Abstract
Much effort in cancer research has focused on the tiny part of our genome that codes for mRNA. However, it has recently been recognized that microRNAs also contribute decisively to tumorigenesis. Studies have also shown that epigenetic silencing by CpG island hypermethylation of microRNAs with tumor suppressor activities is a common feature of human cancer. The importance of other classes of non-coding RNAs, such as long intergenic ncRNAs (lincRNAs) and transcribed ultraconserved regions (T-UCRs) as altered elements in neoplasia, is also gaining recognition. Thus, we wondered whether there were other ncRNAs undergoing CpG island hypermethylation-associated inactivation in cancer cells. We focused on the small nucleolar RNAs (snoRNAs), a subset of ncRNA with a wide variety of cellular functions, such as chemical modification of RNA, pre-RNA processing and control of alternative splicing. By data mining snoRNA databases and the scientific literature, we selected 49 snoRNAs that had a CpG island within ≤ 2 Kb or that were processed from a host gene with a 5′-CpG island. Bisulfite genomic sequencing of multiple clones in normal colon mucosa and the colorectal cancer cell line HCT-116 showed that 46 snoRNAs were equally methylated in the two samples: completely unmethylated (n = 26) or fully methylated (n = 20). Most interestingly, the host gene-associated 5′-CpG islands of the snoRNAs SNORD123, U70C and ACA59B were hypermethylated in the cancer cells but not in the corresponding normal tissue. CpG island hypermethylation was associated with the transcriptional silencing of the respective snoRNAs. Results of a DNA methylation microarray platform in a comprehensive collection of normal tissues, cancer cell lines and primary malignancies demonstrated that the observed hypermethylation of snoRNAs was a common feature of various tumor types, particularly in leukemias. Overall, our findings indicate the existence of a new subclass of ncRNAs, snoRNAs, that are targeted by epigenetic inactivation in human cancer.
Keywords: DNA Methylation, epigenetics, non-coding RNA, small nucleolar RNAs
Introduction
Coding exons account for only 1.5% of the genome,1 despite being the focus of most of the current biomedical research. A large proportion of the genome is made up of non-protein coding regions that might have critical biological important, containing gene regulatory regions (transcriptional and splicing types), matrix attachment sites, origins of replication, other functional elements and non-coding RNAs (ncRNAs).2,3 The physiological and pathological importance of this functional part of the non-protein-coding genome is particularly apparent in a large class of small non-coding RNAs (ncRNAs) known as microRNAs.4 These are about 22 nucleotides long and repress gene expression in a variety of eukaryotic organisms.4 In human cancer, miRNA expression profiles differ between normal tissues and derived tumors and between tumor types.5 miRNAs can act as oncogenes or tumor suppressors, with a key role in tumorigenesis.6-8 Defects in miRNA function have been associated with a failure of miRNA post-transcriptional regulation,9 miRNA transcriptional repression by oncogenic factors,10 loss-of-function genetic alterations in the genes involved in miRNA-processing pathways11-13 and transcriptional silencing associated with hypermethylation of CpG island promoters.14 Thus, as occurs with miRNAs, it is likely that other types of ncRNAs are also involved in human tumorigenesis and are characterized by epigenetic and genetic defects in this disease.3 In this context, Ultraconserved Regions (UCRs), a subset of conserved sequences that are located in intragenic and intergenic regions,15,16 are altered at the transcriptional level in human tumorigenesis.17 Interestingly, transcribed UCRs (T-UCRs) undergo DNA methylation-associated silencing in cancer cells.18
Another important class of ncRNAs that are potentially altered in human cancer are the small nucleolar RNAs (snoRNAs), which are localized in the nucleolus.19 They are responsible for methylation20,21 and pseudouridylation22,23 of rRNA (rRNA) at about 50–100 sites per eukaryotic ribosome. However there is increasing evidence of the targeting of other classes of RNAs, such as mRNAs.24 snoRNAs are divided into two main classes: box C/D and box H/ACA,25 on the basis of their conserved secondary structural characteristics and associated modification reactions.19,26 The C/D box snoRNAs guide the position-specific 2′-O-methylation and are associated with a core of four proteins: fibrillarin (the methyltransferase), NOP56, NOP5/NOP58 and NHPX. The H/ACA snoRNAs direct RNA pseudouridylation of rRNA and are associated with dyskerin (the pseudouridine synthase), GAR1, NHP2 