. J Virol. 2011;85:12304–14. doi: 10.1128/JVI.05691-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watanabe K, Handa H, Mizumoto K, Nagata K. Mechanism for inhibition of influenza virus RNA polymerase activity by matrix protein. J Virol. 1996;70:241–7. doi: 10.1128/jvi.70.1.241-247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perez DR, Donis RO. The matrix 1 protein of influenza A virus inhibits the transcriptase activity of a model influenza reporter genome in vivo. Virology. 1998;249:52–61. doi: 10.1006/viro.1998.9318. [DOI] [PubMed] [Google Scholar]
- 97.Watanabe S, Noda T, Halfmann P, Jasenosky L, Kawaoka Y. Ebola virus (EBOV) VP24 inhibits transcription and replication of the EBOV genome. J Infect Dis. 2007;196(Suppl 2):S284–90. doi: 10.1086/520582. [DOI] [PubMed] [Google Scholar]
- 98.Clinton GM, Little SP, Hagen FS, Huang AS. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978;15:1455–62. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- 99.Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003;84:1613–21. doi: 10.1099/vir.0.19128-0. [DOI] [PubMed] [Google Scholar]
- 100.Curran J, Boeck R, Kolakofsky D. The Sendai virus P gene expresses both an essential protein and an inhibitor of RNA synthesis by shuffling modules via mRNA editing. EMBO J. 1991;10:3079–85. doi: 10.1002/j.1460-2075.1991.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grogan CC, Moyer SA. Sendai virus wild-type and mutant C proteins show a direct correlation between L polymerase binding and inhibition of viral RNA synthesis. Virology. 2001;288:96–108. doi: 10.1006/viro.2001.1068. [DOI] [PubMed] [Google Scholar]
- 102.Suryanarayana K, Baczko K, ter Meulen V, Wagner RR. Transcription inhibition and other properties of matrix proteins expressed by M genes cloned from measles viruses and diseased human brain tissue. J Virol. 1994;68:1532–43. doi: 10.1128/jvi.68.3.1532-1543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwasaki M, Takeda M, Shirogane Y, Nakatsu Y, Nakamura T, Yanagi Y. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J Virol. 2009;83:10374–83. doi: 10.1128/JVI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghildyal R, Mills J, Murray M, Vardaxis N, Meanger J. Respiratory syncytial virus matrix protein associates with nucleocapsids in infected cells. J Gen Virol. 2002;83:753–7. doi: 10.1099/0022-1317-83-4-753. [DOI] [PubMed] [Google Scholar]
- 105.Sleeman K, Bankamp B, Hummel KB, Lo MK, Bellini WJ, Rota PA. The C, V and W proteins of Nipah virus inhibit minigenome replication. J Gen Virol. 2008;89:1300–8. doi: 10.1099/vir.0.83582-0. [DOI] [PubMed] [Google Scholar]