Abstract
Slow deep breathing improves blood oxygenation (SpO2) and affects hemodynamics in hypoxic patients. We investigated the ventilatory and hemodynamic effects of slow deep breathing in normal subjects at high altitude. We collected data in healthy lowlanders staying either at 4559 m for 2–3 days (Study A; N = 39) or at 5400 m for 12–16 days (Study B; N = 28). Study variables, including SpO2 and systemic and pulmonary arterial pressure, were assessed before, during and after 15 minutes of breathing at 6 breaths/min. At the end of slow breathing, an increase in SpO2 (Study A: from 80.2±7.7% to 89.5±8.2%; Study B: from 81.0±4.2% to 88.6±4.5; both p<0.001) and significant reductions in systemic and pulmonary arterial pressure occurred. This was associated with increased tidal volume and no changes in minute ventilation or pulmonary CO diffusion. Slow deep breathing improves ventilation efficiency for oxygen as shown by blood oxygenation increase, and it reduces systemic and pulmonary blood pressure at high altitude but does not change pulmonary gas diffusion.
Introduction
From the point of view of oxygen gas exchange, human lungs are highly inefficient, as suggested by the 50–60 mmHg PO2 gap between atmosphere and arterial blood observed at sea level. Indeed, some animal species can reach much higher altitudes than humans without supplement O2 due to several reasons including a lower PO2 gap between atmosphere and arterial blood [1]. Moreover, in humans hypoxia is often observed in diseases such as lung and cardiac disease or in specific conditions such as high altitude (HA). Indeed, an acute exposure to HA hypoxia, as during hiking or climbing, is associated with reduced PO2 in alveolar air and in arterial blood. In turn, hypoxemia activates a chemoreflex response leading to increased ventilation, which results in hypocapnia and respiratory alkalosis. Exposure to HA is also associated with pulmonary hypertension and lung fluid accumulation, both of which further contribute to hypoxemia and, in some cases, lead to high altitude pulmonary edema (HAPE) [2], [3].
Efficiency of ventilation for oxygen may be improved by changing the respiratory pattern in order to optimize the partitioning between alveolar ventilation and airway ventilation, being that the latter useless in terms of gas exchange. This has been reported by Yoga practice [4] or by regular breathing as obtained during regular rosary praying [5]. Controlled breathing with low rate and high tidal volume, the so called “slow deep breathing”, has also been shown to improve the efficiency of ventilation by increasing alveolar and reducing dead space ventilation [6]. Slow deep breathing may also improve arterial oxygenation by increasing alveolar volume and gas exchange at the alveolar capillary membrane level. The latter particularly increases when interstitial lung fluids are increased. Indeed, it has been reported that paced slow deep breathing improves blood oxygenation in subjects chronically exposed to HA [7] and in patients with congestive heart failure or with chronic pulmonary obstructive disease [6], [8], [9], [10]. Slow deep breathing might also counteract some hemodynamic effects of hypobaric hypoxia at HA, including the increase in systemic blood pressure [11], given the evidence that device-guided slow deep breathing reduces elevated blood pressure in hypertensive patients [12], [13], [14].
Thus, aim of the present study was to assess whether slow deep breathing improves ventilation efficiency in healthy subjects at HA. Specifically, we evaluated if slow deep breathing increases oxygen saturation during acute and prolonged exposure to HA and the mechanisms behind it, being the former but not the latter associated to an increase of extravascular lung fluids [15], [16].
Methods
The present paper reports the data obtained in two groups of subjects and under different hypoxic conditions, i.e. during acute (Study A) and prolonged (Study B) exposure to HA, within the frame of the HIGH altitude CArdiovascular REsearch (HIGHCARE) project. Studies are registered as EudraCT Number [2010-019986-27] (Study A) and EudraCT [2008-000540-14] (Study B).
Design and Setting
Study A was performed at Capanna Regina Margherita (CRM, 4559 m, Monte Rosa, the Alps). The ascent to CRM from sea level (Milan, 140 m) was divided in two days including an overnight stay at 3672 m. Subjects were brought by car and cable car up to 3200 m, and the remaining part of the ascent was covered by hiking. Overall, the ascent from sea level to CRM took about 24 hours. Study tests were performed on the second and on the third full day of permanence at CRM.
In Study B, participants travelled by air from sea level (Milan, 140 m) to Kathmandu (Nepal, 1355 m) where they stayed for three days. Then they were brought again by air transport to 3400 m (Namche Bazaar), where they stayed for another three days. After that, a 5-day trekking led them to Mt. Everest South Base Camp (5400 m) where they remained for 12 days. Study tests were performed on 5th –9th day of permanence at 5400 m (i.e. after a total of 12–16 days of high altitude exposure).