and NOP10.24,26,27 Mutations in the human dyskerin gene, NHP215 and NOP1016 gene are associated with the X-linked genetic disorder, dyskeratosis congenita (DC), where malfunction of rRNA and shortening of telomeres have been observed.28-30 As mutations in the dyskerin gene have also been associated with cancer susceptibility,28-30 it was suggested that snoRNAs were involved in the onset and progression of cancer. One of the first studies to address this possibility reported a snoRNA to be highly expressed in normal brain, but significantly reduced in meningioma.31 Other studies showed that GAS5, a snoRNA-host gene, controls cell survival by inducing or sensitizing cells to apoptosis.32,33 A substantial decrease of GAS5 in breast cancer samples compared with adjacent unaffected normal breast epithelial tissues also suggests that it has a role as a tumor suppressor gene.33
The association between snoRNAs and cancer was underlined by other studies showing that a homozygous 2-bp (TT) deletion of the snoRNA U50 is strongly associated with prostate cancer34 and that U50 undergoes frequent genomic heterozygous deletion and transcriptional downregulation in breast cancer.35 U50 overexpression reduces colony-forming ability in prostate and breast cancer cells.34,35 Taken together, these studies suggest that non-coding snoRNA U50 is important in the development and progression of breast and prostate cancers.34,35 More recently, it was shown that a diversity of snoRNAs are differentially expressed in non-small-cell lung cancer with respect to the corresponding matched tissue,36 encouraging investigation into the possible role of snoRNAs in oncogenesis. Another study has linked at least one snoRNA to the post-transcriptional processing of a protein-coding gene.37 There is also evidence that other snoRNAs might be involved in the regulation of gene expression by giving rise to other regulatory RNA species, such as miRNAs, linking snoRNAs to RNA silencing.38 It would therefore be very interesting to identify the function and mechanisms of the orphan snoRNAs.
The downregulation of tumor suppressor protein-coding genes (e.g., hMLH1, BRCA1, VHL and p16INK4a)39,40 and ncRNAs with growth inhibitory functions, such as miRNAs14 has been closely linked to the presence of CpG island promoter hypermethylation. Thus, we wondered whether the same mechanism could play a role in the loss of adequate snoRNA expression in tumors. Usually, snoRNAs are genomically found in the introns of protein-coding or non-protein-coding host genes, with each intron carrying only one single snoRNA and their transcription being synchronized with that of the host gene. After splicing they are generally processed by debranching and exonucleolytic trimming of the 5′- and 3′-ends,24,41-43 and assembled with specific core proteins that are essential for their localization and correct enzymatic activity, and for preventing their degradation.27 However, intergenic snoRNAs are independently transcribed by RNA polII as independent units,24 and some human intron-encoded snoRNAs may have their own independent promoters.23 Herein, we present a double candidate and genomic approach to unmask snoRNA-associated CpG islands that undergo cancer-specific hypermethylation-associated transcriptional silencing, such as SNORD123, U70C and ACA59B. These findings support a model in which epigenetic disruption of emerging new classes of ncRNAs, such as snoRNAs, is a common feature of human tumorigenesis.
Results and Discussion
snoRNA CpG island DNA methylation analyses
To identify snoRNAs with putative DNA methylation-related inactivation in human tumors, we data-mined the scientific literature on snoRNAs published during 2000–2011, as made available by PubMed.gov, the human genome browser at UCSC44 and the snoRNA-LBME-db database.24 The CpG Island Searcher Program45 was used to determine which snoRNAs were located within ≤2 Kb of a CpG island, since it has been estimated that more than 90% of the human promoters of another type of ncRNA, the microRNAs, are located 1 Kb upstream of the mature transcript.46,47 The DNA methylation status of CpG islands within 2 Kb are also important for regulating the expression of a second type of ncRNA, the T-UCRs.18 We also included snoRNAs that were processed from a host gene RNA containing a 5′-CpG island. Figure 1 illustrates both categories of snoRNA-related CpG islands. Using the described conditions, we selected 49 snoRNAs that had a CpG island within a distance of ≤ 2Kb (15 intergenic independent snoRNAs and 24 within an intron of a host gene) or that were processed from the transcript of a host gene with a 5′-CpG island (n = 10). The characteristics of the 49 selected snoRNAs and the summarized results are shown in Table 1.

