Abstract
Valproic acid (VPA) is used widely to treat epilepsy and bipolar disorder. Women undergoing VPA treatment reportedly have an increased incidence of polycystic ovarian syndrome (PCOS)-like symptoms including hyperandrogenism and oligo- or amenorrhoea. To investigate potential direct effects of VPA on ovarian steroidogenesis we used primary bovine theca (TC) and granulosa (GC) cells maintained under conditions that preserve their ‘follicular’ phenotype. Effects of VPA (7.8–500 µg/ml) on TC were tested with/without LH. Effects of VPA on GC were tested with/without FSH or IGF analogue. VPA reduced (P<0.0001) both basal (70% suppression; IC50 67±10 µg/ml) and LH-induced (93% suppression; IC50 58±10 µg/ml) androstenedione secretion by TC. VPA reduced CYP17A1 mRNA abundance (>99% decrease; P<0.0001) with lesser effects on LHR, STAR, CYP11A1 and HSD3B1 mRNA (<90% decrease; P<0.05). VPA only reduced TC progesterone secretion induced by the highest (luteinizing) LH dose tested; TC number was unaffected by VPA. At higher concentrations (125–500 µg/ml) VPA inhibited basal, FSH- and IGF-stimulated estradiol secretion (P<0.0001) by GC without affecting progesterone secretion or cell number. VPA reversed FSH-induced upregulation of CYP19A1 and HSD17B1 mRNA abundance (P<0.001). The potent histone deacetylase (HDAC) inhibitors trichostatin A and scriptaid also suppressed TC androstenedione secretion and granulosal cell oestrogen secretion suggesting that the action of VPA reflects its HDAC inhibitory properties. In conclusion, these findings refute the hypothesis that VPA has a direct stimulatory action on TC androgen output. On the contrary, VPA inhibits both LH-dependent androgen production and FSH/IGF-dependent estradiol production in this in vitro bovine model, likely by inhibition of HDAC.
Introduction
Epilepsy is a common disorder affecting over 1% of the population, including almost one million women of child bearing age [1]. Therapeutic drugs successfully control seizures in about 70% of patients. However, medication is long-term and side effects are common; these include effects on the reproductive endocrine system of both males and females (reviews: [2], [3], [4]). One of the most widely prescribed anti-epileptic drugs is valproic acid (VPA), a branched-chain fatty acid with anti-convulsant and mood stabilizing properties (review [5]). VPA is also used in the treatment of bipolar disorders, migraines and neuropathic pain. The anti-convulsant and mood stabilizing properties of VPA have been attributed to modulation of voltage-dependent sodium channels, enhancement of GABA inhibitory neurotransmission and/or decreased cerebral glucose metabolism [5].VPA is also known to affect various intracellular signal transduction pathways including MAPK, PKB and PKC-mediated pathways [6], [7] as well as being an inhibitor of type 1 histone deacetylase (HDAC) [8].
Over the past 15 years it has emerged that there is an increased incidence of polycystic ovarian syndrome (PCOS)-like symptoms in epileptic women taking VPA suggesting that the drug can perturb ovarian function and androgen synthesis, possibly as a result of multiple effects on the hypothalamic-pituitary-ovarian axis (reviews: [2], [3], [5], [9]). PCOS is a very common reproductive endocrine disorder affecting 6–8% of women of reproductive age [10], [11]; despite intensive research, its aetiology remains largely unknown. PCOS is usually defined by the presence of hyperandrogenism (in the absence of specific adrenal and/or pituitary disease), oligo- or amenorrhoea and characteristic ‘polycystic’ ovarian morphology as revealed by ultrasonography [11], [12]. However, PCOS is also strongly associated with obesity, insulin resistance and hyperinsulinemia, features of the so-called ‘metabolic syndrome’ [10], [12].
The association between VPA treatment and PCOS-like symptoms was first reported by Isojarvi et al. [13] who found that almost 50% of women treated for epilepsy with VPA had amenorrhea, oligomenorrhoea or prolonged menstrual cycles compared with 19% of women taking carbamazepine, another anti-epileptic drug. In a later study Isojarvi et al. [14] reported that 64% of women receiving VPA had polycystic ovaries and/or hyperandrogenism. In addition to raised serum androgen levels these women were obese, had high fasting serum insulin levels and low levels of serum insulin-like growth factor-binding protein 1. O’Donovan et al. [15] reported that 41% of women taking VPA medication for bipolar disorder exhibited PCOS; more women reported menstrual abnormalities in the VPA group (47%) than in the group not receiving VPA (13%). Other studies [16], [17] have documented higher plasma testosterone and free-androgen levels in women treated with VPA.
Several mechanistic studies, both in vivo and in vitro, have investigated the link between VPA exposure, PCOS-like symptoms and ovarian hyperandrogenism (review: [4]). Most of these have relied on animal models. For instance, Tauboll et al. [18] reported that chronic VPA treatment in rats increased the number of follicular ‘cysts’ and raised total ovarian weight but decreased plasma testosterone concentrations. In contrast, Sveberg Roste et al. [19] found that chronic VPA exposure in female rats did not affect serum androgen levels but dramatically reduced serum estrogen levels, thus raising the androgen: estrogen ratio. Ferin et al. [20] reported that long-term VPA treatment in normally cycling rhesus monkeys had no effect on androgen levels or ovarian morphology. In an in vitro study using propagated human ovarian thecal cells (TC) it was shown that VPA treatment augmented ovarian androgen synthesis and increased transcription of steroidogenic genes [21]. However, Fisseha et al. [22] reported an inhibitory effect of VPA on hCG-induced androgen secretion by rat theca-interstitial cells. Using a porcine TC-GC co-culture model, Tauboll et al. [23] found that VPA increased basal and LH-stimulated androgen secretion by cells from small follicles but decreased LH-stimulated androgen secretion by cells from medium-size follicles. In addition, they showed that VPA reduced basal and FSH-induced estradiol secretion.
Given these inconsistencies in the literature regarding the effects of VPA on the female reproductive endocrine axis, the aim of the present study was to re-evaluate the potential direct ovarian effects of VPA using well-established bovine TC and GC culture models in which the cells retain a non-luteinized ‘follicular’ phenotype and are highly responsive to LH and FSH, respectively. The cow, like the human, is a mono-ovulatory species and human ovaries share many more similarities with bovine ovaries than they do with rodent or porcine ovaries. As such the bovine ovary is regarded as an excellent model for the human ovary [24], [25].