In both studies, each subject underwent: 1) baseline assessment after at least 15 minutes of rest at a spontaneous breathing rate, 2) 15 minutes of slow deep breathing exercise at a rate of 0.1 Hz (6 breaths per minute) paced by a digital metronome (RESPeRATE, Intercure Ltd, Lod, Israel), and 3) recovery (5 minutes – Study A, 30 minutes – Study B) at a spontaneous breathing rate. The study sessions were not preceded by food intake or strenuous exercise, and data collection was performed in sitting position and in conditions of controlled ambient temperature.
The RESPeRATE device used for breath pacing includes a battery-operated hand-held computerized box attached to headphones and to a belt-type respiration sensor. Breathing is guided by a 2-tone melody heard by the subject, who is asked to inhale when a high tone is heard and exhale when a low tone is heard. Breathing rate reduction is mainly obtained by prolonging the expiration phase so that the ratio between inspiration and expiration duration is about 1∶2. In its normal operation mode, the device provides a gradual reduction of breathing rate; however, for the purposes of this study, it was used in a fixed frequency mode at 6 breaths per minute. Before starting the study procedures, the subjects were briefly acquainted with the device. The appropriate performance of the slow breathing was constantly monitored.
Subjects
We enrolled healthy volunteers of both sexes, living permanently at less than 500 m. Subjects were sought among the personnel of the organizing institute and through personal contacts and identified based on their availability for the high-altitude expedition, on their willingness to undergo the testing, and on the physical ability to perform the ascent to high altitude location. Exclusion criteria were: known cardiovascular disease, repeated exposures to altitudes >3000 m in the 8 months preceding the expedition, history of severe mountain sickness, and pregnancy. Professional athletes or climbers were also not included in the study.
The study protocols were approved by the Ethics Committee of Istituto Auxologico Italiano. All subjects gave their written informed consent to participate in the study. During the ascent to high altitude and during the subsequent descent, subjects were assisted by professional Alpine climbers and guides.
Measurements
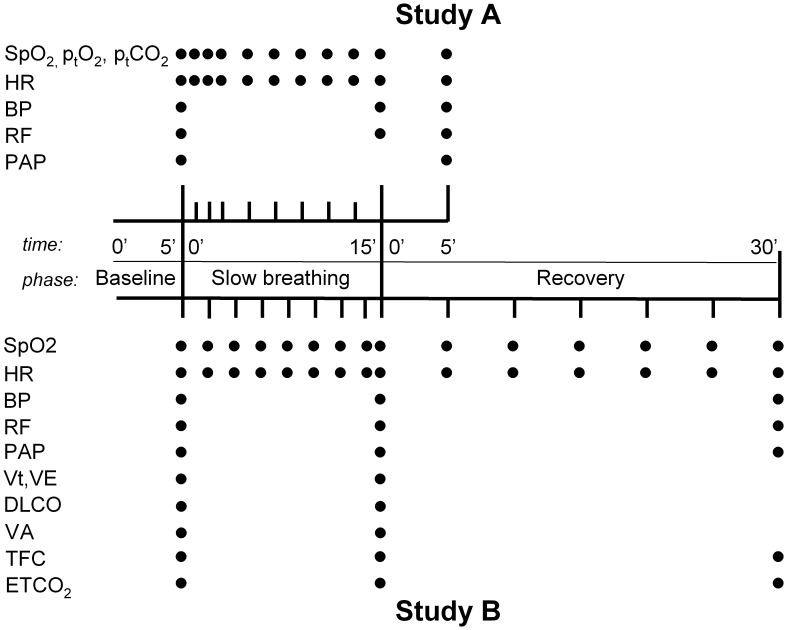
The primary variable of interest in this study was blood oxygen saturation (SpO2), while the secondary variables included arterial blood pressure (BP), heart rate (HR), respiratory frequency (RF) and systolic pulmonary artery pressure (PAP). Additional parameters that were assessed included transcutaneous measurement of PO2 (PtO2) and of PCO2 (PtCO2) in Study A, and end tidal CO2 level in the exhaled air (Pet CO2), thoracic fluid content (TFC) by impedance cardiography, tidal volume (Vt), minute ventilation (VE), alveolar volume (VA), and pulmonary CO diffusion (Dl CO) in Study B. The timing of measurements in both studies is reported in Figure 1.
Figure 1. Schematic representation of the sequence of data collection in studies A and B.