Figure 1. Types of CpG islands associated with snoRNAs in this study. A, Upstream CpG islands of a snoRNA located within 2 Kb. It includes intergenic independent snoRNAs and snoRNAs within an intron of a host gene. B, 5′-CpG islands of host genes for which RNA processing generates the expression of an intronic resident snoRNA.
Table 1. DNA methylation profile of CpG islands associated with snoRNAs. green and red rectangles represent unmethylated and methylated CpG islands, respectively.

We performed bisulfite genomic sequencing of multiple clones using primers encompassing the 49 snoRNA-associated CpG islands to determine the DNA methylation patterns in normal colon mucosa and the colorectal cancer cell line HCT-116. We observed a completely unmethylated status for 23 snoRNA-related CpG islands in normal tissue and colon cancer cells (Table 1). Examples of the bisulfite sequencing analyses are shown in Figure 2. We also found a dense DNA methylation profile for 20 snoRNA-associated CpG islands in normal colon mucosa and HCT-116 colorectal cancer cells (Table 1). Examples of the bisulfite sequencing analyses are shown in Figure 3. Most importantly, we found a cancer-specific hypermethylation event for the snoRNAs SNORD123, U70C and ACA59B. Their associated CpG islands were completely unmethylated in normal colon mucosa and heavily hypermethylated in HCT-116 colorectal cancer cells (Table 1 andFig. 4). For all three cases, the CpG islands studied were in the 5′-region of the host gene where the snoRNA is located: the long non-coding gene LOC100505806 (SNORD123), Astrotactin 2 (U70C) and Solute Carrier Family 47 Member 1 (ACA59B). The DNA methylation results were also confirmed using methylation-specific PCR (Fig. S1).
Figure 2. Bisulfite genomic sequencing of the CpG islands associated with the snoRNAs U98b (host gene PPP2R5A), ACA20/ACA29 (host gene TCP1), U50/U50B (host gene SNHG5) and U32A/U33/U34/U35A (host gene RPL13A) in normal colon and the colorectal cancer cell line HCT-116. CpG dinucleotides are represented as short vertical lines. Eight single clones are represented for each sample. Presence of a methylated or unmethylated cytosine is indicated by a black or white square, respectively. Transcription start sites are represented by vertical black arrows.
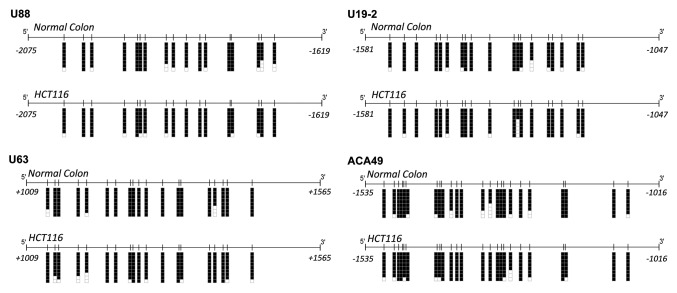
Figure 3. Bisulfite genomic sequencing of the CpG islands associated with the snoRNAs U88, U19–2, U63 and ACA49 in normal colon and the colorectal cancer cell line HCT-116. CpG dinucleotides are represented as short vertical lines. Eight single clones are represented for each sample. Presence of a methylated or unmethylated cytosine is indicated by a black or white square, respectively. Transcription start sites are represented by vertical black arrows.

Figure 4. Bisulfite genomic sequencing of the CpG islands associated with the snoRNAs SNORD123 (host gene is the lncRNA LOC100505806), ACA59B (host gene SLC47A1) and U70C (host gene ASTN2) in normal colon and the colorectal cancer cell line HCT-116. CpG dinucleotides are represented as short vertical lines. Eight single clones are represented for each sample. Presence of a methylated or unmethylated cytosine is indicated by a black or white square, respectively. Transcription start sites are represented by vertical black arrows.
