Abstract
Early nodulin 2 (ENOD2) transcripts and protein are specifically found in the inner cortex of legume nodules, a location that coincides with the site of a barrier to O2 diffusion. The extracellular glycoprotein that binds the monoclonal antibody MAC236 has also been localized to this site. Thus, it has been proposed that these proteins function in the regulation of nodule permeability to O2 diffusion. It would then be expected that the levels of ENOD2 mRNA/protein and MAC236 antigen would differ in nodules with different permeabilities to O2. We examined the expression of ENOD2 and other nodule-expressed genes in Rhizobium meliloti-induced alfalfa nodules grown under 8, 20, or 50% O2. Although there was a change in the amount of MAC236 glycoprotein, the levels of ENOD2 mRNA and protein did not differ significantly among nodules grown at the different [O2], suggesting that neither ENOD2 transcription nor synthesis is involved in the long-term regulation of nodule permeability. Moreover, although nodules from all treatments reduced their permeability to O2 as the partial pressure of O2 (pO2) was increased to 100%, the levels of extractable ENOD2 and MAC236 proteins did not differ from those measured at the growth pO2, further suggesting that if these proteins are involved in a short-term regulation of the diffusion barrier, they must be involved in a way that does not require increased transcription or protein synthesis.
Symbiotic N2 fixation is dependent upon nitrogenase, a very O2-labile enzyme. To protect nitrogenase from inactivation by O2, the [O2] in the infected cells of functional legume nodules is maintained at a very low level. Through leghemoglobin oximetry, infected cell [O2] has been measured at 30 to 50 nm in active N2-fixing nodules (Kuzma et al., 1993), whereas the [O2] in soil water in equilibrium with the atmosphere is about 260 μm. The low [O2] in the infected cells is maintained by a combination of a high rate of bacteroid and mitochondrial respiration as well as a barrier to O2 diffusion thought to be located in the nodule inner cortex (also known as nodule parenchyma; van de Wiel, 1990b) (for review, see Hunt and Layzell, 1993). We will use the terms “nodule inner cortex” and “nodule parenchyma” interchangeably in this paper.
The nodule diffusion barrier was first measured directly by Tjepkema and Yocum (1974), who inserted an O2 microelectrode into soybean nodules and found that the [O2] dropped sharply near the region of the common endodermis. O2 electrodes have since been used to confirm the presence of a barrier to O2 diffusion near the interface between the nodule cortex and central infected zone in pea, French bean, and alfalfa nodules (Witty et al., 1987; Masepohl et al., 1993). This barrier consists of layers of parenchyma cells, some of which (i.e. the boundary layer), have radially aligned cell walls and very few, small intercellular spaces (Parsons and Day, 1990). Because the diffusion coefficient of O2 in air is about 10,000 times greater than in water, and because O2 solubility in water is only 0.03 of that in air, models of gas exchange in nodules predict that a water-filled barrier of about the same thickness as the boundary layer would be sufficient to produce the observed diffusion barrier (Sinclair and Goudriaan, 1981; Hunt et al., 1988).
Nodule permeability to O2 diffusion is controlled by short-term physiological mechanisms and long-term developmental adaptations (Hunt and Layzell, 1993). The latter include changes in nodule anatomy as well as changes in the types or abundance of proteins synthesized in the region functioning as the nodule diffusion barrier. When legume roots are grown at subambient pO2, intercellular spaces in the nodule inner cortex are larger and more abundant. Also, this zone increases or decreases in cell number in proportion to the rhizosphere pO2 present during growth in many plants (Dakora and Atkins, 1989, 1990a, 1990b, 1990c, 1991; Parsons and Day, 1990; James et al., 1991; Arrese-Igor et al., 1993; Atkins et al., 1993).
Short-term physiological regulation of nodule permeability to O2 diffusion occurs within a few minutes following changes in rhizosphere pO2 (King et al., 1988) or other physiological treatments (Hunt and Layzell, 1993). Such a rapid adjustment cannot be accounted for by structural changes or by changes in gene expression. The mechanism responsible for short-term diffusion control remains a topic of conjecture (Thumfort et al., 1994).
We are interested in the molecular mechanisms underlying the establishment of the diffusion barrier and its ability to adjust during development to external pO2. At least three proteins have been localized specifically to the nodule inner cortex, which suggests that they may play a role in the regulation of the diffusion barrier. One of these proteins is a glycoprotein that has been identified by its reaction to the monoclonal antibody MAC236 and related antibodies (VandenBosch et al., 1989). The MAC236 cross-reacting glycoprotein has been immunolocalized primarily in the cell wall and intercellular spaces of the nodule parenchyma in pea and soybean nodules (VandenBosch et al., 1989). The amount of this glycoprotein is related to the rhizosphere pO2 under which the nodules are grown: compared with controls, more of the MAC236 cross-reacting glycoprotein was found occluding intercellular spaces in nodules grown at higher [O2] (40–50%), whereas less was found in nodules grown at lower [O2] (10%) (James et al., 1991; Iannetta et al., 1993b). Exposing lupin nodules to 20 mm KNO3 for 2, 4, or 6 d decreased nodule permeability to O2 and also increased the abundance of the MAC236 cross-reacting glycoprotein within the intercellular spaces of the nodule inner cortex (Iannetta et al., 1993a).
Another protein postulated to be involved in regulation of the diffusion barrier is ENOD2 (Nap and Bisseling, 1990). GmENOD2 transcripts accumulate from early stages of soybean nodule development, specifically in the cells of the nodule parenchyma (Franssen et al., 1987; van de Wiel et al., 1990b). Transcripts for ENOD2 homologs are found in nodules of pea, alfalfa, lupin, cowpea, common bean, and Sesbania rostrata (Dickstein et al., 1988; Szczyglowski and Legocki, 1990; van de Wiel et al., 1990a, 1990b; Padilla et al., 1991; Trese and Pueppke, 1991; Dehio and de Bruijn, 1992), at a location comparable to that described for pea (van de Wiel et al., 1990a, 1990b; Allen et al., 1991) except for lupin nodules (W.M. Karlowski, personal communication). Antibodies raised against a synthetic ENOD2 peptide have since been used to immunolocalize ENOD2 to intercellular spaces of inner cortical cells of pea and soybean nodules (D.J. Sherrier, G.S. Taylor, and K.A. VandenBosch, unpublished results). Proteins that are immunoreactive with MAC236 and the putative ENOD2 protein run at different apparent Mrs, indicating that the two are independent proteins even though they colocalize in the nodule parenchyma. As additional support for their distinctiveness, immunoaffinity-purified ENOD2 does not cross-react with MAC236. Moreover, the 236 protein localizes to infection threads as well as intercellular spaces in contrast to ENOD2, which is found only in intercellular spaces (D.J. Sherrier, G.S. Taylor, and K.A. VandenBosch, unpublished results).
A third protein, peanut lectin, has been localized to the intercellular spaces of peanut nodule parenchyma cells, but not to those of boundary layer cells (VandenBosch et al., 1994). This lectin was also detected in the vacuoles of the nodule parenchyma cells and to a lesser extent within the symbiosome lumen of infected cells (VandenBosch et al., 1994).
In the present study we examined the ability of alfalfa nodules to adapt to changes in pO2 and tested whether adaptation to high or low pO2 is correlated with changes in nodulin gene expression. We hypothesized that the expression of proteins such as ENOD2 or the MAC236 cross-reacting glycoprotein might vary with the rhizosphere pO2 that was present during nodule development.