SpO2, blood oxygen saturation; PtO2, transcutaneous oxygen partial pressure; PtCO2, transcutaneous CO2 partial pressure; HR, heart rate; BP, blood pressure; RF, respiratory frequency; PAP, pulmonary artery pressure; Vt, tidal volume; VE, minute ventilation; Dl CO, pulmonary CO diffusion; VA = alveolar volume; TFC, thoracic fluid content; Pet CO2, end tidal CO2 pressure in the exhaled air.
SpO2 was measured by pulse oximetry (Life Scope I, Nihon Kohden, Tokyo, Japan (Study A) and Tuffsat, GE Healthcare, Milwaukee, WI, USA (Study B)). Resting BP was measured in sitting position according to European Society of Hypertension guidelines (O’Brien et al., 2003) with a validated oscillometric device (OMRON M5-I, Omron, Tokyo, Japan in Study A and Microlife A100Plus Microlife, Windau, Switzerland in Study B), and HR was obtained from a pulse oximeter. Pulse pressure (PP) was calculated as systolic (S) minus diastolic (D) BP. Echocardiography was performed with a Vivid I device (GE Medical Systems, Milwaukee, WI, USA), and pulmonary artery pressure (PAP) was calculated by the analysis of tricuspid regurgitation provided by the continuous Doppler signal (assuming a right atrial pressure of 5 mmHg). Transcutaneous PO2 and PCO2 were continuously measured with TCM4 device (Radiometer, Copenhagen, Denmark) after obtaining stable values with the probe placed on the forearm. Impedance cardiography was performed for 5 minutes in supine position with NICCOMO Cardioscreen (Medis GmbH, Ilmenau, Germany). RF was assessed by counting breaths over 1 minute. Vt, VE, VA and Dl CO were assessed with Sensor Medics 2200 device (Yorba Linda, CA, USA). VA and Dl CO were measured with the single breath – constant expiratory flow technique and calculated according to current guidelines (American Thoracic Society, 1995). Tracer gas for VA measurements was CH4. Dl CO was corrected for hemoglobin and then for differences in inspired O2 (Pi O2), due to differences in barometric pressure between sea level and altitude, and concentration according to the formulas: Dl CO measured x (10.15+ haemoglobin)/1.7×haemoglobin [17] and: Dl CO measured Hb corrected x (1.0+0.0031(Pi O2–150)) [18], respectively. Pet CO2 was measured with Microcap Plus capnograph (Oridion Capnography Inc., Needham, MA, USA).
Sample Size
Both studies were performed within the frame of larger research projects, and therefore the overall sample size was not determined based on the requirements of slow breathing substudies. However, assuming a SD of SpO2 change of 7%, 19 subjects were needed in order to detect a 5% SpO2 change with 90% power and alpha = 0.05. This number was exceeded in both studies.
Data Analysis
Statistica 8.0 (StatSoft, Tulsa, USA) software was used for statistical analysis. Means ± standard deviations (SD) were used for descriptive statistics of continuous variables. Repeated measures analysis of variance was used to assess the changes in the study variables in different phases of the protocol. Bonferroni correction was used for post-hoc comparisons, except for comparisons of SpO2, PtO2 and PtCO2 values obtained at multiple time points during slow breathing and recovery, where, because of the large number of comparisons involved, two-sided Dunnet test was performed, taking the corresponding baseline values as the reference. Pearson’s correlation coefficients were computed to identify the determinants of the size of SpO2 change during slow breathing versus baseline and their relative significance was assessed in multiple linear regression models. Pearson’s chi-square test was used to compare gender composition between subgroups. Throughout the study, a p<0.05 was taken as the level of statistical significance.
Results
Thirty male and nine female subjects (77% and 23%) aged 24–61 years (mean 38.8±11.0) with a mean body mass index (BMI) of 22.9±2.8 kg/m2 were included in study A. The population of Study B (n = 28) was very similar to that of Study A (age 38.9±10.6, BMI 22.9±2.8) except for a higher proportion of female subjects [16 (57%) males and 12 (43%) females].
Changes in Ventilatory Variables
At sea level, all subjects were normotensive and their SpO2 was invariably >95%. At HA, all subjects achieved the required respiratory frequency during device-guided slow breathing exercise.