Hypermethylation of snoRNA-related CpG islands is associated with transcriptional silencing
To demonstrate transcriptional silencing of these snoRNAs in cancer cells in association with the presence of CpG island hypermethylation, we measured transcript levels by quantitative RT-PCR. For the SNORD123, we analyzed the expression of the snoRNA itself, the long non-coding RNA LOC100505806 from which the snoRNA is processed and the mRNA of the Semaphorin 5A (SEMA5A) that it is transcribed in the opposite direction from the same CpG island (Fig. 4). No expression of the SNORD123, LOC100505806 and SEMA5A transcripts could be detected in HCT-116 cells in which the shared CpG island was methylated (Fig. 5A). Normal colon mucosa expressed the three transcripts (Fig. 5A). Methylation-specific PCR analyses of two additional colon cancer cell lines identified a hypermethylated and unmethylated CpG island in SW48 and DLD1 cells, respectively (Fig. S1). Loss of the SNORD123, LOC100505806 and SEMA5A transcripts was observed in the hypermethylated SW48 cells and expression of the three transcripts was found in the unmethylated DLD1 cells (Fig. 5A). The absence of the SNORD123 transcript in HCT-116 and SW48 cells and its presence in DLD1 cells was also validated by Northern-blot analyses (Fig. 5B). For the snoRNAs U70C and ACA59B, no expression of the snoRNA U70C, its host gene ASTN2 and the host gene of ACA59B (SLC47A1) could be detected in HCT-116 cells in which the corresponding CpG island was methylated (Fig. S2). Normal colon mucosa expressed the three transcripts (Fig. S2). Most importantly, the expression for ACA59B (SLC47A1) was restored upon treatment with the DNA demethylating agent 5-aza-2´-deoxycytidine in the HCT-116 cell line (Fig. S2). These results were confirmed using an alternative model of an isogenic HCT-116 cell line in which the two major DNA methyltransferases, DNMT1 and DNMT3b, had been genetically disrupted (HCT116 DKO).48 The CpG island for ACA59B (SLC47A1) was hypomethylated in DKO cells (Fig. S2), but hypermethylated in the HCT116 parental cell line. ACA59B (SLC47A1) expression was restored in DKO cells (Fig. S2), reinforcing the link between CpG island hypermethylation and snoRNA silencing.

Figure 5. Expression analyses of of the transcripts derived from the SNORD123 / LOC100505806 / SEMA5A CpG island. A, Quantitative RT-PCR of SNORD123, LOC10050580 and SEMA5A showed loss of expression in the CpG island hypermethylated HCT-116 and SW48 cells. SNORD123 ACA59B and U70C are expressed in the unmethylated DLD1 cancer cells and in normal colon mucosa. B, northern-blot analysis shows the absence of the SNORD1 transcript in the hypermethylated HCT-116 and SW48 cells and its presence in the unmethylated DLD1 cells.
Profile of snoRNA hypermethylation in different tumor types
To address the cancer-specific hypermethylation event in snoRNAs, we ruled out the possible presence of SNORD123, U70C and ACA59B CpG island methylation in a panel of normal tissues from the colon (n = 5), breast (n = 7) and lung (n = 22), in addition to four blood samples from healthy volunteers, using a DNA methylation microarray approach (Fig. 6).49 The observed high-throughput DNA methylation platform included five, two and four CpG sites corresponding to the bisulfite genomic sequenced CpG islands for SNORD123, U70C and ACA59B, respectively (Fig. 6). The presence of SNORD123, U70C and ACA59B cancer-specific CpG island hypermethylation and transcriptional silencing was not a unique feature of the colorectal cancer cell line HCT-116; analyzing the NCI60 panel of human cancer cell lines (n = 60) from nine tumor types, we also found them in other colon cancer cell line and lung, breast, prostate, ovarian, renal, melanoma, lymphoma and leukemia cells (Fig. 7).
Figure 6. SNORD123, U70C and ACA59B CpG island methylation in normal tissues. DNA methylation microarray analyses of five, two and four CpG sites corresponding to the bisulfite genomic sequenced CpG islands for SNORD123, U70C and ACA59B in normal breast, lung and colon tissues and blood samples. Each square represents a single CpG: green square, unmethylated CpG; red square, methylated CpG. The three snoRNA-associated CpG islands were unmethylated in all the normal tissues tested.