Results
Effect of VPA on Basal and LH-induced Androstenedione and Progesterone Secretion by TC
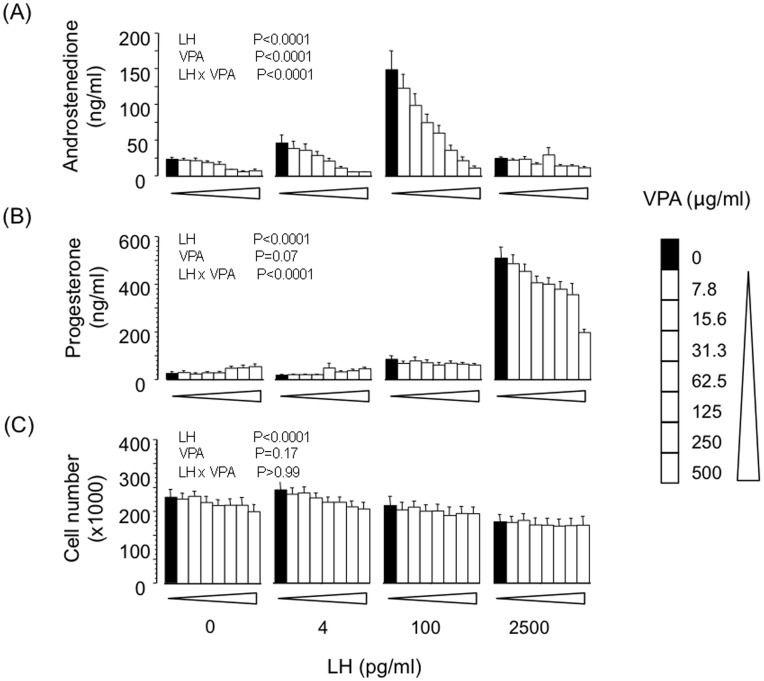
Consistent with our previous observations using this in vitro TC model [26] treatment of cells with an optimal dose-level of LH (100 pg/ml) promoted a robust increase in androstenedione secretion (∼6-fold; P<0.0001) but did not affect progesterone secretion ( Fig. 1 ). At a much higher ‘luteinizing’ dose-level (2500 pg/ml) LH promoted a marked increase in secretion of progesterone but not androstenedione. A small though significant (P<0.0001) decrease in cell number was also elicited by LH. VPA had a marked and dose-dependent suppressive effect on basal androstenedione secretion (IC50 67±10 µg/ml; P<0.0001) and androstenedione secretion induced by the optimal dose-level of LH (IC50 58±10 µg/ml; P<0.0001). VPA also had a modest suppressive effect on progesterone secretion (IC50>250 µg/ml) induced by the highest LH dose-level. VPA had no effect on viable cell number at the end of culture ( Fig. 1 ).
Figure 1. Effects of VPA on basal and LH-induced secretion of (A) androstenedione and (B) progesterone by bovine theca-interna cells; panel (C) shows viable cell number at the end of culture.
Values are means ± SEM (n = 4 independent experiments). Results of 2-way ANOVA are indicated.
Effect of VPA and Basal and LH-induced Gene Expression in TC
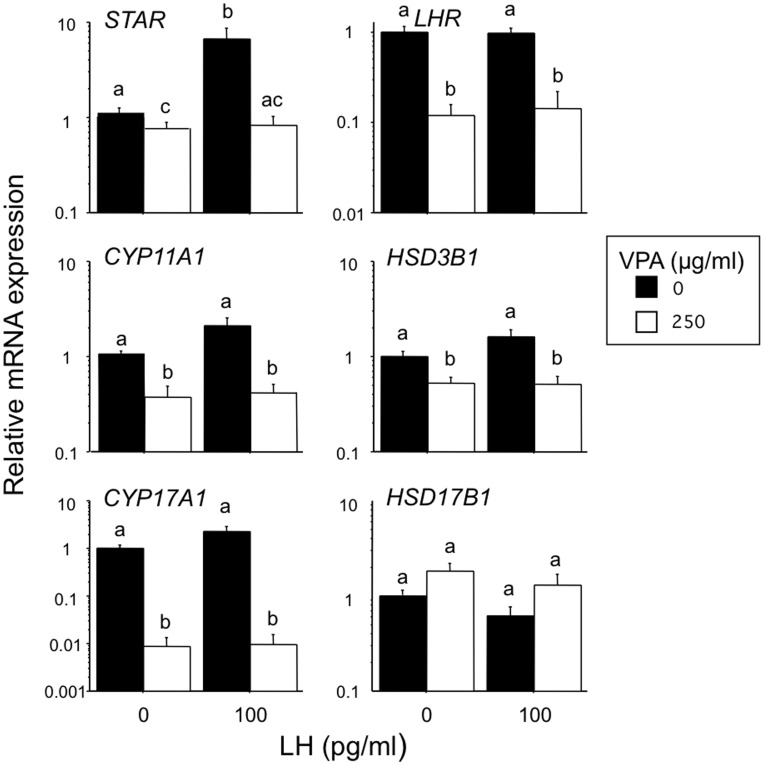
The effects of VPA (250 µg/ml) and LH (100 pg/ml) on the relative abundance of six key mRNA transcripts involved in TC steroidogenesis are shown in Fig. 2 . LH increased the abundance of STAR mRNA (5-fold; P<0.01) and tended to increase CYP17A1 mRNA (3-fold, P>0.05) but did not significantly affect LHR, CYP11A1, HSD3B1 or HSD17B1 mRNA abundance. VPA had a profound suppressive effect on CYP17A1 transcript abundance, both in the presence and absence of LH stimulation (>99% reduction; P<0.0001). VPA also reduced expression of LHR, STAR, CYP11A1 and HSD3B1 under basal and LH-stimulated conditions (∼50% to 90% reduction; P<0.05) but to a much lesser extent than the suppression of CYP17A1 mRNA.
Figure 2. Effects of VPA in the presence and absence of LH on the relative abundance of six key steroidogenic transcripts in bovine theca-interna cells.
Values are means ± SEM (n = 7 independent experiments). Means without a common letter are significantly different (P<0.05).