MATERIALS AND METHODS
Plant Growth and Nodulation Conditions
Alfalfa (Medicago sativa cv Iroquois) seeds were planted in plastic growth pots (volume equals 750 mL) that could be sealed for open-circuit gas-exchange measurements. Each pot contained two to four plants grown in silica sand. Plants were grown at a constant temperature of 20°C, and a photon flux density of 400 μmol quanta m−2 s−1 during a 16-h photoperiod. Pots were flushed twice daily with a modified Hoagland solution containing 5 mm potassium nitrate to inhibit spurious nodule formation. After 4 weeks of growth, the pots were flushed with water and inoculated with a broth culture of Rhizobium meliloti strain 1021. An acrylic lid was sealed onto the pots using a flexible sealant (Qubitac, Qubit Systems, Inc., Kingston, Ontario, Canada). The lid had a slit to accommodate the stems, and the stems were sealed to the lid with sealant. The lid was fitted with a subseal through which nutrient solution could be injected. Each pot received 10 mL of N2-free nutrient solution twice daily. The lid was also fitted with a gas port through which 8, 20, or 50% O2 in N2 was supplied continuously at about 50 cm3 min−1. Gases were humidified before entering the pots to prevent drying of the roots. Gases were vented through a gas exit port at the base of the pot and this port also served as a drain for excess nutrient solution. [O2] in the effluent gas was monitored periodically to ensure that a stable pO2 was supplied to each treatment. Plants were used in gas-exchange experiments 20 d after exposure to each O2 regime.
Gas-Exchange Measurements
Measurements of gas exchange from nodulated roots were made using a computer-controlled, open-flow gas-exchange system as described previously (Hunt et al., 1989). On initial attachment to the system, plants were exposed to a gas mixture containing N2:O2 at the pO2 at which they were grown without exposure to atmospheric pO2.
When H2 evolution in N2:O2 was stable, the gas mixture was switched to Ar:O2 while maintaining a constant pO2. The peak rate of H2 evolution in Ar:O2 was taken as a measure of TNA (Hunt et al., 1987). The rate of CO2 evolution was taken as a measurement of respiration rate. Immediately after TNA was measured, the pO2 in the Ar:O2 mixture was programmed to double every 20 min until 100% O2 was reached. For example, in the 8% O2 treatment, pO2 was increased from 8 to 16%, 16 to 32%, and 32 to 64% in linear ramps, each of 20-min duration, and then increased from 64 to 100% in 11.25 min. In the 20% O2 treatment, pO2 was increased from 20 to 40% and from 40 to 80% in two linear ramps, each of 20-min duration, and then from 80 to 100% in 5 min. In the 50% O2 treatment, pO2 was increased from 50 to 100% in a single 20-min ramp. These changes in pO2 ensured that the nodulated roots were exposed to the same relative change in O2 gradient between the rhizosphere and nodule interior during elevation of pO2. CO2 and H2 evolution were monitored continuously during the increase in pO2, and the maximum rate of H2 evolution was taken as a measurement of PNA (Diaz del Castillo et al., 1992).
After the gas-exchange measurements the nodulated roots were removed from their growth pots, rinsed rapidly to remove growth media, quickly blotted dry, and weighed. The roots and nodules were immersed together in liquid N2, and the nodules were picked frozen and subsequently stored at −80°C until extracted for protein and RNA analysis.
RNA Isolation and Northern Hybridization
Total RNA was isolated from nodules 21 d postinoculation using RNA STAT-60 (Tel-Test “B”, Inc., Friendswood, TX). The RNA was size-fractionated on formaldehyde agarose gels (Sambrook et al., 1989) with 5 μg of RNA loaded per lane. After transfer to Nytran membranes (Schleicher & Schuell), blots were hybridized with α-[32P]dCTP-labeled DNA probes. The probe for ENOD2 transcripts was a 298-bp EcoRI fragment from the plasmid A2ENOD2 (Dickstein et al., 1988). The probe for ENOD40 mRNAs was a 580-bp PstI fragment from the plasmid MsENOD40–2 (Asad et al., 1994). The probe for leghemoglobin mRNA was a 1.0-kb fragment from the plasmid MsLb3 (Löbler and Hirsch, 1992), and the probe for R. meliloti nifHDK transcripts was a 4-kb fragment from the plasmid pRmR2 (Ruvkun and Ausubel, 1981). Blots were also hybridized with Msc27 (Kapros et al., 1992) as an internal control to standardize loading.
Protein Extraction and Western Blotting
For extracts of total soluble protein, nodules were ground in ice-cold homogenization buffer (50 mm Tris, pH 7.5, 0.5 m Suc, 10 mm DTT, and 5 mm PMSF) in 1.5-mL microcentrifuge tubes using a plastic pestle (Kontes, Hayward, CA) attached to a portable electric drill. The nodules were ground with 5 to 10 μL of buffer and 0.3 mg of polyvinylpolypyrolidone per milligram of nodule (wet weight). The tubes were centrifuged at 10,000 rpm at 4°C for 10 min and the supernatants were removed to new tubes. The extracts were stored frozen at −20°C. After aqueous extraction, some nodules were subjected to harsher extraction procedures (Fry, 1988). The protein concentrations of the supernatants were determined by the Bradford assay (Bio-Rad) using BSA as a standard. For competitive ELISA, a different extraction buffer consisting of 40 mm Tris (pH 8.0), 3 mm EDTA, and 1 mm PMSF was used because DTT interfered with antibody binding in the competition assays.
Extracts containing 10 to 20 μg of soluble protein were subjected to SDS-PAGE (Laemmli, 1970) using 12.5% acrylamide gels, then transferred to nitrocellulose in a Transblot apparatus (Hoefer Scientific Products, San Francisco, CA) containing transfer buffer (25 mm Tris, 192 mm Gly, and 20% methanol, pH 8.3) at 100 mA overnight at 4°C. Blots were stained with Ponceau S (Sigma) to locate molecular mass markers, and then incubated with TBS (20 mm Tris, pH 7.5, and 180 mm NaCl) plus 1% BSA for 1 h prior to probing with antibodies. Blots were sealed in plastic pouches with 10 mL of antibodies diluted in TBS plus 1% BSA. Affinity-purified antibody against ENOD2 (D.J. Sherrier, G.S. Taylor, and K.A. VandenBosch, unpublished results) was diluted to 1 μg/mL. MAC236 (gift of N. Brewin, John Innes Institute, Norwich, UK) was diluted 1:100 from hybridoma culture supernatant. Anti-leghemoglobin (gift of C. Vance, University of Minnesota, St. Paul) was diluted 1:1000, and cross-reacting antibodies were first removed by incubating the diluted antiserum with a nitrocellulose filter blotted with an alfalfa root extract. A monoclonal antibody against ribosomal protein P0 (Uchiumi et al., 1990) (gift of T. Uchiumi, Niigata, Japan) was diluted 1:200.
The filters were incubated with antibody solution at 4°C with gentle shaking overnight, and then washed once with TBS, three times with TBS plus 0.05% Nonidet P-40, and once with TBS (5 min each wash). The filters were then incubated with the appropriate secondary antibody diluted 1:1000 in TBS plus 1% BSA. Goat anti-rabbit IgG alkaline phosphatase conjugate was used to detect anti-ENOD2 and anti-leghemoglobin. Goat anti-rat IgG alkaline phosphatase conjugate was used to detect MAC236, and goat anti-mouse IgG alkaline phosphatase conjugate was used to detect anti-ribosomal protein P0. All secondary antibodies were affinity purified (Sigma). The filters were incubated with secondary antibody for 4 to 6 h at 4°C, washed as before, and then incubated in color development buffer until color was apparent. Color development buffer consisted of 50 mm Tris (pH 9.0), 50 mm NaCl, 2.5 mm MgCl2, 330 μg/mL nitroblue tetrazolium, and 165 μg/mL 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt. The filters were rinsed with water and allowed to dry before being photographed.