Figure 7. SNORD123, U70C and ACA59B CpG island methylation in human cancer cell lines. DNA methylation microarray analyses of five, two and four CpG sites corresponding to the bisulfite genomic sequenced CpG islands for SNORD123, U70C and ACA59B in the NCI60 panel of seven different tumor types. Each square represents a single CpG: green square, unmethylated CpG; red square, methylated CpG. The three snoRNA-associated CpG islands were methylated in the originally studied HCT-116 cells, but hypermethylation events were also observed in other classes of malignancies, such as leukemias.
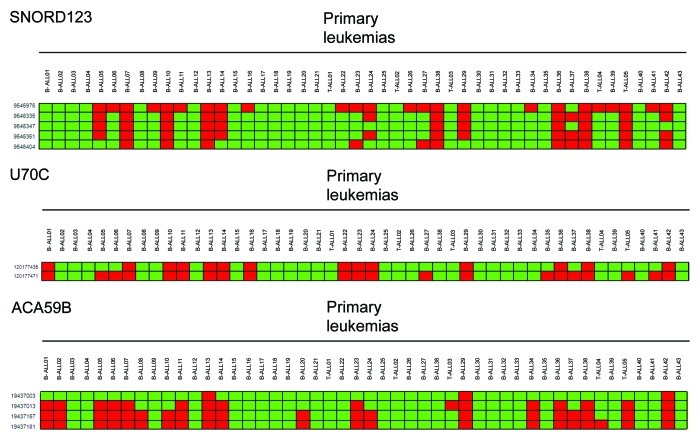
Most importantly, the CpG island hypermethylation of SNORD123, U70C and ACA59B was not an in vitro phenomenon. Having noted the high frequency of CpG island hypermethylation for the three snoRNAs in leukemia cell lines (Fig. 7), we examined the presence of these epigenetic events in 48 primary samples from acute lymphoblastoid leukemia (ALL) patients, 43 had a B-cell phenotype while five were T-ALLs (Fig. 8). Using the described DNA methylation platform, we observed SNORD123, U70C and ACA59B hypermethylation in 27% (13 of 48), 39% (19 of 48) and 29% (14 of 48) of acute lymphoblastoid leukemia cases, respectively (Fig. 8). We did not observe any association between CpG Island hypermethylation of the three studied snoRNAs and disease-free survival or overall survival in these patients (Log rank Mantel-Cox test p > 0.05). We also extended the analyses of snoRNA DNA methylation to primary acute myelogenous leukemia (AML) samples. Among 16 primary acute myelogenous leukemia cases, we observed SNORD123 CpG island hypermethylation in 25% (4 of 16) of samples, while ACA59B and U70C were unmethylated in all cases. Finally, among 20 primary multiple myeloma cases, we observed ACA59B CpG island hypermethylation in 10% (2 of 20) of samples, while SNORD123 and U70C were unmethylated in all cases.
Figure 8. SNORD123, U70C and ACA59B CpG island methylation in primary acute lymphoblastoid leukemias. DNA methylation microarray analyses of five, two and four CpG sites corresponding to the bisulfite genomic sequenced CpG islands for SNORD123, U70C and ACA59B in leukemia patients demonstrated hypermethylation. Each square represents a single CpG: green square, unmethylated CpG; red square, methylated CpG. The B-cell or T-cell phenotype of the studied ALLs is indicated.
Overall, our results reveal the existence of cancer-specific hypermethylation events in CpG islands associated with snoRNAs that lead to their transcriptional inactivation in transformed cells. Despite our increasing knowledge about the biological roles of snoRNAs, one of the main challenges in cancer research into ncRNAs is the identification of a particular function that is relevant for cellular transformation. As coding genes,39,40 microRNAs14 and T-UCRs18 undergoing cancer-specific CpG island hypermethylation-associated silencing are known to have tumor suppressor roles, it is possible that snoRNAs act in a similar manner. This additional level of complexity is really true for the epigenetic silencing of two of the identified snoRNAs, SNORD12350 and ACA59B (also known as SNORA59B),51 because their target RNAs are unknown. ACA59B resides in an intron of the Solute Carrier Family 47 Member 1 (SLC47A1) gene, while SNORD123 is a C/D box snoRNA that resides within a long ncRNA transcript (LOC100505806) while in opposite direction is transcribed from the same CpG island the coding gene SEMA5A, adding another level of complexity in this case. For U70C (also known as SNORA70 or ACA70), the task might be a little easier. U70C was originally cloned from a cervical cancer cell line and belongs to the H/ACA box class of snoRNAs, having the predicted hairpin-hinge-hairpin-tail structure, conserved H/ACA-box motifs, and an association with the GAR1 protein.50,52,53 The snoRNA U70C resides in an intron of the Astrotactin 2 (ASTN2) gene in the sense orientation and it serves as a guide for the pseudouridylation of selected bases of rRNA by forming short duplexes with the 18S rRNA U1692, the target for this snoRNA.50,52,53 A role for 18S rRNA in tumorigenesis is starting to emerge,54-56 and our findings provide additional information about this role.