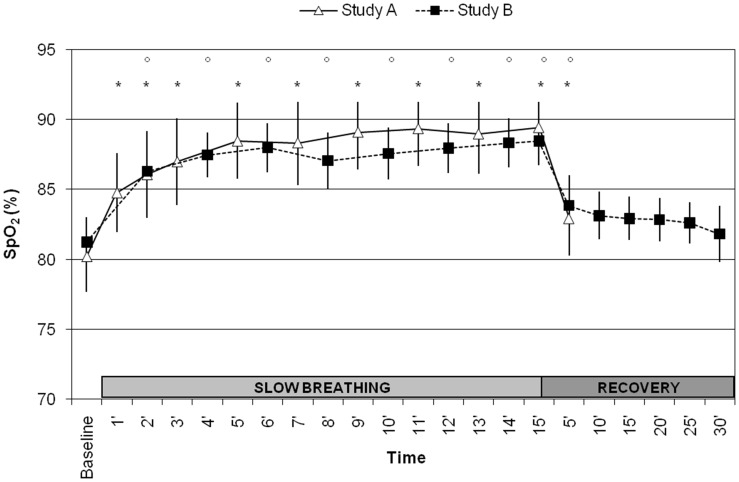
In both studies, slow breathing exercise at HA was associated with a significant increase in SpO2 from baseline value (from 80.2±7.7% to 89.5±8.2% in Study A and from 81.0±4.2% to 88.6±4.5 in Study B, both p<0.001) (Figure 2). A significant increase in PtO2 and a significant reduction in PtCO2 occurred during slow deep breathing (Study A, Table 1), together with a proportional increase in tidal volume and, consequently, with no change in minute ventilation (Study B). No significant changes were induced by slow deep breathing in VA and in Dl CO (Study B). During the recovery phase, all variables returned to baseline values, with the exception of blood oxygenation (SpO2 and PtO2), which remained somewhat higher after 5 minutes of recovery compared with baseline (Table 1, Figure 2).
Figure 2. Changes in SpO2 (upper panel), PtO2 (middle panel) and PtCO2 (lower panel) during slow breathing exercise and recovery.
Differences vs. baseline with p<0.05 are identified with * (Study A) or ° (Study B) symbols. Vertical bars denote 95% CI of the mean.
Table 1. Study variables assessed at high altitude in baseline condition, after 15 minutes of slow breathing exercise and after 5 and 30 minutes of recovery.
| Variable | Study | N | Baseline | Slow breathing (15′) | Recovery | p (overall) | |
| 5′ | 30′ | ||||||
| SpO2 (%) | A | 39 | 80.2±7.7 | 89.5±8.2 *** | 82.9±8.1 *††† | – | <0.001 |
| B | 28 | 81.0±4.2 | 88.6±4.5 *** | 83.8±5.8 *††† | 81.7±5.4 ††† | <0.001 | |
| PtO2 (mmHg) | A | 37 | 42.8±7.6 | 56.9±11.4 *** | 47.6±11.3 **††† | – | <0.001 |
| B | – | – | – | – | – | – | |
| PtCO2 (mmHg) | A | 39 | 26.4±4.0 | 22.5±5.9 *** | 25.8±4.8 ††† | – | <0.001 |
| B | – | – | – | – | – | – | |
| Et CO2 (mmHg) | A | – | – | – | – | – | – |
| B | 28 | 21.4±3.9 | 20.1±4.5 | – | 21.2±3.6 | 0.15 | |
| SBP (mmHg) | A | 39 | 126.0±14.5 | 118.5±16.0 *** | 122.7±16.6 *†† | – | <0.001 |
| B | 28 | 125.2±15.4 | 115.0±14.5 *** | – | 127.9±17.0 ††† | <0.001 | |
| DBP (mmHg) | A | 39 | 75.0±15.2 | 68.7±14.1 *** | 69.8±15.1 *** | – | <0.001 |
| B | 28 | 79.4±9.5 | 78.3±9.5 | – | 83.5±9.2 *†† | <0.001 | |
| PP (mmHg) | A | 39 | 51.0±13.8 | 49.8±13.4 | 52.9±12.6 † | – | 0.052 |
| B | 28 | 45.8±11.4 | 36.7±10.6 *** | – | 44.4±13.1 ††† | <0.001 | |
| HR (bpm) | A | 39 | 81.6±11.4 | 79.3±11.3 | 81.2±12.0 | – | 0.27 |
| B | 28 | 87.6±14.8 | 83.1±12.3 | 84.5±13.3 | 84.1±16.8 | 0.09 | |
| sPAP (mmHg) | A | 9 | 33.7±4.5 | – | 28.7±5.0 ** | – | 0.008 |
| B | 28 | 33.1±10.9 | 29.2±10.3 *** | – | 31.5±10.4 | 0.001 | |
| RF (1/min) | A | 39 | 17.6±5.3 | 6.0±0.1 *** | 17.3±6.2 ††† | – | <0.001 |
| B | 28 | 16.0±3.8 | 6.0±0.2 *** | – | 14.1±4.7 ††† | <0.001 | |
| Vt (l) | A | – | – | – | – | – | – |
| B | 18 | 1.5±0.6 | 3.0±0.7 *** | – | – | <0.001 | |
| VE (l) | A | – | – | – | – | – | – |
| B | 17 | 16.9±6.8 | 17.6±3.8 | – | – | 0.66 | |
| Dl CO (ml/min/mmHg) | A | – | – | – | – | – | – |
| B | 17 | 30.0±6.6 | 34.7±7.1 | – | – | 0.27 | |
| VA (l) | A | – | – | – | – | – | – |
| B | 17 | 6.65±0.85 | 6.71±0.91 | 0.59 | |||
| TFC (ml/kg) | A | – | – | – | – | – | – |
| B | 24 | 36.1±6.4 | 36.0±6.0 | – | 34.9±6.8 | 0.25 | |
Data are separately shown for Study A and B.