Quantitative Competitive ELISA
For quantitative competitive ELISA, nodule extract was diluted in PBS to 5 μg protein/mL. Aliquots of 50 μL were loaded into wells of microtiter plates (Immulon IV, Dynex, Chantilly, VA), which were stored overnight at 4°C. Unbound antigen was removed and plates were incubated for 1 h at 25°C with 100 μL per well of blocking solution (PBS plus 1% BSA). Blocking solution was removed and 25 μL of competitor solution was added followed by 25 μL of antibody solution. Nodule extracts to be tested were usually used at 2 μg protein/mL when testing for ENOD2 and 10 μg protein/mL when testing for the MAC236 glycoprotein. The anti-ENOD2 antibody was used at a final concentration of 0.25 μg/mL, and the MAC236 antibody was used at a final dilution of 1:100. These antibody concentrations were previously determined to give about one-half saturation binding.
To make the ELISA quantitative, a standard curve of competitor was used on each plate. Competitor for ENOD2 was a 21-amino acid synthetic ENOD2 peptide (POHEKPOHENTPOEYQPOHEK, where O = Hyp; D.J. Sherrier, G.S. Taylor, and K.A. VandenBosch, unpublished results) at 5 to 50 ng/mL. This peptide was synthesized based on the deduced amino acid sequence of a PsENOD2 clone (van de Wiel et al., 1990b), with a predicted pattern of posttranslational hydroxylation based on known Pro-rich proteins (Kieliszesky and Lamport, 1994). The repetitive motifs PPHEK and PPEYQ are highly conserved among predicted amino acid sequences of ENOD2 genes in legumes, including alfalfa (Dickstein et al., 1988). A competitor for the MAC236 glycoprotein was an extract from nodules grown at 8% O2, at 5 to 40 μg protein/mL. Controls included wells without competitors. Plates containing competitor/antibody mixtures were incubated overnight at 4°C. Unbound antibody was removed and the plate was washed three times with PBS. Secondary antibody solution, consisting of affinity-purified goat anti-rabbit (for anti-ENOD2) or anti-rat (for MAC236) IgG alkaline phosphatase conjugate (Sigma) diluted 1:1000 in PBS plus 1% BSA, was added (50 μL/well) and the plate was incubated for 4 to 6 h at 4°C. The secondary antibody solution was removed and the plate was washed four times with TBS. The substrate solution was then added and the plates were incubated at 37°C until a yellow color developed. The substrate solution consisted of 104 phosphatase substrate (p-nitrophenyl phosphate, disodium, and hexahydrate; Sigma) at 1 mg/mL in 1 m diethanolamine, pH 9.8, 0.5 mm MgCl2. The reaction was stopped by the addition of 100 μL of 1 m NaOH. A405 was assayed on a plate reader.
Straight line standard curves were generated by a logit-log transformation of the data (Signorella and Hymer, 1984). The logit Y was calculated as:
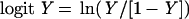 |
1 |
where Y = B/B0 (B is the absorbance at some competitor concentration, and B0 is the mean absorbance of the uncompeted controls). The linear competitor concentration standard curve is described by the equation:
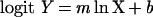 |
2 |
where X is the concentration of competing antigen, and m and b are constants determined by a least-squared linear regression of the logit-log transformed data. The concentration of competing antigen in the ENOD2 assay was expressed in terms of nanograms of ENOD2 peptide equivalents per milliliter. The concentration of competing antigen in the MAC236 assay was expressed in terms of arbitrary units based on the standard curve of 8% nodule extract.
Differences between measurements of ENOD2 and MAC236 were analyzed for significance using a one-sided Student's t test.
Light-Microscopic Analysis of Nodules
Nodules were harvested from plants grown under different O2 regimes as described above, placed in buffered fixative, and subjected to a slight vacuum to facilitate infiltration of fixative. The fixative contained 4% paraformaldehyde and 1% glutaraldehyde in 100 mm sodium phosphate buffer, pH 7.0. The nodules were fixed overnight on ice at ambient pressure. They were then dehydrated in an ethanol series and embedded in London Resin White as previously described (Sherrier and VandenBosch, 1994). Semithin sectioning, immunolabeling, and silver enhancement were also conducted as previously described (Sherrier and VandenBosch, 1994), using affinity-purified anti-ENOD2 IgGs at a concentration of 5 mg/mL as a primary antibody. Labeled sections were counterstained with 1% (w/v) aqueous basic fuchsin to impart contrast to the embedded tissue.
Nodules were also fixed in formaldehyde-acetic acid-alcohol and embedded in paraffin as previously described (McKhann and Hirsch, 1993). Slides were stained for starch with the periodic acid Schiff's reaction (Jensen, 1962). Photographs were taken on a Zeiss Axiophot microscope using Ektachrome Tungsten 160 film, and prepared using Adobe Photoshop.
RESULTS
Gas-Exchange Measurements
TNA and respiration rates were not significantly different in nodulated roots grown at 8, 20, and 50% O2 when assayed at their respective growth pO2 (Table I). Also, the PNA was not significantly different among the three treatments. PNA represents the nitrogenase activity attainable when nitrogenase-linked respiration is not limited by O2. Thus, the degree of O2 limitation of nitrogenase activity in a nodulated root can be indexed by the ratio of TNA to PNA. This ratio is termed OLCN (Diaz del Castillo et al., 1992), and the lower the value, the greater the degree of O2 limitation. All three treatments had OLCN values close to 0.9 (Table I), indicating that despite being grown at different pO2, a similar, small degree of O2 limitation of nitrogenase activity occurred in each.
Table I.
TNA, PNA, respiration rate, and OLCN in nodulated roots of alfalfaa
| Growth pO2 | TNA | PNA | Respiration | OLCN |
|---|---|---|---|---|
| % | μmol g−1FWnrbh−1 | |||
| 8 | 4.29 ± 0.33 | 4.62 ± 0.27 | 9.19 ± 0.49 | 0.93 ± 0.03 |
| 20 | 4.67 ± 0.30 | 5.18 ± 0.33 | 10.90 ± 0.39 | 0.90 ± 0.03 |
| 50 | 5.14 ± 0.52 | 5.68 ± 0.40 | 12.52 ± 1.12 | 0.90 ± 0.04 |
Results are the means ± se of four replicates, and specific activities are expressed per gram fresh weight of nodulated root tissue per hour. Each replicate comprised two to four attached nodulated roots sealed within their growth pots.
FWnr, Fresh weight of nodulated root.
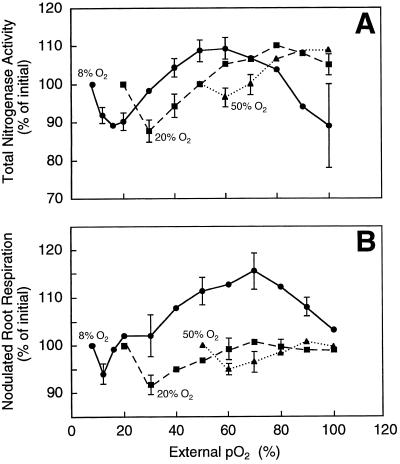
Although PNA values were similar in nodulated roots grown at different pO2, nitrogenase sensitivity to elevated pO2 differed among treatments. At all three pO2, nitrogenase activity declined during the initial few minutes of the O2 ramp (Fig. 1A). This was attributed to an Ar-induced inhibition of nitrogenase activity. After the initial Ar-induced decline in nitrogenase activity, activity in all three treatments increased above initial levels. PNA values were attained at 60, 80, and 90% O2 in the nodulated roots grown at 8, 20, and 50% O2, respectively. In the 8% O2 treatment, PNA was followed by a decline in nitrogenase activity at pO2 above 60%. In the other two treatments, there were no significant differences between the PNA values and the nitrogenase activities measured at 100% O2.
Figure 1.
