Abstract
The PH domain-containing proteins Slm1 and Slm2 were originally identified as substrates of the rapamycin-insensitive TOR complex 2 (TORC2) and as mediators of signaling by the lipid second messenger phosphatidyl-inositol-4,5-bisphosphate (PI4,5P2) in budding yeast S. cerevisiae. More recently, these proteins have been identified as critical effectors that facilitate phosphorylation and activation of the AGC kinases Ypk1 and Ypk2 by TORC2.1 Here, we review the molecular basis for this regulation as well as place it within the context of recent findings that have revealed Slm1/2 and TORC2-dependent phosphorylation of Ypk1 is coupled to the biosynthesis of complex sphingolipids and to their levels within the plasma membrane (PM) as well as other forms of PM stress. Together, these studies reveal the existence of an intricate homeostatic feedback mechanism, whereby the activity of these signaling components is linked to the biosynthesis of PM lipids according to cellular need.
Keywords: budding yeast, rapamycin, lipids, kinases, signal transduction
Regulation of cell growth in eukaryotic organisms is controlled in part by the evolutionarily conserved target of rapamycin (TOR) signaling network. TOR is large protein kinase within the PI3 kinase-like family of protein kinases and functions within two distinct complexes, termed TORC1 and TORC2, whereby TORC1 is uniquely inhibited by the immunosuppressant rapamycin (recently reviewed in ref. 2). Several studies have revealed that an important function for these complexes is the specific recognition and phosphorylation of distinct AGC kinases (named for their similarity to mammalian protein kinases A, G and C).3 In mammalian cells, mTORC1 has been shown to recognize S6K1, while mTORC2 recognizes AKT (PKB) and SGK.4-6 For AKT and SGK, plasma membrane (PM) localization has been found to be a crucial step in their activation. This is accomplished in part by the presence of lipid-targeting domains, where AKT contains an N terminal pleckstrin homology (PH) domain and SGK1, which differs from other SGK isoforms at the N terminus, contains a Phox homology (PX)-like sequence that regulates its PM localization.7,8 Both mTORC1 and mTORC2 phosphorylate residues within so-called turn and hydrophobic motifs (TM and HM, respectively).5,6 For many, but not all, of these AGC kinases, phosphorylation at these sites is necessary for subsequent phosphorylation at a conserved serine within their activation loop (A-loop) by the PDK1 kinase.7,9
This specificity of the TOR complexes to recognize distinct AGC kinases extends to budding yeast, where TORC1 has been shown to specifically phosphorylate Sch9, the presumptive ortholog of S6K1, whereas TORC2 specifically phosphorylates Ypk2, a presumptive ortholog of Akt and/or SGK.10,11 We have demonstrated recently,1 as have others,12,13 that the closely related Ypk1 kinase is also a specific target of TORC2. For our studies, we developed a phospho-specific antibody directed against the HM site of Ypk1.1 Using this antibody, we observed a decrease in phosphorylation at the HM site in a strain that harbors a temperature sensitive (ts) mutation in the essential TORC2 component Avo3 following a shift of cells to non-permissive temperatures. Interestingly, under these conditions we also observed reduced A-loop phosphorylation by the Pkh1/2 kinases, orthologs of PKD1, using a phospho-specific antibody directly against this site. This latter result suggests that phosphorylation of Ypk1 by TORC2 increases its efficiency as a target for Pkh1/2, in support of a two-step model for AGC kinase activation.
Both TORC2 and Pkh1/2 are at or within close proximity to the PM, where they adopt distinct punctate patterns of localization.14 A number of studies suggest further that these kinases associate with unique membrane environments, whereby TORC2 associates with what has been termed the MCT (membrane compartment specific for TORC2), whereas Pkh1/2 associate with the MCC (membrane compartment specific for the Can1 amino acid transporter).14,15 The MCC is also very closely associated with eisosomes, large PM-associated protein complexes that have been proposed to be involved in PM organization.16 Thus, to be phosphorylated and activated, Ypk1/2 must likely interact with these components at these specific sites within the PM. However, unlike AKT and SGK1, there is no known lipid-targeting motif within Ypk1/2, and therefore, how they might be recruited to these sites of activation has remained mysterious. To address this issue, we focused our efforts on two other previously identified TORC2 substrates, namely, the PH domain-containing proteins Slm1 and Slm2. These seemed to be likely candidates to be involved in Ypk1/2 regulation, because previous findings have suggested a close correlation between processes regulated by Ypk1/2 and Slm1/2, including maintenance of actin polarization and sphingolipid metabolism.17,18 Moreover, by constructing and characterizing ATP-analog sensitive (AS) alleles of Ypk1 and Ypk2, we determined that these kinases negatively regulate a concise set of stress responsive genes, including genes regulated by the transcription factor Crz1, whose activity is positively controlled by the Ca2+-dependent phosphatase calcinceurin.19 This was an important observation, because previous studies have linked TORC2 to the regulation of calcineurin via Slm1/2 by an undetermined mechanism.20 Based on our findings, we hypothesized that Slm1/2 could play an essential role in the function of Ypk1/2 and provide a mechanism for negative regulation of calcineurin.