Effect of VPA on Basal, FSH- and IGF-induced Estradiol and Progesterone Secretion by GC
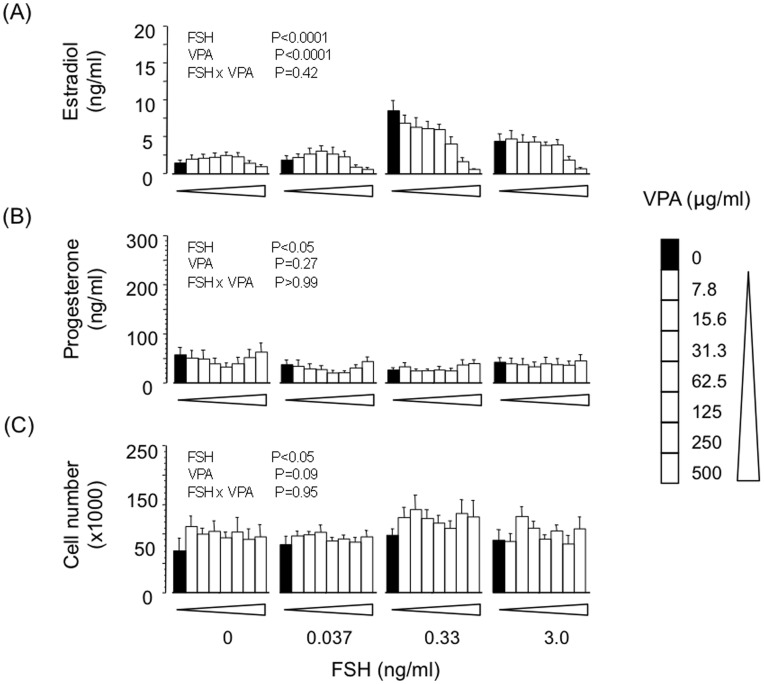
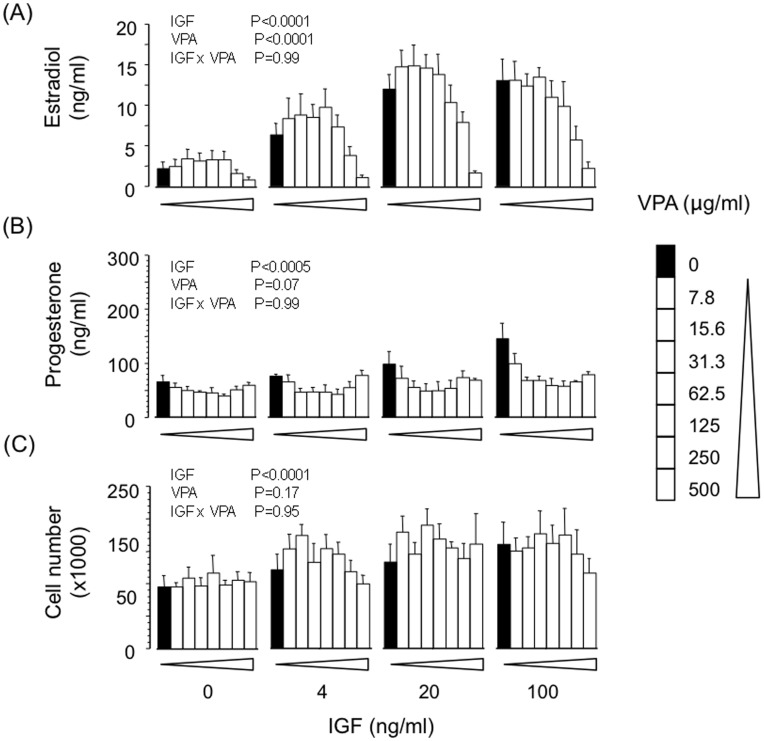
VPA dose-dependently suppressed basal (IC50 ∼250 µg/ml; P<0.01) and FSH-induced (IC50 ∼200 µg/ml; P<0.0001) secretion of estradiol but did not influence progesterone secretion or viable cell number at the end of culture ( Fig. 3 ). Similarly, VPA dose-dependently reversed the stimulatory effect of IGF analogue on estradiol secretion (IC50 ∼250 µg/ml; P<0.001) without affecting progesterone secretion or cell number ( Fig. 4 ).
Figure 3. Effects of VPA on basal and FSH-induced secretion of (A) estradiol and (B) progesterone by bovine granulosa cells; panel (C) shows viable cell number at the end of culture.
Values are means ± SEM (n = 4 independent experiments). Results of 2-way ANOVA are indicated.
Figure 4. Effects of VPA on basal and LR3-IGF-1 (IGF)-induced secretion of (A) estradiol and (B) progesterone by bovine granulosa cells; panel (C) shows viable cell number at the end of culture.
Values are means ± SEM (n = 4 independent experiments). Results of 2-way ANOVA are indicated.
Effect of VPA on Basal, FSH-induced and IGF-induced Gene Expression in GC
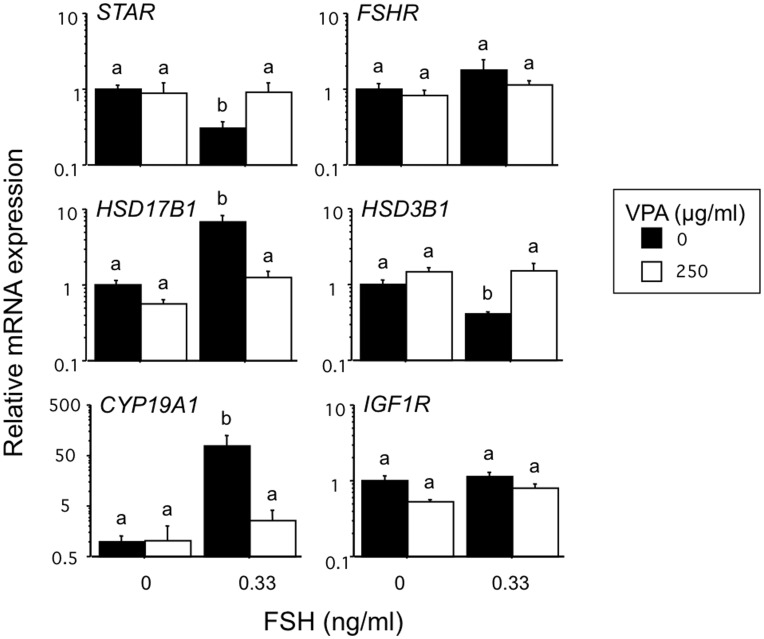
As anticipated FSH greatly increased the relative abundance of CYP19A1 (76-fold; P<0.0001) and HSD17B1 (7-fold; P<0.01) mRNA in GC, paralleling the FSH-induced increase in estradiol secretion by the cells ( Fig. 5 ). Concomitantly, FSH slightly reduced the abundance of STAR and HSD3B1 mRNA, consistent with the small reduction in progesterone secretion observed. VPA reversed the FSH-induced upregulation of CYP19A1 and HSD17B1 expression (P<0.001). Likewise the FSH-induced reduction in STAR and HSD3B1 expression was reversed by VPA treatment. Neither FSH or VPA, alone or in combination, had any significant effect on abundance of FSHR or IGF1R transcripts.