Nitrogenase activities (A) and respiration rates (B) of nodulated roots of alfalfa exposed to gradual increases in pO2 in a balance of Ar. Increases in pO2 were from initial growth pO2 of 8% (•), 20% (▪), and 50% (▴). Each point represents the mean of four replicates ± representative ses. Absolute initial values for TNA and respiration rates are shown in Table I.
In general, the ramps in [O2] affected the respiration rates of nodulated roots in a fashion similar to the effect on nitrogenase activities in all three pO2 treatments (Fig. 1B). In each case, an initial decline in respiration rate was followed by a recovery as the O2 ramps progressed. However, only in the case of the 8% O2 treatment did the respiration rate increase significantly above initial levels. Also, this treatment was the only one to show a decline in respiration rate at pO2 values greater than that at which the maximum respiration rate was reached.
Expression of Nodulin Genes
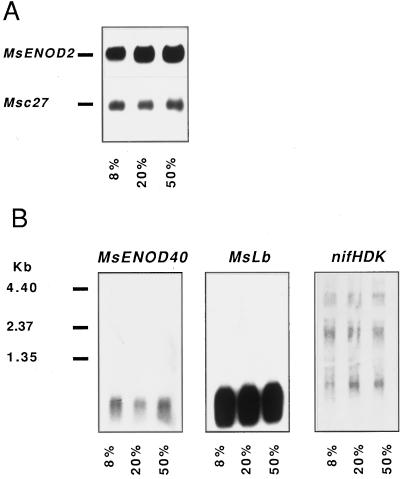
Total RNA was extracted from frozen alfalfa nodules that developed under conditions of 8, 20, or 50% O2. There was little difference in the amount of total RNA per gram fresh weight recovered from the three treatments (1.4, 1.32, and 1.23 mg/g fresh weight for 8, 20, or 50% O2-grown nodules, respectively). Northern-blot analysis (Fig. 2A) revealed that steady-state levels of ENOD2 mRNA remained constant between 8 and 50% pO2. Levels of leghemoglobin and ENOD40 mRNAs were also not affected by [O2], nor was the expression of the R. meliloti nifHDK genes (Fig. 2B).
Figure 2.
RNA blot of total RNA from alfalfa nodules grown under 8, 20, or 50% O2. A, Blot probed for MsENOD2 and Msc27 mRNAs. B, Blots probed for MsENOD40, MsLb3, and R. meliloti nifHDK mRNAs. Five micrograms of total nodule RNA was loaded per lane.
Immunological Assays of Nodulins
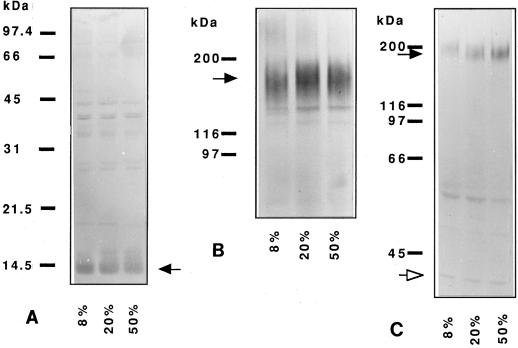
Because there was no apparent effect of O2 level on the level of accumulation of nodulin transcripts, we assessed the abundance of leghemoglobin, ENOD2, and the MAC236 glycoprotein in nodule extracts using immunological techniques. Although total soluble protein content as a fraction of nodule fresh weight (in milligrams per gram) was highest in the control nodules (25.7 versus 19.6% for nodules developed under conditions of 8% O2 and 17.4% for 50% O2-grown nodules), all immunological comparisons were based on using the same amount of protein from each extract. Western blots of total soluble protein from nodules grown in 8, 20, or 50% O2 were probed with rabbit anti-leghemoglobin antiserum, rabbit anti-ENOD2 antiserum, and the rat monoclonal antibody MAC236. Anti-leghemoglobin antiserum showed significant cross-reaction to proteins other than leghemoglobin (data not shown). Cross-adsorption of this antiserum to alfalfa root proteins removed much of the cross-reaction, and when subsequently used, it was clear that the quantity of leghemoglobin was similar under all pO2s tested (Fig. 3A).
Figure 3.
Immunological detection of different proteins on western blots. A, Leghemoglobin. Blot probed with anti-leghemoglobin antiserum that was previously exposed to alfalfa root proteins to remove cross-reacting antibodies. The leghemoglobin band is indicated by the arrow at 15 kD. Twenty micrograms of total protein was loaded per lane. B, ENOD2. Extracts from nodules grown at 8, 20, and 50% O2 were subjected to electrophoresis on a 7.5% acrylamide gel and blotted onto nitrocellulose. The blot was probed with anti-ENOD2 antibody at 1 μg/mL. The major ENOD2 protein band is indicated by the arrow. C, MAC236 glycoprotein. Blot probed with MAC236 at 1:100 and monoclonal anti-ribosomal protein P0 at 1:200 dilution. The MAC236 glycoprotein is indicated by the black arrow, and the P0 protein (which served as a loading control) is indicated by the white arrow.
Western blots of nodule extracts were probed with the anti-ENOD2 antiserum. The antibody reacted primarily to a broad band at about 155 kD and one or two smaller bands at about 140 and 120 kD (Fig. 3B). In some extracts, a fainter band could be seen at about 70 kD (data not shown). There was little difference in the intensity or pattern of bands between nodules grown at 8 and 50%; a slightly higher amount of ENOD2 antigen was detected in the 20%-grown nodules (Fig. 3B).
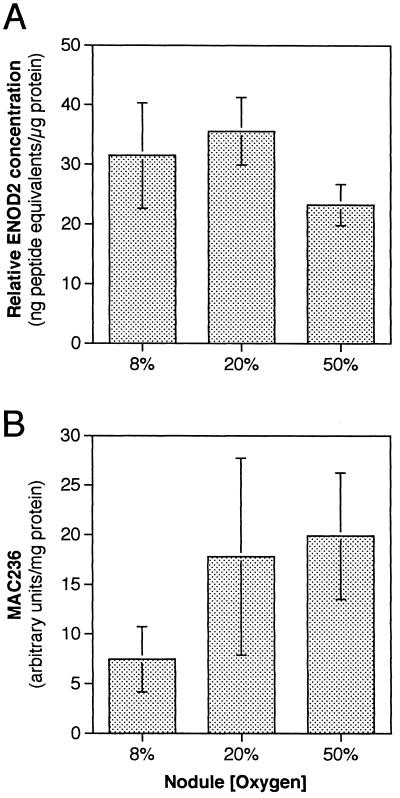
Nodule extracts were also assayed for ENOD2 protein by an indirect competitive ELISA with anti-ENOD2 antibodies (Fig. 4A), using a synthetic 20-amino acid peptide as a standard. Comparison of the competition by nodule extracts with the standard curve allowed us to measure the quantity of ENOD2 in units of “peptide equivalents.” This method more accurately quantifies relative antigen concentrations than the minimal dilution method employed by Iannetta et al. (1993b). There was no significant difference in the relative quantity of extractable ENOD2 protein between nodules grown at 8 and 20% pO2, or between those grown at 8 and 50% pO2. However, there was approximately 25% less ENOD2 in extracts of nodules grown at 50% pO2, and this was significant at the 99% confidence level determined by using a one-sided Student's t test.
Figure 4.
Quantification of MAC236 glycoprotein and ENOD2. A, Relative quantity of ENOD2 in nodules. B, Relative quantity of MAC236 glycoprotein in nodules. Bars represent the mean of three to four assays on each of six individual nodules. Error bars are sd for the six nodules.