To better understand the functional relationship between Ypk1/2 and Slm1/2, we examined the role of the Slm proteins in regulating the activity of Ypk1, which we monitored by measuring phosphorylation of the Ypk1 target Fpk1. Because loss of both Slm1 and Slm2 is lethal, we constructed a strain that was also deleted for SAC7, the gene encoding the Rho1 GTPase activating protein (GAP), which was been shown to suppress the lethality of a slm1Δ slm2Δ strain.18 In this strain lacking Slm1/2, we observed that phosphorylation of Fpk1 was significantly reduced compared with WT or slm1Δ sac7Δ cells, suggesting that Ypk1 activity is in fact impaired in the absence of Slm1/2. Additionally, we examined the phosphorylation state of the A-loop and HM sites in Ypk1 in the absence of Slm1/2 using the phosphospecific antibodies described above. Here, we observed decreased phosphorylation at both sites, revealing that Slm1/2 are important for Ypk1 activation.
To test whether the Slm proteins are required specifically for TORC2-dependent events, we constructed a mutant allele of Ypk1, Ypk1D242A, based on the identification of a similar mutation in Ypk2 that allows cells to live in the absence of proper TORC2 function but still requires Pkh1/2 activity.11 We observed that this TORC2-independent or “bypass” allele of Ypk1 suppressed the lethality of slm1Δ slm2Δ cells, suggesting Slm1/2 is indeed required for TORC2-dependent activation of Ypk1. We tested this further by combining the D242A allele with mutations at both the TM and HM sites that prevent their phosphorylation and observed that this Ypk1 triple mutant also rescued the lethality of a slm1Δ slm2Δ strain. Thus, the TORC2-independent allele of Ypk1 obviates a requirement for both TORC2 and Slm1/2 activity. Importantly, the D242A allele does not suppress a non-phosphorylatable mutation within the A-loop of Ypk1, demonstrating a continued requirement for Pkh1/2. Taken together, these data indicate that Slm1/2 is specifically required for phosphorylation and activation of Ypk1 by TORC2.
These findings raised the question as to how the Slm1/2 proteins might contribute, mechanistically, to Ypk1 activation. All presently available evidence supports a model wherein one important role for Slm1/2 is to recruit Ypk1 to the PM membrane for phosphorylation by TORC2. This model is based on a number of findings, including an altered localization pattern of Ypk1 in the absence of the Slms. Thus, in agreement with previous studies,21,22 we observed that GFP tagged-Ypk1 localizes throughout the cell, with an enriched pool at the PM. In slm1Δ slm2Δ sac7Δ cells, however, Ypk1 is localized exclusively within the cytoplasm, and no detectable PM enrichment is observed. Because Slm1 and Slm2 associate with the PM, we also tested whether Slm1 and Ypk1 interact physically. For this, we performed co-precipitation experiments from whole cell extracts, where we observed that a portion of Ypk1 and Slm1 do indeed associate, consistent with the notion that physical interactions between these proteins is necessary for proper PM targeting of Ypk1.1