-p<0.05, **-p<0.01, ***, p<0.001 vs. baseline; † - p<0.05, †† - p<0.01, †††, p<0.001 vs. slow breathing.
SpO2, blood oxygen saturation; ptO2– transcutaneous oxygen partial pressure; ptCO2– transcutaneous CO2 partial pressure; Et CO2– end tidal CO2 pressure in the exhaled air; SBP – systolic blood pressure; DBP – diastolic blood pressure; PP – pulse pressure; HR – heart rate; sPAP – systolic pulmonary artery pressure; RF – respiratory frequency; Vt – tidal volume; VE – minute ventilation; VA – alveolar volume; Dl CO - pulmonary CO diffusion; TFC – thoracic fluid content.
Changes in Hemodynamic Variables
A significant reduction in SBP was found in both studies at the end of the slow breathing session, with SBP values which remained lower than at baseline after 5 minutes (Study A) but not after 30 minutes (Study B) of recovery. The corresponding changes in DBP and PP are shown in Table 1. HR tended to decrease during slow deep breathing, but the changes were not significant. Pulmonary artery pressure was significantly reduced at the end of slow deep breathing exercise (Study B) and after 5 minutes of recovery (Study A). There were no significant changes in thoracic fluid content as quantified by impedance cardiography in Study B (Table 1).
Predictors of Response to Slow Breathing Exercise
In Study A and B, the only variable which significantly predicted the increase of SpO2 induced by slow deep breathing was the SpO2 baseline value itself. This relationship was inverse in nature (Study A: r = −0.38, p = 0.017, Study B: r = −0.48, p = 0.010), i.e. a lower baseline SpO2 predicted a larger increase in SpO2 with the slow breathing exercise.
Discussion
Our paper offers for the first time information on the respiratory and hemodynamic effects of device-guided slow deep breathing in healthy lowlanders exposed to HA. Our main result is that in healthy subjects exposed to HA, i.e. to a low ambient-air PO2, the change in breathing pattern from a spontaneous rate to a paced frequency of 6 breaths per minute was associated with an improvement of ventilation efficiency, as shown by the significant increase in blood oxygen saturation. This was the case both for acute (Study A) and prolonged (Study B) exposure to HA hypoxia. This increase occurred rapidly and was maintained throughout the slow deep breathing period. Most of the improvement of blood oxygenation was lost within 5 minutes after restoration of spontaneous breathing pattern, and no differences compared with baseline were evident after 30 minutes. Our study extends the previous reports on the benefits of slow deep breathing intervention in other hypoxic conditions, such as in patients with chronic pulmonary obstructive disease [10], in hypoxic patients with heart failure [6], and in subjects living permanently at HA [7]. In all these studies, the observed changes were less pronounced than in our paper, a discrepancy which is explained by the higher baseline SpO2 values they reported (about 90%) as compared to the conditions of our studies (about 80%).
In the present study, we showed for the first time the time course of the response to slow deep breathing, showing that the maximum effect is reached after about 5 minutes and is subsequently maintained. Moreover, we reported for the first time data on the recovery period. In Study B, we extended the recovery period to 30 minutes, which allowed us to observe a progressive reduction of slow deep breathing effects, which are at their highest after 5 minutes, but some continue up to 30 minutes after its termination.