Western blots of nodule extracts were probed with the monoclonal antibody MAC236 together with a monoclonal antibody against ribosomal protein P0 (used as a loading control). A glycoprotein recognized by MAC236 was present in alfalfa nodules and increased in quantity with increasing pO2 (Fig. 3C). It should be noted that this, and similar antibodies, also react with protein in infection threads (VandenBosch et al., 1989). Our study did not determine whether the increase in alfalfa nodules was in nodule parenchyma cells, infection thread-containing cells, or both. The apparent molecular mass of the MAC236 glycoprotein in alfalfa nodules was 195 kD. This was somewhat smaller than the size of 240 kD reported for lupin nodules (de Lorenzo et al., 1993), but larger than the 95 kD reported for pea nodules (VandenBosch et al., 1989). After aqueous extraction of the nodules, the remaining cell wall debris were subjected to harsher extraction procedures, but only a small amount of additional MAC236 glycoprotein was recovered (data not shown).
Competitive ELISA with MAC236 (Fig. 4B) revealed that the MAC236 glycoprotein increased 2.4-fold in nodules grown at 20% compared with those grown at 8% O2 (significant at the 99% confidence level). There was no significant difference in the abundance of the MAC236 glycoprotein between nodules grown at 20 and 50% O2 based on the large ses. However, as shown in the western-blot analysis (Fig. 3C), there was a trend toward increased MAC236 protein levels as the [O2] was raised.
All of the nodules from the experiments described above were from plants harvested after O2 ramping. In a separate gas-exchange experiment, nodules taken from plants harvested before O2 ramping were compared with nodules from plants harvested after O2 ramping. MAC236 glycoprotein was assayed in nodules that developed under 20% O2, and ENOD2 was assayed in nodules that developed under 8 and 50% O2. In no case was there a significant difference in the amount of extractable antigen between nodules harvested before and after O2 ramping (Table II).
Table II.
Effect of O2 ramping on quantity of extractable ENOD2 and MAC236 glycoproteina
| Growth pO2 | Antigen | Pre-Ramp | Post-Ramp |
|---|---|---|---|
| % | |||
| 8 | ENOD2 | 55 ± 5b | 63 ± 39b |
| 50 | ENOD2 | 62 ± 32b | 66 ± 26b |
| 20 | MAC236 | 11 ± 2c | 13 ± 4c |
Individual nodules were assayed by competitive ELISA. Results are the means ± ses of assays on eight nodules from each of two plants for each treatment.
Units of nanograms of ENOD2 peptide equivalents per microgram of nodule protein.
Arbitrary units of MAC236 antigen per microgram of nodule protein.
Localization of Nodulins in Nodules Grown under Different [O2]
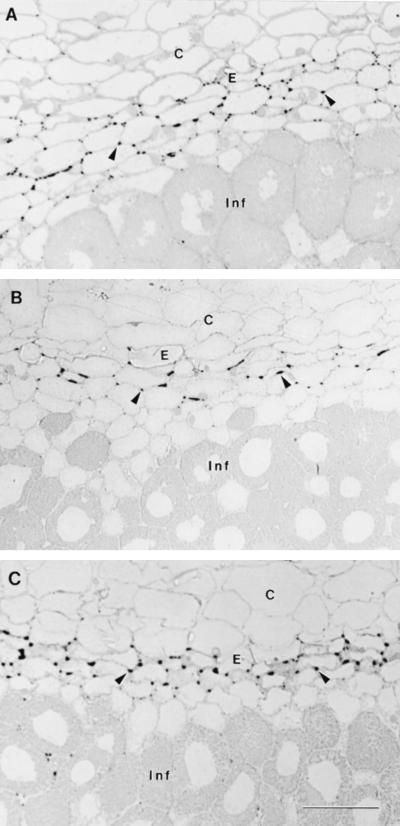
Longitudinal sections of nodules were compared to discern whether the ENOD2 protein was distributed differently in nodules grown under different [O2]. An antibody against distinctive, conserved motifs of ENOD2 was used in an immunolabeling silver-enhancement protocol. The distribution of immunoreactive protein in alfalfa nodules from all three growing conditions resembled that seen previously in pea nodules (D.J. Sherrier, G.S. Taylor, and K.A. VandenBosch, unpublished results). Labeled protein was abundant in intercellular spaces in the nodule parenchyma and was adjacent to the nodule endodermis starting in interzone II-III (data not shown) and continuing into zone III (Fig. 5, A–C). Interzone II-III lies between the prefixation zone II and the N2-fixation zone III (Vasse et al., 1990). Label was heaviest at the cell corners and was sometimes observed lining the nodule parenchyma cells. No qualitative or obvious quantitative differences in labeling patterns were seen among the different O2 treatments. In all cases, label was found in intercellular spaces, in two to four cell layers of the nodule parenchyma. ENOD2 protein was occasionally detected in the intercellular spaces of the central infected zone (Fig. 5C), but it accumulated to much lower levels and occurred at a much lower frequency than what was observed in the nodule parenchyma. Omission of primary antibody or incubation in preimmune serum resulted in a lack of specific labeling (data not shown).
Figure 5.
Immunolabeling of ENOD2 protein in alfalfa nodules grown under different pO2s. Following silver enhancement, immunogold labeling appeared as an opaque black deposit on intercellular spaces in the nodule parenchyma (arrowheads). The regions of the nodules depicted were comparable and are all in upper zone III. C, Outer cortex; E, nodule endodermis; Inf, infected cells in zone III. All views are magnified ×315. Bar, 50 μm. A, Labeled section of a nodule grown in 8% O2. B, Labeled section of a nodule grown in 20% O2. C, Labeled section of a nodule grown in 50% O2.
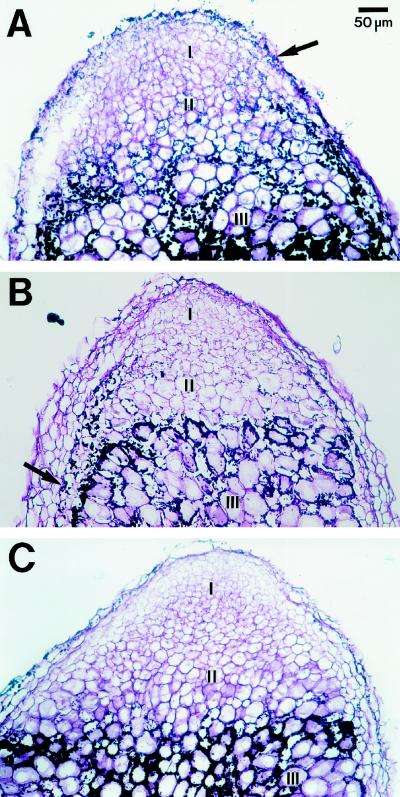
In contrast to the immunolabeling results, a dramatic difference in the patterns of starch accumulation was observed for the different treatments. Vasse et al. (1990) noted that starch deposition in amyloplasts occurs in a clearly demarcated cell layer (interzone II-III). In the present study, we utilized periodic acid Schiff's staining to visualize the abundant, plate-like amyloplasts in interzone II-III in nodules of plants grown in 20% O2. Small, round amyloplasts were present in uninfected cells of the central region and also in the boundary layer cells (arrow, Fig. 6B). There was a gradual decrease in starch abundance from interzone II-III toward the base of the nodule grown in 20% O2 (data not shown).
Figure 6.
Periodic acid Schiff's staining of paraffin-embedded alfalfa nodules that had been grown under different [O2]. Zones I, II, and the periphery of zone III are shown. A, Longitudinal section of a nodule grown in 8% O2. The arrow points to small starch grains in the nodule cortex. B, Longitudinal section of a nodule grown in 20% O2. The arrow points to starch grains in the boundary layer. C, Longitudinal section of a nodule grown in 50% O2.