As these findings suggested that Slm-mediated recruitment to the PM is essential for TORC2 phosphorylation of Ypk1, we tested whether independently targeting Ypk1 to the PM would result in phosphorylation by TORC2. First, we fused the PH domain of Slm1 alone onto GFP- and HA-tagged versions of Ypk1, where we observed that in slm1Δ slm2Δ sac7Δ cells, the fusion protein localizes uniformly at the PM. Moreover, unlike WT Ypk1, this fusion protein is phosphorylated at both the HM and A-loop sites in these cells. We also fused the entire Slm1 protein to Ypk1, which we observed localizes in a punctate pattern at the PM, very similar to WT Slm1. This fusion is also phosphorylated at both the HM and A-loop sites in slm1Δ slm2Δ sac7Δ cells. Importantly, both of the fusion proteins tested are completely functional, based on their ability to replace WT Ypk1 in a plasmid shuffle assay. Taken together, these findings reveal that Slm1/2 play an essential role in the activation of Ypk1, in part by facilitating its localization at the PM, allowing for phosphorylation by TORC2 and, subsequently, by Pkh1/2.1
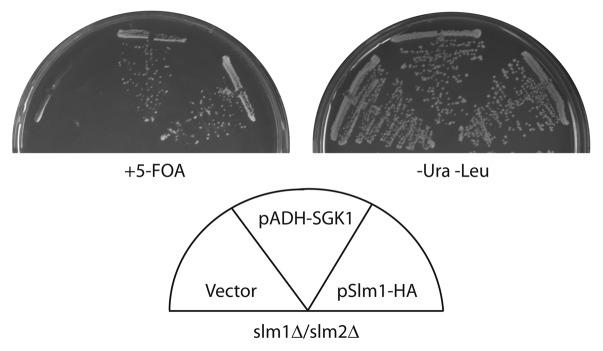
To extend these findings, we were intrigued by results of a previous study demonstrating that SGK1, a mammalian ortholog of Ypk1/2 and a target of mTORC2, can rescue the lethality of ypk1Δ ypk2Δ cells, indicating it can substitute functionally for these kinases.23 Importantly, SGK1 has a lipid-targeting domain that facilitates its interaction with the PM in mammalian cells, where no orthologs of Slm1/2 have been identified to date. Accordingly, we tested whether SGK1 could also rescue the lethality of slm1Δ slm2Δ cells. Here, we used a plasmid shuffle approach and observed that expression of SGK1 can indeed support growth of cells lacking Slm1/2 (Fig. 1). These findings reinforce the idea that targeting to the membrane is an important step in the activation of AGC kinases that are targets of TORC2/mTORC2.
Figure 1. SGK rescue of slm1Δslm2Δ. (A) Strain PLY1357 (slm1Δslm2Δ pPL421) was transformed with a control vector (pPL420), pADH-SGK (a generous gift of J. Thorner23) or pPL422 (pRS315Met25-Slm1-3HA), described in reference 1. The resulting transformants were streaked out onto SCD minus uracil and leucine or onto 5-Fluoroorotic acid (5-FOA) solid agar plates and grown at 30°C for ~2 days.
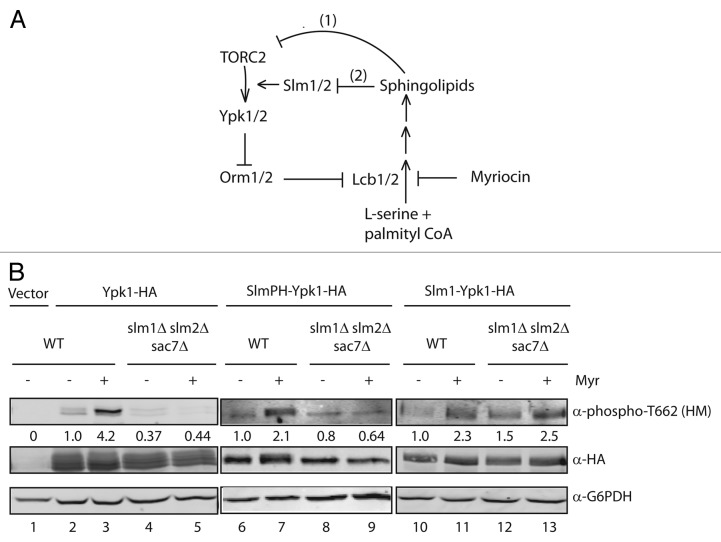
While our findings demonstrate the importance of the Slm proteins for PM recruitment and activation of Ypk1 (and presumably for Ypk2 as well), an outstanding question concerns the nature of the upstream signals and molecular events that regulate interactions between TORC2, Slm1/2 and Ypk1/2. In this regard, two recently published papers provide evidence that levels of complex sphingolipids within the PM provide an important link to Ypk1 regulation.12,13 Sphingolipids play an essential cellular role as a structural component of the PM, and we have shown previously that their biosynthesis is regulated by TORC2 signaling.24 Thorner and colleagues have now shown that TORC2-dependent phosphorylation of Ypk1 is negatively regulated by sphingolipids. In particular, these authors found that treating cells with myriocin, which inhibits the first step of sphingolipid biosynthesis, results in a significant increase in phosphorylation of Ypk1 at position T662.13 It was proposed that this increase in Ypk1 activity is part of a homeostatic response to a decrease in sphingolipid biosynthesis. Specifically, Ypk1 phosphorylates and represses the activity of two proteins, Orm1 and Orm2, which are, in turn, negative regulators of the first step in sphingolipid biosynthesis.25 Thus, Ypk1-dependent phosphorylation and inhibition of Orm1/2 is proposed to enable cells to respond to sphingolipid depletion. The molecular target for sphingolipids was not identified in this study, although it was speculated that they may directly inhibit TORC2 activity13 (Fig. 2A, scheme 1).