Figure 5. Effects of VPA in the presence and absence of FSH on the relative abundance of six key steroidogenic transcripts in bovine granulosa cells.
Values are means ± SEM (n = 4 independent experiments). Means without a common letter are significantly different (P<0.05).
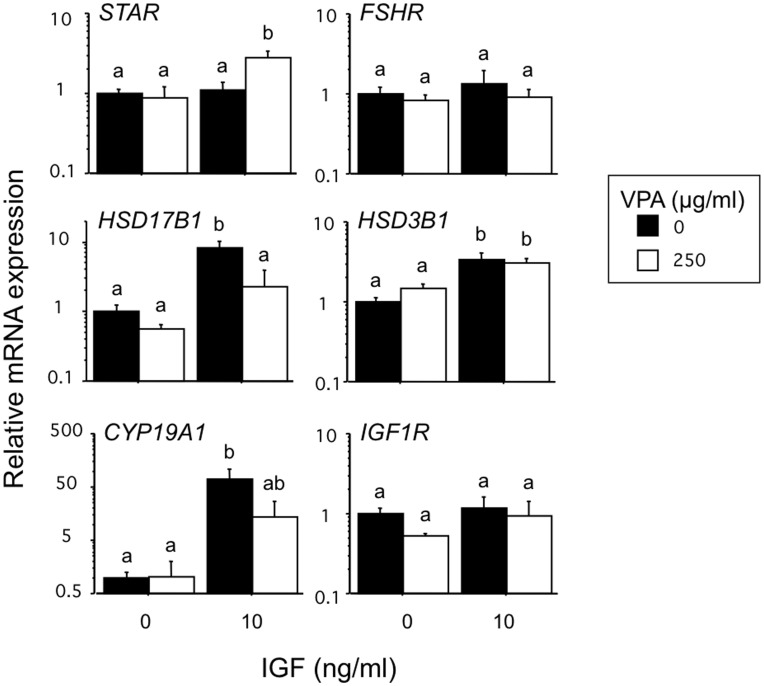
As shown in Fig. 6 treatment of GC with IGF analogue also upregulated the abundance of CYP19A1 (71-fold; P<0.0001) and HSD17B1 (8-fold; P<0.01) transcripts consistent with the observed IGF-induced increase in estradiol secretion. Co-treatment with VPA partially reversed these increases in CYP19A1 and HSD17B1 mRNA. Neither IGF analogue or VPA, alone or in combination, had any significant effect on abundance of FSHR or IGF1R transcripts in GC.
Figure 6. Effects of VPA in the presence and absence of LR3-IGF on the relative abundance of six key steroidogenic transcripts in bovine granulosa cells.
Values are means ± SEM (n = 4 independent experiments). Means without a common letter are significantly different (P<0.05).
Comparison of the Effect of Three Different HDAC Inhibitors on TC Androgen Secretion and GC Estrogen Secretion
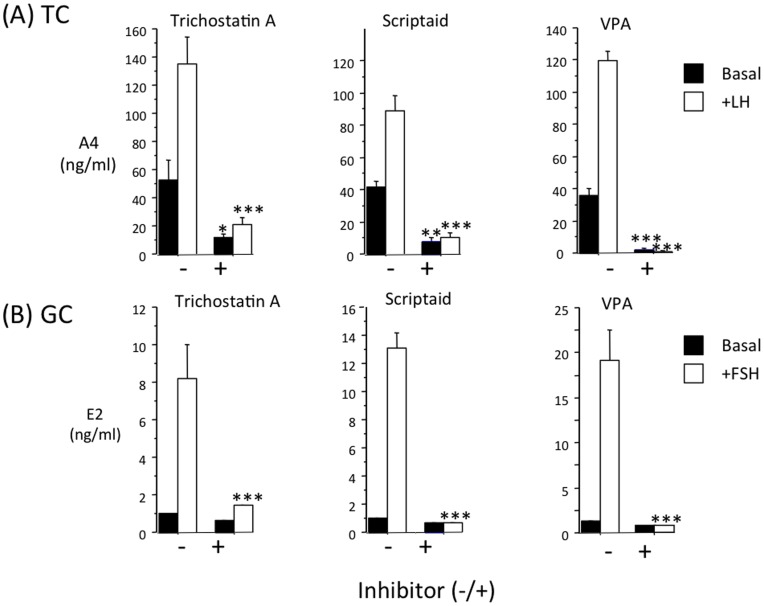
To explore the possibility that the suppressive action of VPA on TC androgen secretion and GC estrogen secretion reflects its HDAC inhibitory properties, we conducted a further experiment to compare the effects of VPA (250 µg/ml; ∼2 mM) with those of two highly potent HDAC inhibitors, scriptaid and trichostatin A at 5 nM and 500 nM, respectively. As shown in Fig. 7 all three compounds inhibited basal and LH-induced secretion of androstenedione by TC and FSH-induced secretion of estrogen by GC.
Figure 7. Three different HDAC inhibitors (trichostatin A; 5 nM), scriptaid; 500 nM) and VPA; 2 mM) have similar inhibitory effects on (A) basal and LH-induced androstenedione secretion by bovine theca cells and (B) basal and FSH-induced estrogen secretion by bovine granulosa cells.
Values are means ± SEM (n = 4 independent cultures). *p<0.05, ***p<0.001 compared to corresponding group without inhibitor.
Discussion
In view of considerable evidence that women undergoing VPA treatment for epilepsy and bipolar disorder show an increased incidence of PCOS-like symptoms including hyperandrogenemia and menstrual cycle disturbances (reviewed by: [2], [3], [5]), research is clearly warranted to try to clarify the mechanism(s) through which this widely prescribed drug might elicit such an effect on ovarian function. Potentially VPA could perturb ovarian function by acting at one or more levels of the hypothalamic-pituitary-ovarian axis, or by modifying some other organ that influences ovarian function indirectly (e.g. pancreatic insulin secretion). This study, together with several previous studies discussed below, sought evidence for a direct action of VPA on ovarian steroidogenesis. Importantly, the present in vitro data presented firmly refute the hypothesis that VPA has a direct stimulatory effect on thecal androgen production.