Starch was even more abundant in nodules grown in 8% O2. Amyloplasts were found in the uninfected cells of interzone II-III, zone II, and along the periphery of zone I (the meristem), as well as in the cells of the nodule cortex (Fig. 6A). Moreover, amyloplasts were evident throughout the entire length of the nodule (data not shown). There was a copious accumulation of both plate-like amyloplasts in the infected cells surrounding the central region and small, round amyloplasts in the boundary layer. In contrast, starch grains were sparse in nodules grown in 50% pO2 (Fig. 6C). Very few amyloplasts were observed in the nodule, except in interzone II-III, and in both infected and uninfected cells. Few periodic acid Schiff's positive staining bodies were observed in either zone I or zone II in these nodules, and an insignificant number of amyloplasts were observed in the more mature regions of the nodule, with the exception of the senescent zone.
DISCUSSION
Gas Exchange
Although O2 supply to the central infected zone of legume nodules often limits nitrogenase activity under normal and adverse environmental conditions (Hunt et al., 1987, 1989; Hunt and Layzell, 1993), nitrogenase activity did not differ among nodules that were grown and assayed at 8, 20, and 50% O2. Also, respiration rates and the degree of O2 limitation of nitrogenase activity were similar in all three O2 treatments. Taken together, these results indicate that [O2] in the central zone of nodules from the three treatments was similar, despite large differences in the O2 gradient between the rhizosphere and the central zone. This could only be achieved if the nodules from the three treatments had very different permeabilities to O2. Using a simplification of Fick's Law (Sheehy et al., 1983):
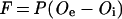 |
3 |
in which F represents nodule O2 consumption (assuming equivalence with CO2 production), P represents nodule permeability to O2, Oe represents rhizosphere [O2], and Oi represents the [O2] in the nodule central zone, maintaining F and Oi constant would require that nodule permeability would change in inverse proportion with increases in Oe. Therefore, the nodules grown at 8% O2 would have had permeabilities to O2 diffusion approximately 2.5 times greater than those grown at 20% O2 and 6.3 times greater than those grown at 50% O2.
The nodules grown at each pO2 were able to regulate permeability in response to changing rhizosphere pO2, evidenced by maintenance of high nitrogenase activities as pO2 was increased to 100%. Respiration rate changed little with elevated pO2. Therefore, increased respiratory O2 consumption would have had a very little role in maintaining a central zone [O2] conducive to nitrogenase activity. Applying the Fick's Law analog in Equation 3 above, these results suggest that between the initial growth pO2 and the pO2 at which PNA was reached, permeabilities of nodules grown at 8, 20, and 50% O2 decreased to approximately 13, 25, and 50% of initial values, respectively. Therefore, any developmental changes in alfalfa nodules that allow them to adapt to long-term changes in rhizosphere pO2 do not change the ability of the nodules to regulate diffusion resistance physiologically during short-term changes in pO2. A similar conclusion was reached in earlier studies with soybean (Atkins et al., 1993).
Nodule Structure
In a previous study, alfalfa plants nodulated under conditions of 1% pO2 had macroscopically observable outgrowths of loosely packed cells that resembled aerenchyma on both roots and nodules (Arrese-Igor et al., 1993). The presence of loosely packed cells is likely to affect gas permeability. However, we did not observe aerenchymatous tissue on nodules or roots in our experiments. A concentration of 8% pO2 is apparently not low enough to induce this kind of modification. Nevertheless, the nodule tissues did appear less compact in the hypoxically grown nodules, whereas tissues in the 50% pO2-grown nodules appeared more compact. Even zone I, the nodule meristem, appeared to consist of smaller, more densely packed cells in nodules grown in 50% pO2 (compare Fig. 6C). Parsons and Day (1990) observed a similar increase in tissue compactness for soybean nodules formed under high O2.
A major anatomical difference among the different treatments was the level of starch grain abundance. Like Arrese-Igor et al. (1993), we found that nodules developed under low-O2 conditions amassed numerous starch granules. These authors suggested that the accumulation of starch in indeterminate nodules such as alfalfa might be related to stress. However, on the basis of nitrogenase activity and respiration measurements, there was no evidence that the nodules at 8% O2 were exposed to stressful conditions. Moreover, nodules grown under 50% O2 accumulated very little starch, and these nodules had metabolic activity similar to those at 8 and 20% O2. One interpretation for the presence of excess starch is that the available carbon exceeded the respiratory demand of the tissue and accordingly accumulated as starch. In any case, starch accumulation is a relatively long-term response and does not explain the short-term regulation of the nodule to changes in rhizosphere pO2, although Layzell et al. (1990) proposed that carbohydrates may be involved in osmotic regulation of the diffusion barrier.
Protein Levels and Nodulin Expression
The amount of protein per nodule fresh weight was higher in the control nodules (20% pO2) than in the nodules grown at 8 or 50% pO2. This is in agreement with measurements of soluble protein in soybean nodules grown at 10, 21, and 40% pO2 (James et al., 1991). It cannot presently be determined whether this represents a difference in the abundance of one protein or many. Leghemoglobin, the most abundant protein in nodules, facilitates the diffusion of O2 across the gradient that exists between the infected cell surface and the bacteroids (Appleby, 1984). We found no apparent difference in the abundance of leghemoglobin, when visualized on western blots, among the different pO2 treatments. This is consistent with measurements of leghemoglobin in other N2-fixing symbioses (Dakora et al., 1991). Because there was no significant difference in the respiration rate of the nodules grown at all three pO2, it is not surprising that leghemoglobin levels were similar among the treatments.
It has been shown previously that nodules of soybean and cowpea grown at sub- and supra-ambient pO2 maintain different permeabilities by a change in thickness of the cortical diffusion barrier and by a change in the proportion of the nodule parenchyma that is composed of open (unoccluded) intercellular spaces (Dakora and Atkins, 1990c, 1991; Parsons and Day, 1990). Other studies at higher growth [O2] demonstrated that the occlusion of intercellular spaces is correlated with the deposition of a glycoprotein recognized by the monoclonal antibody MAC236 (James et al., 1991; Iannetta et al., 1993b, 1995). We found that a glycoprotein recognized by MAC236 was also present in alfalfa nodules, and that its abundance increased as pO2 increased from 8 to 20% O2 during development. Although there was no significant difference between the abundance of MAC236 glycoprotein in nodules grown at 20 and 50% O2, there was a trend toward increasing amounts of MAC236 protein in the nodules exposed to higher [O2] (compare Fig. 3C). Iannetta et al. (1995) found that the MAC236 antibody cross-reacted with increasing amounts of material in the intercellular spaces of white lupin nodules exposed for 15 or 30 min to 50% O2, whereas control nodules showed little or no labeling of the cell walls. They proposed that an MAC236-type glycoprotein could be involved in rapidly regulating permeability in lupin nodules. However, MAC236 also cross-reacts with glycoproteins from infection threads and infection droplets (VandenBosch et al., 1989), and as pointed out by James et al. (1997), indeterminate nodules such as those on alfalfa have a large number of infection threads in contrast to white lupin nodules, which have few.
The levels of ENOD2 and MAC236-extractable protein did not change pre- versus post-ramp in the ramping experiments. Given that the duration of the ramped increases in pO2 were only 71, 45, and 20 min for nodules grown at 8, 20, and 50% O2, respectively, it is unlikely that the short-term decreases in nodule permeability could be caused by increased synthesis of either MAC236 glycoprotein or ENOD2 protein. However, it is possible that the MAC236 or ENOD2 proteins play a role in the short-term regulation of nodule permeability by altering the gas:water content of intercellular spaces in response to changes in [O2]. For example, one could envision a mechanism whereby a cell wall matrix glycoprotein was rapidly cross-linked in response to changes in O2, thereby creating a gel or adhesive that would hold water and displace gas within intercellular spaces. If this were to occur in the innermost layers of the inner cortex or in the outermost layers of the central infected zone, then the result could be a wider, more convoluted path of O2 diffusion and a rapid, physiologically induced change in nodule permeability to O2 diffusion. Nevertheless, this hypothesis is challenged in that Van Cauwenberghe et al. (1994), using a cryoplaning technique, did not observe any measurable change in water content of the intercellular spaces in the inner cortex of soybean nodules.