The enormous task of understanding the mechanisms by which snoRNA epigenetic silencing contributes to the origin and progression of human tumors still lies ahead. In the meantime, our observation that epigenetic inactivation by CpG island hypermethylation of a subset of snoRNAs, such as SNORD123, U70C and ACA59B, occurs across a wide spectrum of human cancer cell lines and primary tumors of diverse cellular and tissue origin provides clear support for the concept that major disruption of ncRNA programming is a common feature of cancer cells.
Materials and Methods
Cell lines, culture conditions and primary study samples
The human cancer cell lines examined in this study were obtained from the American Type Culture Collection (ATCC). HCT-116 and DKO cells were a generous gift from Dr. Bert Vogelstein (Johns Hopkins Kimmel Comprehensive Cancer Center). Cell were maintained in appropriate media and treated with 1 μM 5-aza-2´-deoxycytidine (Sigma) for 48 h to achieve demethylation.18 DNA samples from normal tissues and primary leukemias were obtained at the time of the clinically indicated surgical procedures. All patients gave written consent to participate in the study and the Ethics Committee of each hospital approved the study protocol.
DNA methylation analyses
The CpG Island Searcher Program45 was used to determine which snoRNAs were located within 2 Kb of a CpG island. DNA methylation status was established by PCR analysis of bisulfite-modified genomic DNA, which induces chemical conversion of unmethylated, but not methylated, cytosine to uracil. Two procedures were used. First, methylation status was established by bisulfite genomic sequencing of the corresponding CpG islands. Eight independent clones were analyzed. The second analysis used methylation-specific PCR with primers specific for either the methylated or modified unmethylated DNA. The primers used are described in Table S1.
Quantification of snoRNAs with real-time PCR
Quantitative real-time PCR was performed to quantify the level of snoRNAs, as described previously.18 Briefly, to quantify SEMA5A and the snoRNA-host genes 1 μg of purified and DNase-treated (TURBO DNA-free, Ambion) total RNA was reverse-transcribed using Thermoscript RT and random primer hexamers. cDNA was amplified by real-time PCR using SYBR (Applied Biosystems) green detection. Reverse transcription using a custom-designed TaqMan microRNA Reverse Transcription Kit (Applied Biosystems) was used to quantify the SNORD123 and U70C, providing specificity for the mature RNA target. Reactions were performed in an Applied Biosystems 7900HT Fast Real-Time PCR system in 384-well plates. Expression values of ASTN2, LOC100505806, SLC47A1 and SEMA5A were normalized to HPRT1 and expression values of U70C and SNORD123 to RNU19, respectively. Total RNA was extracted from three independent experiments and real-time PCR reactions were performed in triplicate. The primers used are described in Table S1.
Northern-blot
Fifteen micrograms of total RNA were loaded in a 15% denaturating polyacrylamide gel containing 8 M Urea in 0.5XTBE buffer system. Decade Marker (Ambion) was prepared according to manufacturer’s protocol, using [γ-32P] ATP (PerkinElmer) and simultaneously loaded into the gel. Both RNA and marker were resolved by gel electrophoresis and transferred onto Hybond-N+ membrane (Amersham) in 0.5XTBE, followed by UV-cross linking (1200 Jules). Both SNORD123 and 5.8S rRNA probes were radiolabeled with 25 μCie using T4 kinase (Invitrogen) and purified with Nucaway Spin columns (Ambion). The membrane was prehybridized in hybridization buffer for 1h and hybridized overnight in the same solution at 45°C containing the SNORD123 probe previously heated at 95°C for 2 min. The membrane was washed at low stringency followed by film exposure. The membrane was then hybridized with the 5.8S rRNA probe using the same conditions followed by quantification using phosphorimager technology. All the probes used are described in Table S1.