Baseline SpO2 was similar in both studies despite the difference in altitudes of about 900 m. This may be explained by the fact that, at the same altitude, locations closer to the equator (like Nepal) are characterized by a somewhat higher atmospheric pressure, compared with locations at higher latitudes (like the Alps). Another possible explanation is the longer acclimatization time in Study B, associated with lower lung fluid content than in Study A. Indeed, prolonged residence at HA is associated with a progressive reduction in the initially increased interstitial lung liquid content, and with an improvement in alveolo-capillary gas exchange [15], [16]. Interestingly, slow deep breathing induced similar SpO2 changes both under acute and prolonged exposure to HA regardless of lung fluid content (Figure 2). Thus, the effects of slow deep breathing on SpO2 are likely to be independent from lung fluid content.
Our study provides some information on the possible mechanisms responsible for SpO2 increase during slow deep breathing. In particular, ventilatory variables assessed in Study B indicate that this increase is not due to changes in minute ventilation during slow deep breathing, and indeed, the reduced respiratory rate is compensated by a proportionally increased tidal volume, but total ventilation is the same. However, the reduction of PtCO2 during slow deep breathing exercise in Study A and the SpO2 increase in both studies suggest that slow deep breathing improves the efficiency of ventilation. The lack of reduction of Pet CO2 in Study B (table 1) is not in contrast with this interpretation of our findings but merely a technical consequence of the measurement technique. Indeed, Pet CO2 pressure, due to the shape of the CO2 curve during expiration, is higher with lower respiratory frequency. Therefore, a reduction in PaCO2 may actually have occurred during slow deep breathing in both studies. However, several other mechanisms may be hypothesized to participate in explaining our findings. First of all, the increased tidal volume might have mechanically modified the characteristics of the alveolar wall, thus facilitating gas exchange in a condition such as HA, where some degree of interstitial pulmonary edema is likely [3], [19]. Indeed, deep tidal volumes may lead to increase of fluid clearance by lymphatics [20] and to increase of venous return and, therefore, cardiac output. However, the latter does not seem to be a major component at least under prolonged exposure to HA (Study B), since slow deep breathing induced no significant change in lung CO diffusion (Dl CO), which is cardiac-output dependent, nor in thoracic fluid content as assessed by impedance cardiography. Besides, the increase in tidal volume [21] occurring with slow deep breathing may lead to alveolar recruitment and thus to a net increase in the surface available for gas exchange. However, anatomical VA was unchanged, but we measured with VA the total VA which is not the alveolar space actually used during spontaneous or paced breathing. We are likely to have increased the used alveolar volume with slow deep breathing and, consequently, we have reduced dead space minute ventilation and the dead space to tidal volume ratio to a percentage, as previously reported for heart failure patients during slow deep breathing exercise [7]. Furthermore, the transcutaneous PO2 and PCO2 values (table 1), if used as surrogates for arterial values in the conventional alveolar gas equation, show a reduction in the Alveolar-arterial PO2 difference consistent with improvements in ventilation-perfusion mismatch. In addition, pulmonary arterial pressure was reduced by slow deep breathing in both studies, as in congestive heart failure patients, suggesting pulmonary perfusion improvement [9]. Moreover, because slow deep breathing is associated to a reduction of sympathetic tone (see below), the improvement of ventilation/perfusion matching may also originate by more respiratory sinus arrhythmia [22]. Finally, the reduction of sympathetic tone could lead to a reduction in metabolic rate, which, possibly combined with an increase of cardiac output, may lead to an increase of mixed venous PO2 and thus less admixture. All together, our data suggest that the benefits from slow deep breathing exercise are due to an improvement in ventilation mechanics, in pulmonary perfusion and in ventilation/perfusion matching, and possibly to a reduction of the metabolic rate.
Another interesting result of our study is that slow deep breathing affected not only pulmonary, but also systemic hemodynamics. As shown in a recent paper by our group [11], exposure to HA is associated with an increase in arterial blood pressure over 24 hours, likely due to the elevated sympathetic drive in this condition, related to the chemoreflex response to hypoxia. Slow deep breathing induced a reduction in blood pressure (mainly in SBP) at HA, which partly persisted early in the recovery phase (Study A) and was no longer present after 30 minutes of recovery (Study B). This acute blood pressure lowering effect of slow deep breathing may be related to the ability of this manoeuvre to increase baroreflex and reduce chemoreflex sensitivity [8], [23], resulting in a sympathetic inhibitory action, as recently directly shown by Oneda et al. [24]. The blood pressure reduction observed in our study is in line with data obtained in previous studies that proposed regular and repeated performance of slow deep breathing exercise at sea level as a nonpharmacological approach to the treatment of hypertension [12], [13], [14]. These studies have also emphasized that this effect may originate from an enhanced sensitivity of the baroreflex and/or a reduced sensitivity of the chemoreflex [4], [23].