The question now is whether either ENOD2 or MAC236 (or both) is cross-linked in response to O2. Although the immunohistological study did not show a significant change in ENOD2 levels in nodules grown at the different [O2], a slight decrease in ENOD2 levels was detected using western-blot and ELISA analyses. This decrease may reflect a change in extractable protein due to cross-linking, and thus may not necessarily exclude a role for ENOD2 in the diffusion barrier. The deduced amino acid sequence of ENOD2 is similar to the sequence of SbPRP2 from soybean, a protein that is rapidly insolubilized in cell walls following wounding or pathogen infection (Bradley et al., 1992). These authors also reported cross-linking of the MAC265 antigen upon elicitor treatment. MAC265 and MAC236 colocalize to the same tissues in legumes. The two monoclonals recognize either neutral (MAC265) or acidic (MAC236) components of a 95-kD antigen (VandenBosch et al., 1989). Thus, it is reasonable to suggest that ENOD2 acts in concert with other cell wall components, such as the MAC236 antigen.
Based on our results, changes in ENOD2 gene expression or protein synthesis do not appear to be involved in the regulation of O2 diffusion either in the short term or the long term. The data for the MAC236 glycoprotein, on the other hand, are inconclusive. The larger question now is whether ENOD2 is absolutely required for nodule development and/or the proper functioning of the diffusion barrier. To this end, we have generated ENOD2-antisense alfalfa plants and have found that when grown at different [O2], they nodulate normally, suggesting that full expression of ENOD2 is not required for a functioning nodule (K.L. Wycoff, S. Hunt, D.B. Layzell, and A.M. Hirsch, unpublished results). Moreover, although some of the antisense lines appear to lack sense transcripts, several express ENOD2 protein, albeit at lower levels based on western-blot analysis (K.L. Wycoff, S. Hunt, D.B. Layzell, and A.M. Hirsch, unpublished results). However, a conundrum is that ENOD2 is also expressed in mycorrhizal roots (van Rhijn et al., 1997), which a priori would not be expected to have an O2 diffusion barrier. ENOD2 expression in these roots may be reflective of a change in endogenous hormone levels brought about by colonization by mycorrhizal fungi. Indeed, expression of the ENOD2 gene in uninoculated alfalfa roots is up-regulated by cytokinin treatment (van Rhijn et al., 1997). Thus, the major role for ENOD2 in nodule development is still obscure and must await either the isolation of an ENOD2 mutant plant or the discovery of a nodulating legume that lacks the ENOD2 gene.
ACKNOWLEDGMENTS
The authors wish to thank Bruce Brand, Jennifer Kuo, and Pieternel van Rhijn. We thank W.M. Karlowski for his helpful comments on the manuscript. Margaret Kowalczyk is acknowledged for her help with the figures.
Abbreviations:
- ENOD2
early nodulin 2
- ENOD40
early nodulin 40
- OLCN
O2-limitation coefficient of nitrogenase
- pO2
partial pressure of O2
- PNA
potential nitrogenase activities
- TNA
total nitrogenase activities
Footnotes
This research was supported by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (grant no. 92-37305-7717 to K.L.W. and grant nos. 92-37305-7815 and 95-37305-2366 to K.V.B.), the Natural Sciences and Engineering Research Council (Canada) (research grant to D.B.L.), and the National Science Foundation (grant no. 90-23888 to A.M.H.).
LITERATURE CITED
- Allen T, Raja R, Dunn K. Cells expressing ENOD2 show differential spatial organization during the development of alfalfa root nodules. Mol Plant Microbe Inter. 1991;4:139–146. doi: 10.1094/mpmi-4-139. [DOI] [PubMed] [Google Scholar]
- Appleby CA. Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol. 1984;35:443–478. [Google Scholar]
- Arrese-Igor C, Royal M, de Lorenzo C, de Felipe MR, Aparicio-Tejo PM. Effect of low rhizosphere oxygen on growth, nitrogen fixation and nodule morphology in lucerne. Physiol Plant. 1993;89:55–63. [Google Scholar]
- Asad S, Fang Y, Wycoff K, Hirsch AM. Isolation and characterization of cDNA and genomic clones of MsENOD40: transcripts are detected in meristematic cells of alfalfa. Protoplasma. 1994;183:10–23. [Google Scholar]
- Atkins CA, Hunt S, Layzell DB. Gaseous diffusive properties of soybean nodules cultured with non-ambient pO2. Physiol Plant. 1993;87:89–95. [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Appleby CA, Atkins CA. Effect of pO2 on the formation and status of leghemoglobin in nodules of cowpea and soybean. Plant Physiol. 1991;95:723–730. doi: 10.1104/pp.95.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora FD, Atkins CA. Diffusion of oxygen in relation to structure and function in legume root nodules. Aust J Plant Physiol. 1989;16:131–140. [Google Scholar]
- Dakora FD, Atkins CA. Effect of pO2 on growth and nodule functioning of symbiotic cowpea (Vigna unguiculata L. Walp.) Plant Physiol. 1990a;93:948–955. doi: 10.1104/pp.93.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora FD, Atkins CA. Effect of pO2 during growth on the gaseous diffusional properties of nodules of cowpea. Plant Physiol. 1990b;93:956–961. doi: 10.1104/pp.93.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora FD, Atkins CA. Morphological and structural adaptation of nodules of cowpea to functioning under sub- and supra-ambient oxygen pressure. Planta. 1990c;182:572–582. doi: 10.1007/BF02341034. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Atkins CA. Adaptation of nodulated soybean (Glycine max L. Merr.) to growth in rhizospheres containing nonambient pO2. Plant Physiol. 1991;96:728–736. doi: 10.1104/pp.96.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C, de Bruijn FJ. The early nodulin gene SrENOD2 from Sesbania rostrata is inducible by cytokinin. Plant J. 1992;2:117–128. doi: 10.1046/j.1365-313x.1992.t01-51-00999.x. [DOI] [PubMed] [Google Scholar]
- de Lorenzo C, Iannetta PPM, Fernandez-Pascual M, James EK, Lucas MM, Sprent JI, Witty JF, Minchin FR, de Felipe MR. Oxygen diffusion in lupin nodules II: mechanisms of diffusion barrier operation. J Exp Bot. 1993;44:1469–1474. [Google Scholar]
- Diaz del Castillo L, Hunt S, Layzell DB. O2 regulation and O2-limitation of nitrogenase activity in root nodules of pea and lupin. Plant Physiol. 1992;86:269–278. [Google Scholar]
- Dickstein R, Bisseling T, Reinhold VN, Ausubel FM. Expression of nodule-specific genes in alfalfa root nodules blocked at an early stage of development. Genes Dev. 1988;2:677–687. doi: 10.1101/gad.2.6.677. [DOI] [PubMed] [Google Scholar]
- Franssen HJ, Nap J-P, Gloudemans T, Stiekema W, van Dam H, Govers F, Louwerse J, von Kammen A, Bisseling T. Characterization of cDNA for nodulin-75 of soybean: a gene product involved in early stages of root nodule development. Proc Natl Acad Sci USA. 1987;84:4495–4499. doi: 10.1073/pnas.84.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC (1988) The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Longman Scientific and Technical, Essex, UK
- Hunt S, Gaito S, Layzell DB. Model of gas exchange and diffusion in legume nodules. II. Characterization of the diffusion barrier and estimation of the infected cell concentration of CO2, H2 and N2. Planta. 1988;173:128–141. doi: 10.1007/BF00394497. [DOI] [PubMed] [Google Scholar]