Figure 2. Myriocin-induced TORC2 phosphorylation of Ypk1 requires Slm1/2. (A) Model for sphingolipid regulation of TORC2 activity. Scheme 1 refers to the model described in13 describes a regulation of TORC2 activity by level of sphingolipids, while Scheme 2 refers to the model from12 describing sphingolipid regulation of Slm1/2 localization as a major influence on the ability of TORC2 to phosphorylate Ypk1. See text for details. (B) WT (SEY6210) and slm1Δslm2Δsac7Δ (PLY1447) strains expressing empty vector (pPL420), Ypk1-HA (pPL433) or SlmPH-Ypk1-HA (pPL495) were grown at 30°C as described in reference 1. Protein extracts were prepared using the NaOH cell lysis method30 and loaded onto SDS-PAGE gels and transferred to nitrocellulose membrane. Membranes were probed with α-HA (Covance; 12CA5, 1:5000), α-phospho-Ypk1 (T662) (1:20,000; described in ref. 1) and α-G6PDH (Sigma-Aldrich; 1:100,000) primary antibodies, and visualized using the appropriate secondary antibodies conjugated to IRDye (LI-COR Biosciences; 1:5000) on the Odyssey Infrared Imaging System (LI-COR Biosciences). Quantification below the blot describes the difference relative to WT after normalizing to the α-HA signal.
More recently, Loewith and colleagues have demonstrated that Slm1/2 provide an important link to sphingolipid-mediated regulation of Ypk1.12 In agreement with our findings described above, these authors confirm that the Slm proteins are required for TORC2-dependent phosphorylation of Ypk1. Moreover, they observe that Slm1/2 are also required for myriocin-induced hyper-phosphorylation of T662, suggesting the Slm proteins are part of the regulatory scheme proposed by Thorner and coworkers (Fig. 2A, scheme 2). Furthermore, it was observed that inhibition of sphingolipid biosynthesis results in a significant movement of Slm1 from a location that is predominantly at eisosomes to a location that is predominantly at the MCT compartment associated with TORC2. The authors propose a model wherein depletion of sphingolipids leads to PM stress that causes Slm1 to relocalize to facilitate Ypk1 activation. According to our findings, we would argue that this re-localization of Slm1/2 is important primarily because it directly mediates physical interactions between Ypk1/2 and TORC2. More recently, it has been demonstrated that heat-stress induced sphingolipid biosynthesis is also mediated in part by Ypk1-dependent phosphorylation of Orm1/2.26 We predict this step will also turn out to require the activity of the Slm proteins, in particular given that the phosphorylation state of these proteins have previously been shown to be regulated by heat stress.27
An important question raised by the above findings is whether Ypk1 association with the PM is sufficient for myriocin-induced hyper-phosphorylation by TORC2, or whether the Slm proteins possess an additional function that is influenced by myriocin. We reasoned we could address this question using the Slm1-Ypk1 fusion proteins described above, where the Slm1PH-Ypk1 is uniformly distributed within the PM and the Slm1-Ypk1 fusion adopts an eisosome-like punctate localization pattern at the PM.1 We first confirmed that WT Ypk1 is dependent on the Slm proteins for myriocin-induced phosphorylation via TORC2, in that we observe robust phosphorylation of T662 following drug treatment in WT cells but not in slm1Δ slm2Δ sac7Δ cells (Fig. 2B, compare lanes 3 and 5). We next examined the phosphorylation state of the SlmPH-Ypk1 and Slm1-Ypk1 fusion proteins, where, in contrast to WT Ypk1, both fusions remain phosphorylated at T662 in slm1Δ slm2Δ sac7Δ cells (Fig. 2B, compare lanes 4, 8 and 12). We observed that myriocin treatment stimulated T662 phosphorylation of both fusions in WT cells, but, remarkably, the drug only stimulated phosphorylation of the Slm1-Ypk1 fusion in slm1Δ slm2Δ sac7Δ cells (Fig. 2B, compare lanes 8 and 9, 12 and 13). We conclude from these results that myriocin-induced hyperphosphorylation of Ypk1 requires an intact Slm1 protein that is capable of associating with eisosomes. This latter finding may provide an explanation for our observation that while both of the Slm1-Ypk1 fusion proteins are fully functional as Ypk1 kinases, only the complete Slm1-Ypk1 fusion complements the lethality of a slm1Δ slm2Δ double mutant (our unpublished results). Thus, one possibility is that the ability to stimulate Ypk1 phosphorylation in response to PM stress represents an essential function for the Slm proteins and may involve direct interactions with TORC2.