Infinium 450K DNA methylation array
The DNA methylation levels at 10 CpG sites encompassing the SNORD123-associated CpG island were determined using the Infinium 450K DNA methylation microarray, as previously described.49 Briefly, DNA was quantified by Quant-iT™ PicoGreen dsDNA Reagent (Invitrogen) and the integrity was analyzed in a 1.3% agarose gel. Bisulfite conversion of 600 ng of each sample was done according to the manufacturer’s recommendations for the Illumina Infinium Assay. Effective bisulfite conversion was checked for three controls that were converted simultaneously with the samples. Four µl of bisulfite-converted DNA were used to hybridize on an Infinium HumanMethylation 450 BeadChip, following the Illumina Infinium HD Methylation protocol. The chip was analyzed using an Illumina HiScan SQ fluorescent scanner. The intensities of the images were measured using GenomeStudio (2010.3) Methylation module (1.8.5) software. The methylation score of each CpG is represented as a β value.
Supplementary Material
Acknowledgements
This work was supported by the European Research Council (ERC) Advanced Grant EPINORC, the Ministerio de Ciencia e Innovación (MICINN) grant SAF2011–22803, Fondo de Investigaciones Sanitarias Grant PI08–1345 and the Health Department of the Catalan Government (Generalitat de Catalunya). HF is a Portuguese Foundation for Science and Technology PhD Fellow (SFRH / BD / 33887 / 2009. ME is an ICREA Research Professor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental Material can be found at www.landesbioscience.com/journals/rnabiology/article/19353
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/19353
References
- 1.International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–45. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 2.Mattick JS. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays. 2003;25:930–9. doi: 10.1002/bies.10332. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 4.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Hammond SM. MicroRNAs as tumor suppressors. Nat Genet. 2007;39:582–3. doi: 10.1038/ng0507-582. [DOI] [PubMed] [Google Scholar]
- 8.Melo SA, Esteller M. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 2011;585:2087–99. doi: 10.1016/j.febslet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–7. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet. 2009;41:365–70. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo SA, Moutinho C, Ropero S, Calin GA, Rossi S, Spizzo R, et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell. 2010;18:303–15. doi: 10.1016/j.ccr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Serra P, Esteller M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene. 2011;354:1–14. doi: 10.1038/onc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–5. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 16.Katzman S, Kern AD, Bejerano G, Fewell G, Fulton L, Wilson RK, et al. Human genome ultraconserved elements are ultraselected. Science. 2007;317:915. doi: 10.1126/science.1142430. [DOI] [PubMed] [Google Scholar]
- 17.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–29. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Lujambio A, Portela A, Liz J, Melo SA, Rossi S, Spizzo R, et al. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene. 2010;29:6390–401. doi: 10.1038/onc.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerbi SA. Small nucleolar RNA. Biochem Cell Biol. 1995;73:845–58. doi: 10.1139/o95-092. [DOI] [PubMed] [Google Scholar]
- 20.Cavaillé J, Nicoloso M, Bachellerie JP. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature. 1996;383:732–5. doi: 10.1038/383732a0. [DOI] [PubMed] [Google Scholar]
- 21.Kiss-László Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077–88. doi: 10.1016/S0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- 22.Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/S0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Zhou X, Wang X, Zhu D, Zhang Y. Identification and characterization of human snoRNA core promoters. Genomics. 2010;96:50–6. doi: 10.1016/j.ygeno.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Lestrade L, Weber MJ. snoRNA-LBME-db, a comprehensive database of human H/ACA and C/D box snoRNAs. Nucleic Acids Res. 2006;34(Database issue):D158–62. doi: 10.1093/nar/gkj002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–34. doi: 10.1016/S0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- 26.Bachellerie JP, Cavaillé J, Hüttenhofer A. The expanding snoRNA world. Biochimie. 2002;84:775–90. doi: 10.1016/S0300-9084(02)01402-5. [DOI] [PubMed] [Google Scholar]