Using well defined in vitro model systems based on primary cultures of bovine TC [26], [27], [28], [29] and GC [30], [31], [32], [33] the present study shows that VPA has a direct, dose-dependent inhibitory action on ovarian steroidogenesis, suppressing both basal and LH-induced androgen production by TC as well as FSH- and IGF-induced estrogen production by GC. With regard to TC, our findings concur with those of a recent study [22] documenting a VPA-induced inhibition of basal and hCG-induced androgen secretion by rat theca-interstitial cells. Moreover, chronic VPA treatment in vivo was shown to decrease plasma testosterone concentrations in rats [18]. However, these findings contradict those of a study on propagated (4th passage) human TC [21] showing that VPA stimulated basal and forskolin-induced androgen secretion, and raised cellular levels of CYP17A1 (cytochrome P450c17) and CYP11A1 (cytochrome P450scc) protein. In another in vitro study utilizing a porcine TC/GC co-culture model [23] VPA was found to stimulate androgen secretion by small follicles but to inhibit androgen secretion by medium-size follicles. The reasons for these inconsistencies amongst in vitro studies in different laboratories are currently unknown but species differences are likely a major contributory factor, along with methodological differences with regard to culture conditions (cell harvesting, plating density, culture media, use of serum supplementation, culture duration, extent to which cells have been propagated) that may affect the behaviour and/or phenotype of cells in vitro. In the case of the porcine TC/GC co-culture model, another factor complicating the interpretation of these data is the ability of porcine TC to synthesize considerable amounts of estrogen [34]. Bovine TC, like those of human, mouse, rat and sheep, do not express CYP19A1 and are incapable of estrogen synthesis.
The short term primary cultures of bovine TC and GC used in the present study are believed to provide a physiologically relevant model in the sense that the cells retain a non-luteinized (‘follicular’) phenotype over the 6-day culture period under chemically defined, serum-free conditions. These cells are highly responsive to gonadotrophin stimulation in terms of LH-induced androgen secretion by TC, FSH- and IGF-induced estradiol, inhibin and activin secretion by GC and expression of genes involved in steroidogenesis including LHR and CYP17A1 in TC and FSHR and CYP19A1 in GC [26], [27], [28], [29], [31], [32], [33], [35], [36].
Examination of steady-state mRNA levels in control and VPA-treated TC revealed a clear-cut inhibition by VPA of five key genes involved in the steroidogenic response, most notably CYP17A1 (>99% suppression) with a much lesser degree of inhibition of LHR, STAR, CYP11A1 and HSD3B1 transcript abundance. Likewise, Fisseha et al. [22] showed that VPA reduced CYP17A1 expression by rat theca-interstitial cells cultured under both basal and hCG-stimulated conditions, although effects on LHR, STAR, CYP11A1 and HSD3B1 expression were not reported. These authors also showed a comparable inhibitory effect of VPA on the androgen response to 8-bromo-cyclic adenosine-3′5′-monophosphate (8-Br-cAMP), indicating that VPA exerts its suppressive action at a level distal to cAMP generation following LH/CG receptor activation.
The profound suppression of both basal and LH-induced androgen secretion we observed can best be accounted for by the severe reduction in CYP17A1 expression since progesterone secretion was not suppressed by VPA, except in the presence of a very high dose-level of LH that, as anticipated, promoted cell luteinisation as evidenced by a marked increase in progesterone secretion and a loss of androgen secretion [26], [37]. Indeed, VPA tended to increase progesterone secretion in cells incubated without LH or with a very low concentration of LH (4 pg/ml), indicating that steps in the steroidogenic pathway proximal to CYP17A1 catalysis were not rate-limiting under these conditions. The lack of effect of VPA on viable cell number recorded at the end of culture indicates that VPA does not affect cell proliferation and/or survival during the 4-day treatment period.
With regard to GC, our finding that VPA also suppressed basal, FSH-induced and IGF-induced secretion of estradiol-17β by cultured GC is consistent with several in vitro and in vivo studies. Using GC harvested from women undergoing laparotomy procedures for infertility treatment Tauboll et al. [38] recently reported that VPA suppressed basal and FSH-induced estradiol secretion. Similarly, in the porcine TC/GC co-culture study of Tauboll et al. [23] VPA reduced basal and FSH-induced estrogen secretion. Chronic in vivo exposure of rats to VPA greatly reduced circulating estrogen levels, although androgen levels were not affected [19]. However, long-term in vivo treatment of rhesus monkeys with VPA had no effect on circulating estradiol or androgen levels and did not affect menstrual cyclicity or ovarian morphology [20].
QPCR analysis of GC levels of six key transcripts involved in steroidogenesis revealed that VPA did not affect the abundance of FSHR or IGF1R mRNA suggesting that the inhibitory action of VPA on estrogen secretion did not involve downregulation of either receptor. However, VPA completely reversed FSH-induced expression of CYP19A1 and HSD17B1 and partially reversed IGF-induced CYP19A1 and HSD17B1 expression. An inhibitory effect of VPA on CYP19A1 in FSH-treated human GC was also observed by Tauboll et al. [38]. As discussed below the enzymes encoded by CYP19A1 and HSD17B1 both play key roles in the synthesis of estradiol-17β by GC.
VPA was some 4-fold more potent in suppressing TC androgen production (IC50 ∼60 µg/ml) than GC estradiol production (IC50 ∼250 µg/ml), the former value being within the therapeutic serum range for human subjects of about 50–100 µg/ml [39]. Since TC-derived androgens are an obligatory substrate for GC the CYP19A1 (cytochrome P450 aromatase) enzyme, and hence follicular estrogen synthesis [40], [41], in an in vivo context this implies a dual inhibitory action of VPA on follicular estrogen production primarily by depriving GC of aromatase substrate and secondarily by inhibiting FSH- and IGF-induced upregulation of CYP19A1 and HSD17B1 expression by GC. Androstenedione is the principle androgen produced by bovine (and human) TC, and the17β-hydroxysteroid dehydrogenase enzyme (HSD17B1) is required to convert the product of its aromatization, estrone, into estradiol-17β, the principle ovarian estrogen [40], [41]. Despite the above, on the basis of the relative IC50 values, it is more likely that the predominant direct ovarian effect of VPA at therapeutically relevant concentrations is exerted on TC rather than GC.
The extent to which observations made using different animal models, including the present bovine ovarian cell culture systems, can be extrapolated to human ovarian function is uncertain. However, in contrast to rodent and porcine ovaries used for many of the previous VPA-associated studies, there are striking similarities between human and bovine ovaries in terms of morphology, follicle size-range, developmental time-line, steroidogenic activity, ovulation rate and physiological regulation by endocrine and intraovarian factors [24], [25], [42], [43]. Given these similarities, and considering the practical and ethical constraints associated with accessing human ovarian tissue for in vitro studies, the likelihood is that insights gained from pharmacological studies using bovine in vitro models are indeed relevant to translational and biomedical research on human ovarian function.