Our understanding of how TORC2 signaling is modulated is still at an early stage. Thus, the identification of a role for Slm1/2 in mediating TORC2-dependent phosphorylation of Ypk1 represents an important advance in the field. Moreover, the fact that this regulation is responsive to PM stress, including the levels of complex sphingolipids, provides an important clue as to the physiological relevance of interactions between these components. A number of outstanding questions remain, however, regarding the function and regulation of Slm1/2. For example, the Slms are themselves targets of TORC2, and their phosphorylation state is known to be regulated by levels of the phosphoinositide PI(3,4)P2.18,28 Interestingly, TORC2 is also partially required for Slm1 localization.18 Thus, how other PM lipids and TORC2 phosphorylation affect Slm1/2 mobilization and recruitment of Ypk1 to TORC2 remains to be determined. In addition, how these events might be linked to other upstream regulatory interactions, including the proposed role of ribosomes in modulating TORC2 activity,29 is another important area for future study.
Glossary
Abbreviations:
- PH
pleckstrin homology
- PM
plasma membrane
- TOR
target of rapamycin
- TORC2
TOR complex 2
- AS
analog-sensitive alleles
- TM
turn motif
- HM
hydrophobic motif
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21752
References
- 1.Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T. Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci USA. 2012;109:1536–41. doi: 10.1073/pnas.1117563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–96. [PubMed] [Google Scholar]
- 4.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 7.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–60. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pao AC, McCormick JA, Li H, Siu J, Govaerts C, Bhalla V, et al. NH2 terminus of serum and glucocorticoid-regulated kinase 1 binds to phosphoinositides and is essential for isoform-specific physiological functions. Am J Physiol Renal Physiol. 2007;292:F1741–50. doi: 10.1152/ajprenal.00027.2007. [DOI] [PubMed] [Google Scholar]
- 9.Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20:4380–90. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–74. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, et al. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol. 2005;25:7239–48. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, et al. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol. 2012;14:542–7. doi: 10.1038/ncb2480. [DOI] [PubMed] [Google Scholar]
- 13.Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2011;108:19222–7. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berchtold D, Walther TC. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell. 2009;20:1565–75. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinska K, Malinsky J, Opekarova M, Tanner W. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci. 2004;117:6031–41. doi: 10.1242/jcs.01493. [DOI] [PubMed] [Google Scholar]
- 16.Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- 17.Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol. 2006;26:5861–75. doi: 10.1128/MCB.02403-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audhya A, Loewith R, Parsons AB, Gao L, Tabuchi M, Zhou H, et al. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 2004;23:3747–57. doi: 10.1038/sj.emboj.7600384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–44. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulet JM, Martin DE, Loewith R, Hall MN. Mutual antagonism of target of rapamycin and calcineurin signaling. J Biol Chem. 2006;281:33000–7. doi: 10.1074/jbc.M604244200. [DOI] [PubMed] [Google Scholar]
- 21.Roelants FM, Torrance PD, Bezman N, Thorner J. Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell. 2002;13:3005–28. doi: 10.1091/mbc.E02-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, et al. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol. 2000;20:4411–9. doi: 10.1128/MCB.20.12.4411-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casamayor A, Torrance PD, Kobayashi T, Thorner J, Alessi DR. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr Biol. 1999;9:186–97. doi: 10.1016/S0960-9822(99)80088-8. [DOI] [PubMed] [Google Scholar]
- 24.Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, et al. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab. 2008;7:148–58. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–53. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Miao Y, Yamane Y, Zhang C, Shokat KM, Takematsu H, et al. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Mol Biol Cell. 2012;23:2388–98. doi: 10.1091/mbc.E12-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daquinag A, Fadri M, Jung SY, Qin J, Kunz J. The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress. Mol Cell Biol. 2007;27:633–50. doi: 10.1128/MCB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadri M, Daquinag A, Wang S, Xue T, Kunz J. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol Biol Cell. 2005;16:1883–900. doi: 10.1091/mbc.E04-07-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–68. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Dilova I, Aronova S, Chen JC, Powers T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1.Rtg3p-dependent target genes. J Biol Chem. 2004;279:46527–35. doi: 10.1074/jbc.M409012200. [DOI] [PubMed] [Google Scholar]