Our study has a few limitations, which need to be acknowledged. Firstly, based on previous studies [13], [14], [23], [25], the duration of the slow deep breathing manoeuvre was 15 minutes, and therefore the effects of a more prolonged or repeated slow deep breathing at HA remain unexplored. Secondly, none of our subjects had HAPE, and therefore we were not able to directly test the efficacy of slow deep breathing in such condition. Thirdly, due to logistic limitations, we were not able to directly assess alveolar ventilation and dead space ventilation, but such information can be extrapolated from the present study data and from a previous report in hypoxic patients with heart failure [6].
In conclusion, slow deep breathing induced a significant improvement in ventilation efficiency as shown by SpO2 increase in healthy subjects exposed to HA. This improvement was most likely due to a reduction of dead space ventilation and an increase in alveolar ventilation, and was associated to a reduction of both pulmonary and systemic BP levels, both elevated at HA. This intervention is easy and cheap. The usefulness of slow deep breathing should however be tested in large scale studies on hypoxemic patients and at HA in subjects with HAPE.
Acknowledgments
We express our gratitude towards: Club Alpino Italiano (C.A.I.), division of Varallo Sesia, the Capanna Regina Margherita staff, and the Alpine Guides of Varallo Sesia for their valuable organizational support; Dr Luca Grappiolo for the careful administrative management of the project; Dr Licia Pietrobon and Dr Ellen Tosazzi for their effective secretarial support.
We also wish to acknowledge the contribution to these studies given by our late colleague Dr Giulio Savia.
We also thank the other members of the HIGHCARE group: Manuela Bartesaghi (Istituto Auxologico Italiano, Milano), Giovanna Branzi (Istituto Auxologico Italiano, Milano), Gianluca Caldara (Università di Milano-Bicocca & Istituto Auxologico Italiano, Milano), Andrea Cappugi (Centro di Bioclimatologia - Università di Firenze), Paolo Castiglioni (Polo Tecnologico, Fondazione Don Gnocchi, Milano), Francesca Contini (Istituto Auxologico Italiano, Milano), Marco Di Rienzo (Polo Tecnologico, Fondazione Don Gnocchi, Milano), Alessia Giglio (Università di Milano-Bicocca & Istituto Auxologico Italiano, Milano), Francesca Gregorini (Università di Milano-Bicocca & Istituto Auxologico Italiano, Milano), Elena Kashina (Center for Cardiovascular Research (CCR), Institute of Pharmacology Charité - Universitätsmedizin Berlin, Germany), Fulvio Magni (Università Milano Bicocca, Milano), Veronica Mainini (Università Milano Bicocca, Milano), Gabriella Malfatto (Istituto Auxologico Italiano, Milano), Paolo Mazzoleni (Polo Tecnologico, Fondazione Don Gnocchi, Milano)
Paolo Meriggi (Polo Tecnologico, Fondazione Don Gnocchi, Milano), Pietro Amedeo Modesti (Clinica Medica e Cardiologia – Università di Firenze), Marco Morabito (Centro di Bioclimatologia - Università di Firenze), Barbara Poletti (Istituto Auxologico Italiano, Università degli Studi di Milano)
Alberto Piperno (Università di Milano-Bicocca, Milano), Stefano Rapi (Clinica Medica e Cardiologia – Università di Firenze), Paolo Salvi (University of Nancy, France), Giulio Savia (Istituto Auxologico Italiano, Piancavallo), Margherita Tamplenizza (Università Milano Bicocca), Mariaconsuelo Valentini (Università di Milano-Bicocca & Istituto Auxologico Italiano, Milano).
Funding Statement
Study A was supported by institutional funds of Istituto Auxologico Italiano, while Study B was supported with an unrestricted grant from Boehringer-Ingelheim Germany and Banca Intesa San Paolo, Milan, Italy. Sapio Life Italy s.r.l., GE Healthcare, and Intercure Ltd. supplied the devices necessary for this study free of charge. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Piiper J, Scheid P (1992) Modeling of gas exchange in vertebrate lungs, gills, and skin. In: Marcel Dekker I, editor. Physiological adaptation in vertebrates Respiration, circulation, and metabolism. New york.