With regard to the mechanism through which VPA suppresses androgen secretion by theca cells, our observation that two highly potent HDAC inhibitors, scriptaid (pan-HDAC inhibitor) and trichostatin A (type 1and 2 HDAC inhibitor) also suppressed androstenedione secretion, supports the notion that the action of VPA is due to its activity as a type 1 HDAC inhibitor [8], [44], [45]. Since TC expression of LH receptor and each of the steroidogenic proteins suppressed by VPA (STAR, CYP11A1, CYP17A1 and HSD3B1) is known to be partially dependent on the common transcription factor, steroidogenic factor-1 (SF-1; NR5A1) [46], we speculate that repressed transcription and/or posttranslational modification of SF-1 could provide a plausible explanation for the inhibition of androgen synthesis observed under both basal and LH-stimulated conditions. In support of this, a previous study using steroidogenic adrenocortical cell lines found that VPA (and other HDAC inhibitors) targets SF-1 for ubiquitination and degradation [47]. Clearly, further mechanistic studies are required to delineate the cellular and molecular pathways through which VPA and other HDAC inhibitors modulate ovarian steroidogenesis at the level of both theca and granulosa cells. The observation that all three HDAC inhibitors also inhibited FSH-induced estradiol secretion by GC suggests a similar mechanism of action on this cell type.
In conclusion, the present findings do not support the hypothesis that VPA has a direct action on the ovary to raise thecal androgen production. Conversely, our evidence firmly indicates a VPA-induced inhibition of both TC androgen production and GC estrogen production arising primarily from a suppression of CYP17A1 and CYP19A1 mRNA levels in the respective cell-types. Irrespective of the mechanism of action of VPA, our findings underscore the need for further in vivo studies, particularly clinical studies in humans, to re-evaluate whether this widely prescribed drug does indeed promote hyperandrogenism through a direct stimulatory action on ovarian theca cells.
Materials and Methods
Bovine Ovaries and Cell Culture
Bovine GC and TC were isolated from adult bovine ovaries obtained from a local abattoir (St Merryn Meat Ltd) as described in detail elsewhere [26], [27], [32]. For each experiment cells pooled from approximately 50 individual 4–6 mm follicles were routinely plated out in 96-well tissue culture plates (Nunclon, Life Technologies Ltd, Paisley, UK) at 75,000 viable cells/well (estimated using trypan blue) and cultured for 6 days under defined serum-free conditions. The culture medium used for both cell-types consisted of McCoy’s 5A modified medium supplemented with 1% (v/v) antibiotic-antimycotic solution, 10 ng/ml bovine insulin, 2 mM L-glutamine, 10 mM HEPES, 5 µg/ml apo-transferrin, 5 ng/ml sodium selenite and 0.1% (w/v) BSA (all purchased from Sigma). In the case of GC the culture medium was supplemented with androstenedione (Sigma UK Ltd, Poole, Dorset, UK) at 10−7 mol/l as a substrate for CYP19A1 (cytochrome P450 aromatase). Medium was removed after 48 h and 96 h and replaced with fresh media containing treatments as indicated below. Conditioned media were retained for assay and at the end of the culture period viable cell number was determined by neutral red dye uptake assay to provide an assessment of cell proliferation/survival.
In culture experiments in which total RNA was to be extracted for PCR analysis, cells were seeded into 24-well plates (105 cells/ml) with 3 replicate wells per treatment. At the end of culture cell lysates were prepared using Tri-Reagent (Sigma) and pooled lysates from replicate wells were stored at −80C until total RNA isolation.
Treatments
Ovine LH (NIADDK oLH-S-16) and FSH (NIADDK oFSH-19SIAPP) were obtained from NHPP, Torrance, CA, USA. Recombinant IGF-1 analogue (Long R3 IGF-1) and VPA, were purchased from Sigma. Treatments were dissolved in Hank’s balanced salt solution containing 0.1% (w/v) BSA and stock solutions sterilized using 0.2 µm membrane filters before dilution in sterile culture medium. Scriptaid (Sigma) and trichostatin A (Sigma) were dissolved in DMSO (10 mM) before further dilution in sterile culture medium.
Steroid Assays
Concentrations of androstenedione in TC-conditioned media were determined by radioimmunoassay as reported previously [26]. The detection limit was 0.1 ng/ml and mean intra- and inter-assay CVs were 7% and 10% respectively. Concentrations of estradiol-17β in GC-conditioned media were determined by radioimmunoassay [48]. The detection limit of the assay was 2 pg/ml and mean intra- and inter-assay CVs were 6% and 9% respectively. Concentrations of progesterone in conditioned media from both cell-types were determined by competitive ELISA [49]. The detection limit was 0.1 ng/ml and mean intra- and inter-assay CVs were 8% and 11% respectively.
Purification of RNA, cDNA Synthesis and Real-time PCR
Total RNA was isolated from cultured cells using Tri-Reagent (Sigma). Briefly, cell monolayers were lysed in Tri Reagent (0.5 ml/well) and after aqueous phase separation, RNA was precipitated in isopropanol, washed in 75% (v/v) ethanol and the RNA pellet re-suspended in 50 µl nuclease-free water. Potential genomic DNA contamination was removed with an RNase-free DNase kit (RQ1; Promega UK Ltd, Southampton, UK). The Tri Reagent extraction process was repeated and the final RNA pellet re-suspended in 20 µl nuclease-free water; RNA quantity and quality were evaluated by spectrophotometry at 260/280 nm. First strand cDNA was synthesized from 1 µg of RNA template using the Reverse-iT reverse transcription kit (used according to manufacturers protocol; Abgene, Epsom, Surrey, UK) in a 20 µl reaction primed with random hexamers.
Primers (see table 1 ) were designed to amplify target sequences using Primer Express software (version 1.5, Applied Biosystems). In primer validation experiments melt curve analysis and agarose gel electrophoresis were used to verify that each primer pair generated a single product of the predicted size. cDNA template log-dilution curves were used to demonstrate satisfactory PCR efficiency (>85%) and linearity. PCR assays were carried out in a volume of 24 µl, comprising 10 µl cDNA template (equivalent to 20 ng reverse-transcribed RNA), 1µl each forward and reverse primers (final concentration 0.4 µM) and 12 µl QuantiTect SYBR Green QPCR 2× Master Mix (Qiagen, UK). Samples were processed on an ABI PRISM® 7700 Sequence Detection System (Perkin Elmer-Applied Biosystems, Warrington, UK) with the following thermal cycling conditions: 2 min at 50°C, 15 min at 95°C (one cycle) followed by 15 s at 95°C and 1 min at 60°C (40 cycles).