- 27.Reichow SL, Hamma T, Ferré-D’Amaré AR, Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35:1452–64. doi: 10.1093/nar/gkl1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–8. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 29.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–29. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang LS, Lin SY, Lieu AS, Wu TL. Differential expression of human 5S snoRNA genes. Biochem Biophys Res Commun. 2002;299:196–200. doi: 10.1016/S0006-291X(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 32.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5) J Cell Sci. 2008;121:939–46. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 33.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 34.Dong XY, Guo P, Boyd J, Sun X, Li Q, Zhou W, et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics. 2009;36:447–54. doi: 10.1016/S1673-8527(08)60134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong XY, Rodriguez C, Guo P, Sun X, Talbot JT, Zhou W, et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet. 2008;17:1031–42. doi: 10.1093/hmg/ddm375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horsthemke B, Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am J Med Genet A. 2008;146A:2041–52. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 37.Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–2. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- 38.Bratkovič T, Rogelj B. Biology and applications of small nucleolar RNAs. Cell Mol Life Sci. 2011;68:3843–51. doi: 10.1007/s00018-011-0762-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–9. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 41.Kiss T, Filipowicz W. Exonucleolytic processing of small nucleolar RNAs from pre-mRNA introns. Genes Dev. 1995;9:1411–24. doi: 10.1101/gad.9.11.1411. [DOI] [PubMed] [Google Scholar]
- 42.Tycowski KT, Shu MD, Steitz JA. A mammalian gene with introns instead of exons generating stable RNA products. Nature. 1996;379:464–6. doi: 10.1038/379464a0. [DOI] [PubMed] [Google Scholar]
- 43.Hoeppner MP, White S, Jeffares DC, Poole AM. Evolutionarily stable association of intronic snoRNAs and microRNAs with their host genes. Genome Biol Evol. 2009;1:420–8. doi: 10.1093/gbe/evp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takai D, Jones PA. The CpG island searcher: a new WWW resource. In Silico Biol. 2003;3:235–40. [PubMed] [Google Scholar]
- 46.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci U S A. 2007;104:17719–24. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Ruan J, Wang G, Zhang W. Characterization and identification of microRNA core promoters in four model species. PLoS Comput Biol. 2007;3:e37. doi: 10.1371/journal.pcbi.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–6. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 49.Sandoval J, Heyn HA, Moran S, Serra-Musach J, Pujana MA, Bibikova M, et al. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics. 2011;6:692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 50.Yang JH, Zhang XC, Huang ZP, Zhou H, Huang MB, Zhang S, et al. snoSeeker: an advanced computational package for screening of guide and orphan snoRNA genes in the human genome. Nucleic Acids Res. 2006;34:5112–23. doi: 10.1093/nar/gkl672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiss AM, Jády BE, Bertrand E, Kiss T. Human box H/ACA pseudouridylation guide RNA machinery. Mol Cell Biol. 2004;24:5797–807. doi: 10.1128/MCB.24.13.5797-5807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Y, Li S. Genome-wide analyses of retrogenes derived from the human box H/ACA snoRNAs. Nucleic Acids Res. 2007;35:559–71. doi: 10.1093/nar/gkl1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber MJ. Mammalian small nucleolar RNAs are mobile genetic elements. PLoS Genet. 2006;2:e205. doi: 10.1371/journal.pgen.0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahon M, Ayllón V, Panov KI, O’Connor R. Ribosomal 18 S RNA processing by the IGF-I-responsive WDR3 protein is integrated with p53 function in cancer cell proliferation. J Biol Chem. 2010;285:18309–18. doi: 10.1074/jbc.M110.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Q, Wu J, Zhang Y, Liu Y, Kong R, Hu L, et al. 1A6/DRIM, a novel t-UTP, activates RNA polymerase I transcription and promotes cell proliferation. PLoS One. 2010;5:e14244. doi: 10.1371/journal.pone.0014244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uemura M, Zheng Q, Koh CM, Nelson WG, Yegnasubramanian S, De Marzo AM. Overexpression of ribosomal RNA in prostate cancer is common but not linked to rDNA promoter hypomethylation. Oncogene. 2011;319:1–10. doi: 10.1038/onc.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.