Abstract
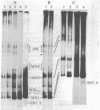
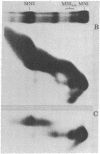
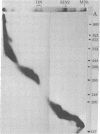
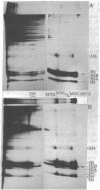
Staphyloccal nuclease digests of HeLa chromatin fractionated on low ionic strength nucleoprotein gels have been further analyzed by second-dimension DNA and protein gel electrophoresis. In vivo radioactive labeling of chromatin components and use of longer gels allowed a higher sensitivity and resolution than has been previously reported for this approach. A number of nonhistone protein spots and about 20 DNA spots can be detected in the mononucleosomal region of the second-dimension gel. In particular, there are three DNA spots identical in DNA size that correspond to three discrete kinds of core mononucleosomes resolved on the first-dimension nucleoprotein gel. Analysis of protein composition shows that the most rapidly migrating particle contains all four core histones but no A24 semihistone (A24 is a covalent conjugate of histone H2A and a specific nonhistone protein, ubiquitin), whereas the other two core mononucleosomes contain A24 semihistone. Thus, one can now quantitatively separate the A24-lacking core mononucleosomes from those containing A24, making it possible to directly address the question of whether A24 is associated with nucleosomes containing a specific subset of DNA sequences. Additional features of two-dimensional nucleoprotein-DNA patterns are "whiskers," which run slower than core mononucleosomes in the nucleoprotein dimension and both faster and slower than core-length DNA in the DNA dimension. In more extensive digests, "secondary whiskers" are observed, which run faster than core mononucleosomes in both dimensions and appear to coincide with previously described subnucleosomal particles SN7 and SN8 [Bakayev, V., Bakayeva, T. & Varshavsky, A. (1977) Cell 11, 619-629]. Possible mechanisms of whisker formation are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburger W., Hörz W., Zachau H. G. Nuclease cleavage of chromatin at 100-nucleotide pair intervals. Nature. 1976 Dec 9;264(5586):517–522. doi: 10.1038/264517a0. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafus N. L., Albright S. C., Todd R. D., Garrard W. T. A method for mapping the distributions of modified and variant histones among mono- and polynucleosomes. J Biol Chem. 1978 Apr 25;253(8):2568–2574. [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Schmatchenko V. V., Georgiev G. P. Non-histone proteins in mononucleosomes and subnucleosomes. Eur J Biochem. 1978 Nov 2;91(1):291–301. doi: 10.1111/j.1432-1033.1978.tb20965.x. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Ballal N. R., Kang Y. J., Olson M. O., Busch H. Changes in nucleolar proteins and their phosphorylation patterns during liver regeneration. J Biol Chem. 1975 Aug 10;250(15):5921–5925. [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Histone 1 is proximal to histone 2A and to A24. Proc Natl Acad Sci U S A. 1979 May;76(5):2190–2194. doi: 10.1073/pnas.76.5.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Levinger L. F., Birinyi F. Dimeric histone interactions and histone packing. J Biol Chem. 1980 Jan 25;255(2):748–754. [PubMed] [Google Scholar]
- Derynck R., Fiers W. A two-dimensional electrophoretic procedure for the separation of DNA restriction fragments. J Mol Biol. 1977 Feb 25;110(2):387–404. doi: 10.1016/s0022-2836(77)80078-8. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Daskal Y., Busch H. A reciprocal relationship between contents of free ubiquitin and protein A24, its conjugate with histone 2A, in chromatin fractions obtained by the DNase II, Mg++ procedure. Biochem Biophys Res Commun. 1978 Oct 16;84(3):786–793. doi: 10.1016/0006-291x(78)90773-8. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Musso R., Busch H. Presence of protein A24 in rat liver nucleosomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5492–5495. doi: 10.1073/pnas.74.12.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R., Burch J. B., Kunkel G. Semihistone protein A24 replaces H2A as an integral component of the nucleosome histone core. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1030–1034. doi: 10.1073/pnas.76.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. O., Goldknopf I. L., Guetzow K. A., James G. T., Hawkins T. C., Mays-Rothberg C. J., Busch H. The NH2- and COOH-terminal amino acid sequence of nuclear protein A24. J Biol Chem. 1976 Oct 10;251(19):5901–5903. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Reiser J., Renart J., Stark G. R. Transfer of small DNA fragments from polyacrylamide gels to diazobenzyloxymethyl-paper and detection by hybridization with DNA probes. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1104–1112. doi: 10.1016/0006-291x(78)90656-3. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Overall pathway of mononucleosome production. J Biol Chem. 1979 Apr 25;254(8):3074–3083. [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Levy W. B., Dixon G. H. Free ubiquitin is a non-histone protein of trout testis chromatin. Nature. 1978 Nov 9;276(5684):196–198. doi: 10.1038/276196a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Palter K., Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975 Sep;6(1):85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]