Table 1. List of primers used for real-time PCR.
| Target | Accessionnumber | Forward primer5' to 3' | Reverse primer5' to 3' | Ampliconsize (bp) |
| FSHR | NM_174061.1 | GCCAGCCTCACCTACCCCAGC | AATTGGATGAAGGTCAGAGGTTTGCC | 75 |
| LHR | NM_174381.1 | ATTGCCTCAGTCGATGCCCAGACC | AAAAAGCCAGCCGCGCTGC | 92 |
| IGF1R | XM_606794.3 | ACCTCCCAGCCTAAGCAAAATGATCC | TCTTCGGCCACCATGCAGTTCC | 123 |
| STAR | NM_174189 | TTTTTTCCTGGGTCCTGACAGCGTC | ACAACCTGATCCTTGGGTTCTGCACC | 103 |
| CYP11A1 | NM_176644 | CAGTGTCCCTCTGCTCAACGTCC | TTATTGAAAATTGTGTCCCATGCGG | 99 |
| HSD3B1 | NM_174343.2 | GCCACCTAGTGACTCTTTCCAACAGCG | TGGTTTTCTGCTTGGCTTCCTCCC | 111 |
| CYP17A1 | NM_174304 | GACAAAGGCACAGACGTTGTGGTCA | TGATCTGCAAGACGAGACTGGCATG | 301 |
| CYP19A1 | NM_174305.1 | CGCCACTGAGTTGATTTTTGCTGAGA | TAAGGCTTTGCGCATGACCAGGTC | 301 |
| HSD17B1 | NM_001102365 | CGCATATTGGTGACCGGGAGCATA | AATCGCCAGACTCTCGCACAAACC | 108 |
| ACTB | NM_173979.3 | ATCACCATCGGCAATGAGCGGTTC | CGGATGTCGACGTCACACTTCATGA | 128 |
The ΔΔCt method [50] was used for comparison of the relative abundance of each mRNA transcript in TC and GC. Ct values for each transcript in a given sample were first normalized to β-actin Ct value (which was uniform across experimental all groups: ANOVA P>0.1). Resultant ΔCt values for individual replicates within each treatment group were then normalized to the average ΔCt value of the respective vehicle-treated control group. These ΔΔCt values were finally converted to fold-differences using the formula: fold-difference = 2(−ΔΔCt).
Statistical Analysis
Results for hormone secretion (during final 96–144 h period of culture) and cell number at the end of culture were analysed using two-way analysis of variance (ANOVA) and are presented as means ± SEM based on 4 independent culture experiments. To reduce heterogeneity of variance, hormone data were log-transformed prior to statistical analysis. QPCR data (from n = 7 independent TC batches, n = 4 independent GC batches) were statistically analysed (ANOVA and post-hoc Fisher’s PLSD test) as ΔCt values before conversion to fold-difference values for graphical presentation.
Acknowledgments
We wish to thank K Briley and E Perks for technical assistance.
Funding Statement
This work was supported by the Biotechnology and Biological Sciences Research Council (www.bbsrc.ac.uk/) (grants BBS/B/10439 and BB/G017174/1 to PGK). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Foldvary N (2001) Treatment issues for women with epilepsy. Neurol Clin 19: 409–425. [DOI] [PubMed] [Google Scholar]
- 2. Harden CL (2005) Sexuality in women with epilepsy. Epilepsy Behav 7 Suppl 2S2–6. [DOI] [PubMed] [Google Scholar]
- 3. Isojarvi J (2008) Disorders of reproduction in patients with epilepsy: antiepileptic drug related mechanisms. Seizure 17: 111–119. [DOI] [PubMed] [Google Scholar]
- 4. Tauboll E, Roste LS, Svalheim S, Gjerstad L (2008) Disorders of reproduction in epilepsy–what can we learn from animal studies? Seizure 17: 120–126. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds MF, Sisk EC, Rasgon NL (2007) Valproate and neuroendocrine changes in relation to women treated for epilepsy and bipolar disorder: a review. Curr Med Chem 14: 2799–2812. [DOI] [PubMed] [Google Scholar]
- 6. Watterson JM, Watson DG, Meyer EM, Lenox RH (2002) A role for protein kinase C and its substrates in the action of valproic acid in the brain: implications for neural plasticity. Brain Res 934: 69–80. [DOI] [PubMed] [Google Scholar]
- 7. Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, et al. (2001) The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem 276: 31674–31683. [DOI] [PubMed] [Google Scholar]
- 8. Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, et al. (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20: 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilo L, Meo R (2008) Polycystic ovary syndrome in women using valproate: a review. Gynecol Endocrinol 24: 562–570. [DOI] [PubMed] [Google Scholar]
- 10.Franks S, McCarthy MI, Hardy K (2006) Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl 29: 278–285; discussion 286–290. [DOI] [PubMed]
- 11. Sheehan MT (2004) Polycystic ovarian syndrome: diagnosis and management. Clin Med Res 2: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasquali R, Gambineri A, Pagotto U (2006) The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG 113: 1148–1159. [DOI] [PubMed] [Google Scholar]
- 13. Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV (1993) Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med 329: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 14. Isojarvi JI, Laatikainen TJ, Knip M, Pakarinen AJ, Juntunen KT, et al. (1996) Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 39: 579–584. [DOI] [PubMed] [Google Scholar]
- 15. O’Donovan C, Kusumakar V, Graves GR, Bird DC (2002) Menstrual abnormalities and polycystic ovary syndrome in women taking valproate for bipolar mood disorder. J Clin Psychiatry 63: 322–330. [DOI] [PubMed] [Google Scholar]
- 16. Murialdo G, Galimberti CA, Gianelli MV, Rollero A, Polleri A, et al. (1998) Effects of valproate, phenobarbital, and carbamazepine on sex steroid setup in women with epilepsy. Clin Neuropharmacol 21: 52–58. [PubMed] [Google Scholar]
- 17. Stephen LJ, Kwan P, Shapiro D, Dominiczak M, Brodie MJ (2001) Hormone profiles in young adults with epilepsy treated with sodium valproate or lamotrigine monotherapy. Epilepsia 42: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 18. Tauboll E, Isojarvi JI, Harbo HF, Pakarinen AJ, Gjerstad L (1999) Long-term valproate treatment induces changes in ovarian morphology and serum sex steroid hormone levels in female Wistar rats. Seizure 8: 490–493. [DOI] [PubMed] [Google Scholar]
- 19. Sveberg Roste L, Tauboll E, Isojarvi JI, Pakarinen AJ, Huhtaniemi IT, et al. (2002) Effects of chronic valproate treatment on reproductive endocrine hormones in female and male Wistar rats. Reprod Toxicol 16: 767–773. [DOI] [PubMed] [Google Scholar]
- 20. Ferin M, Morrell M, Xiao E, Kochan L, Qian F, et al. (2003) Endocrine and metabolic responses to long-term monotherapy with the antiepileptic drug valproate in the normally cycling rhesus monkey. J Clin Endocrinol Metab 88: 2908–2915. [DOI] [PubMed] [Google Scholar]