- Hunt S, King BJ, Canvin DT, Layzell DB. Steady and non-steady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987;84:164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, King BJ, Layzell DB. Effects of gradual increases in O2 concentration on nodule activity in soybean. Plant Physiol. 1989;91:315–321. doi: 10.1104/pp.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Layzell DB. Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Physiol Mol Biol. 1993;44:483–511. [Google Scholar]
- Iannetta PPM, de Lorenzo C, James EK, Fernandez-Pascual M, Sprent JI, Lucas MM, Witty JF, de Felipe MR, Minchin FR. Oxygen diffusion in lupin nodules I: visualization of diffusion barrier operation. J Exp Bot. 1993a;44:1461–1467. [Google Scholar]
- Iannetta PPM, James EK, McHardy PD, Sprent JI, Minchin FR. An ELISA procedure for quantification of relative amounts of intercellular glycoprotein in legume nodules. Ann Bot. 1993b;71:85–90. [Google Scholar]
- Iannetta PPM, James EK, Sprent JI, Minchin FR. Time-course of changes involved in the operation of the oxygen diffusion barrier in white lupin nodules. J Exp Bot. 1995;46:565–575. [Google Scholar]
- James EK, Minchin FR, Iannetta PPM, Sprent JI. Temporal relationships between nitrogenase and intercellular glycoprotein in developing white lupin nodules. Ann Bot. 1997;79:493–503. [Google Scholar]
- James EK, Sprent JI, Minchin FR, Brewin NJ. Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ. 1991;14:467–476. [Google Scholar]
- Jensen WA. Botanical Histochemistry. San Francisco, CA: W.H. Freeman; 1962. [Google Scholar]
- Kapros T, Bögre L, Németh K, Bak L, Györgyey J, Wu SC, Dudits D. Differential expression of histone H3 gene variants during cell cycle and somatic embryogenesis in alfalfa. Plant Physiol. 1992;98:621–625. doi: 10.1104/pp.98.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M, Lamport DTA. Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 1994;5:157–172. doi: 10.1046/j.1365-313x.1994.05020157.x. [DOI] [PubMed] [Google Scholar]
- King BJ, Hunt S, Weagle GE, Walsh KB, Pottier RH, Canvin DT, Layzell DB. Regulation of O2 concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988;87:296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzma MM, Hung S, Layzell DB. Role of oxygen in the limitation and inhibition of nitrogenase activity and respiration rate in individual soybean nodules. Plant Physiol. 1993;101:161–169. doi: 10.1104/pp.101.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layzell DB, Hunt S, Palmer GR. Mechanism of nitrogenase inhibition in soybean nodules. Plant Physiol. 1990;92:1101–1107. doi: 10.1104/pp.92.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M, Hirsch AM. An alfalfa (Medicago sativa L.) cDNA encoding an acidic leghemoglobin (MsLb3) Plant Mol Biol. 1992;20:733–736. doi: 10.1007/BF00046457. [DOI] [PubMed] [Google Scholar]
- Masepohl B, Witty JF, Riedel K-U, Klipp W, Pühler A. Rhizobium meliloti mutants defective in symbiotic nitrogen fixation affect the oxygen gradient in alfalfa (Medicago sativa) root nodules. J Exp Bot. 1993;44:419–426. [Google Scholar]
- McKhann HI, Hirsch AM. In situ localization of specific mRNAs in plant tissues. In: Thompson JE, Glick BR, editors. Methods in Plant Molecular Biology and Biotechnology. Boca Raton, FL: CRC Press; 1993. pp. 173–205. [Google Scholar]
- Nap J-P, Bisseling T. The roots of nodulins. Physiol Plant. 1990;79:407–414. [Google Scholar]
- Padilla JE, Miranda J, Sánchez F. Nodulin regulation in common bean nodules induced by bacterial mutants. Mol Plant Microbe Inter. 1991;4:433–439. [Google Scholar]
- Parsons R, Day DA. Mechanism of soybean nodule adaptation to different oxygen pressures. Plant Cell Environ. 1990;13:501–512. [Google Scholar]
- Ruvkun GB, Ausubel FM. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci USA. 1981;77:191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sheehey JE, Minchin FR, Witty JF. Biological control of the resistance to oxygen flux in nodules. Ann Bot. 1983;52:565–571. [Google Scholar]
- Sherrier DJ, VandenBosch KA. Localization of repetitive proline-rich proteins in the extracellular matrix of pea root nodules. Protoplasma. 1994;183:148–161. [Google Scholar]
- Signorella AP, Hymer WC. An enzyme-linked immunosorbent assay for rat prolactin. Anal Biochem. 1984;136:372–381. doi: 10.1016/0003-2697(84)90232-x. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981;67:143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Legocki AB. Isolation and nucleotide sequence of cDNA clone encoding nodule-specific (hydroxy)proline-rich protein LENOD2 from yellow lupin. Plant Mol Biol. 1990;15:361–363. doi: 10.1007/BF00036922. [DOI] [PubMed] [Google Scholar]
- Thumfort PP, Atkins CA, Layzell DB. A re-evaluation of the role of the infected cell in the control of O2 diffusion in legume nodules. Plant Physiol. 1994;105:1321–1333. doi: 10.1104/pp.105.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema JD, Yocum CC. Measurement of oxygen partial pressure within soybean nodules. Planta. 1974;119:351–360. doi: 10.1007/BF00388335. [DOI] [PubMed] [Google Scholar]
- Trese AT, Pueppke SG. Cloning of cowpea (Vigna unguiculata) genes that are regulated during initiation of nodulation. Mol Plant Microbe Inter. 1991;4:46–51. [Google Scholar]
- Uchiumi T, Traut RR, Kominami R. Monoclonal antibodies against acidic phosphoproteins P0, P1, and P2 of eukaryotic ribosomes as functional probes. J Biol Chem. 1990;265:89–95. [PubMed] [Google Scholar]
- Van Cauwenberghe OR, Hunt S, Newcomb W, Canny MJ, Layzell DB. Evidence that short-term regulation of nodule permeability does not occur in the inner cortex. Physiol Plant. 1994;91:477–487. [Google Scholar]
- VandenBosch KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin NJ. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989;8:335–342. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBosch KA, Rodgers LR, Sherrier D, Kishinevsky BD. A peanut nodule lectin in infected cells and in vacuoles and the extracellular matrix of nodule parenchyma. Plant Physiol. 1994;104:327–337. doi: 10.1104/pp.104.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wiel C, Norris JH, Bochenek B, Dickstein R, Bisseling T, Hirsch AM. Nodulin gene expression and ENOD2 localization in effective, nitrogen-fixing and ineffective, bacteria-free nodules of alfalfa. Plant Cell. 1990a;2:1009–1017. doi: 10.1105/tpc.2.10.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wiel C, Scheres B, Franssen H, van Lierop MJ, van Lammeren A, van Kammen A, Bisseling T. The early nodulin transcript ENOD2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J. 1990b;9:1–7. doi: 10.1002/j.1460-2075.1990.tb08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn P, Fang Y, Galili S, Shaul O, Atzmon N, Wininger S, Eshead Y, Lum M, Li Y, To V and others. Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction pathways used in forming arbuscular mycorrhizae and Rhizobium-induced nodules may be conserved. Proc Natl Acad Sci USA. 1997;94:5467–5472. doi: 10.1073/pnas.94.10.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J, deBilly F, Camut S, Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Skøt L, Revsbech NP. Direct evidence for changes in the resistance of legume root nodules to O2 diffusion. J Exp Bot. 1987;38:1129–1140. [Google Scholar]