- 2. Vock P, Fretz C, Franciolli M, Bartsch P (1989) High-altitude pulmonary edema: findings at high-altitude chest radiography and physical examination. Radiology 170: 661–666. [DOI] [PubMed] [Google Scholar]
- 3. Maggiorini M, Leon-Velarde F (2003) High-altitude pulmonary hypertension: a pathophysiological entity to different diseases. Eur Respir J 22: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 4. Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L (2000) Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet 356: 1495–1496. [DOI] [PubMed] [Google Scholar]
- 5. Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, et al. (2001) Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ 323: 1446–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernardi L, Spadacini G, Bellwon J, Hajric R, Roskamm H, et al. (1998) Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 351: 1308–1311. [DOI] [PubMed] [Google Scholar]
- 7. Keyl C, Schneider A, Gamboa A, Spicuzza L, Casiraghi N, et al. (2003) Autonomic cardiovascular function in high-altitude Andean natives with chronic mountain sickness. J Appl Physiol 94: 213–219. [DOI] [PubMed] [Google Scholar]
- 8. Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, et al. (2002) Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 105: 143–145. [DOI] [PubMed] [Google Scholar]
- 9. Parati G, Malfatto G, Boarin S, Branzi G, Caldara G, et al. (2008) Device-guided paced breathing in the home setting: effects on exercise capacity, pulmonary and ventricular function in patients with chronic heart failure: a pilot study. Circ Heart Fail 1: 178–183. [DOI] [PubMed] [Google Scholar]
- 10. Raupach T, Bahr F, Herrmann P, Luethje L, Heusser K, et al. (2008) Slow breathing reduces sympathoexcitation in COPD. Eur Respir J 32: 387–392. [DOI] [PubMed] [Google Scholar]
- 11. Bilo G, Caldara G, Styczkiewicz K, Revera M, Lombardi C, et al. (2011) Effects of selective and nonselective beta-blockade on 24-h ambulatory blood pressure under hypobaric hypoxia at altitude. J Hypertens 29: 380–387. [DOI] [PubMed] [Google Scholar]
- 12. Meles E, Giannattasio C, Failla M, Gentile G, Capra A, et al. (2004) Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens 17: 370–374. [DOI] [PubMed] [Google Scholar]
- 13. Parati G, Carretta R (2007) Device-guided slow breathing as a non-pharmacological approach to antihypertensive treatment: efficacy, problems and perspectives. J Hypertens 25: 57–61. [DOI] [PubMed] [Google Scholar]
- 14. Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, et al. (2001) Treating hypertension with a device that slows and regularises breathing: a randomised, double-blind controlled study. J Hum Hypertens 15: 271–278. [DOI] [PubMed] [Google Scholar]
- 15. Agostoni P, Caldara G, Bussotti M, Revera M, Valentini M, et al. (2010) Continuous positive airway pressure increases haemoglobin O2 saturation after acute but not prolonged altitude exposure. Eur Heart J 31: 457–463. [DOI] [PubMed] [Google Scholar]
- 16. Agostoni P, Swenson ER, Bussotti M, Revera M, Meriggi P, et al. (2011) High-altitude exposure of three weeks duration increases lung diffusing capacity in humans. J Appl Physiol 110: 1564–1571. [DOI] [PubMed] [Google Scholar]
- 17. Cotes JE, Dabbs JM, Elwood PC, Hall AM, McDonald A, et al. (1972) Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusiong capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci 42: 325–335. [DOI] [PubMed] [Google Scholar]
- 18. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, et al. (2005) Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735. [DOI] [PubMed] [Google Scholar]
- 19. Anand IS, Malhotra RM, Chandrashekhar Y, Bali HK, Chauhan SS, et al. (1990) Adult subacute mountain sickness–a syndrome of congestive heart failure in man at very high altitude. Lancet 335: 561–565. [DOI] [PubMed] [Google Scholar]
- 20. Staub NC (1974) Pulmonary edema. Physiol Rev 54: 678–811. [DOI] [PubMed] [Google Scholar]
- 21. Wasserman K (1978) Breathing during exercise. N Engl J Med 298: 780–785. [DOI] [PubMed] [Google Scholar]
- 22. Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T (1996) Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation 94: 842–847. [DOI] [PubMed] [Google Scholar]
- 23. Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, et al. (2005) Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46: 714–718. [DOI] [PubMed] [Google Scholar]
- 24. Oneda B, Ortega KC, Gusmao JL, Araujo TG, Mion D Jr (2010) Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens Res 33: 708–712. [DOI] [PubMed] [Google Scholar]
- 25. Rosenthal T, Alter A, Peleg E, Gavish B (2001) Device-guided breathing exercises reduce blood pressure: ambulatory and home measurements. Am J Hypertens 14: 74–76. [DOI] [PubMed] [Google Scholar]