- 21. Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, et al. (2004) Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology 145: 799–808. [DOI] [PubMed] [Google Scholar]
- 22. Fisseha S, Towns R, Harada M, Peegel H, Menon KM (2010) Inhibitory effect of valproic acid on ovarian androgen biosynthesis in rat theca-interstitial cells. Endocrine 37: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tauboll E, Gregoraszczuk EL, Kolodziej A, Kajta M, Ropstad E (2003) Valproate inhibits the conversion of testosterone to estradiol and acts as an apoptotic agent in growing porcine ovarian follicular cells. Epilepsia 44: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 24. Gilling-Smith C, Willis DS, Franks S (1997) Oestradiol feedback stimulation of androgen biosynthesis by human theca cells. Hum Reprod 12: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 25. Mihm M, Evans AC (2008) Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod Domest Anim 43 Suppl 248–56. [DOI] [PubMed] [Google Scholar]
- 26. Glister C, Richards SL, Knight PG (2005) Bone morphogenetic proteins (BMP) −4, −6, and −7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 146: 1883–1892. [DOI] [PubMed] [Google Scholar]
- 27. Glister C, Kemp CF, Knight PG (2004) Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, −6 and −7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 127: 239–254. [DOI] [PubMed] [Google Scholar]
- 28. Glister C, Satchell L, Knight PG (2010) Changes in expression of bone morphogenetic proteins (BMPs), their receptors and inhibin co-receptor betaglycan during bovine antral follicle development: inhibin can antagonize the suppressive effect of BMPs on thecal androgen production. Reproduction 140: 699–712. [DOI] [PubMed] [Google Scholar]
- 29. Kendall NR, Marsters P, Guo L, Scaramuzzi RJ, Campbell BK (2006) Effect of copper and thiomolybdates on bovine theca cell differentiation in vitro. J Endocrinol 189: 455–463. [DOI] [PubMed] [Google Scholar]
- 30. Glister C, Groome NP, Knight PG (2003) Oocyte-mediated suppression of follicle-stimulating hormone- and insulin-like growth factor-induced secretion of steroids and inhibin-related proteins by bovine granulosa cells in vitro: possible role of transforming growth factor alpha. Biol Reprod 68: 758–765. [DOI] [PubMed] [Google Scholar]
- 31. Glister C, Satchell L, Knight PG (2011) Granulosal and thecal expression of bone morphogenetic protein- and activin-binding protein mRNA transcripts during bovine follicle development and factors modulating their expression in vitro. Reproduction 142: 581–591. [DOI] [PubMed] [Google Scholar]
- 32. Glister C, Tannetta DS, Groome NP, Knight PG (2001) Interactions between follicle-stimulating hormone and growth factors in modulating secretion of steroids and inhibin-related peptides by nonluteinized bovine granulosa cells. Biol Reprod 65: 1020–1028. [DOI] [PubMed] [Google Scholar]
- 33. Gutierrez CG, Campbell BK, Webb R (1997) Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol Reprod 56: 608–616. [DOI] [PubMed] [Google Scholar]
- 34. Tsang BK, Ainsworth L, Downey BR, Marcus GJ (1985) Differential production of steroids by dispersed granulosa and theca interna cells from developing preovulatory follicles of pigs. J Reprod Fertil 74: 459–471. [DOI] [PubMed] [Google Scholar]
- 35. Portela VM, Machado M, Buratini J Jr, Zamberlam G, Amorim RL, et al. (2010) Expression and function of fibroblast growth factor 18 in the ovarian follicle in cattle. Biol Reprod 83: 339–346. [DOI] [PubMed] [Google Scholar]
- 36. Silva JM, Hamel M, Sahmi M, Price CA (2006) Control of oestradiol secretion and of cytochrome P450 aromatase messenger ribonucleic acid accumulation by FSH involves different intracellular pathways in oestrogenic bovine granulosa cells in vitro. Reproduction 132: 909–917. [DOI] [PubMed] [Google Scholar]
- 37. Kayani AR, Glister C, Knight PG (2009) Evidence for an inhibitory role of bone morphogenetic protein(s) in the follicular-luteal transition in cattle. Reproduction 137: 67–78. [DOI] [PubMed] [Google Scholar]
- 38. Tauboll E, Gregoraszczuk EL, Wojtowicz AK, Milewicz T (2009) Effects of levetiracetam and valproate on reproductive endocrine function studied in human ovarian follicular cells. Epilepsia 50: 1868–1874. [DOI] [PubMed] [Google Scholar]
- 39. Dutta S, Cloyd JC, Granneman GR, Collins SD (2003) Oral/intravenous maintenance dosing of valproate following intravenous loading: a simulation. Epilepsy Res 53: 29–38. [DOI] [PubMed] [Google Scholar]
- 40. Hillier SG (2001) Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol 179: 39–46. [DOI] [PubMed] [Google Scholar]
- 41. Hillier SG, Whitelaw PF, Smyth CD (1994) Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 100: 51–54. [DOI] [PubMed] [Google Scholar]
- 42.Campbell BK, Souza C, Gong J, Webb R, Kendall N, et al.. (2003) Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl 61: 429–443. [PubMed]
- 43. Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17: 121–155. [DOI] [PubMed] [Google Scholar]
- 44. Vanhaecke T, Papeleu P, Elaut G, Rogiers V (2004) Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: toxicological point of view. Curr Med Chem 11: 1629–1643. [DOI] [PubMed] [Google Scholar]
- 45. Kramer OH (2009) HDAC2: a critical factor in health and disease. Trends Pharmacol Sci. 30: 647–655. [DOI] [PubMed] [Google Scholar]
- 46. Schimmer BP, White PC (2010) Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol 24: 1322–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen WY, Weng JH, Huang CC, Chung BC (2007) Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1). Mol Cell Biol 27: 7284–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tannetta DS, Feist SA, Bleach EC, Groome NP, Evans LW, et al. (1998) Effects of active immunization of sheep against an amino terminal peptide of the inhibin alpha C subunit on intrafollicular levels of activin A, inhibin A and follistatin. J Endocrinol 157: 157–168. [DOI] [PubMed] [Google Scholar]
- 49. Bleach EC, Glencross RG, Feist SA, Groome NP, Knight PG (2001) Plasma inhibin A in heifers: relationship with follicle dynamics, gonadotropins, and steroids during the estrous cycle and after treatment with bovine follicular fluid. Biol Reprod 64: 743–752. [DOI] [PubMed] [Google Scholar]
- 50. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]