Abstract
Purpose of the Study:
I examine whether 5 aspects of a significant other’s death quality (pain, decision-making capacity, location, problems with end-of life care, and preparation) affect whether one does advance care planning (ACP). I also identify specific aspects of others’ deaths that respondents say triggered their own planning.
Design and Methods:
Data are from the New Jersey End of Life study, a survey of 305 adults age 55+ seeking care at 2 major New Jersey medical centers. I estimate multivariate logistic regression models for a subsample of 253 participants who recently lost a loved one and provide descriptive findings from an open-ended question regarding the motivation for one’s ACP.
Results:
Multivariate analyses revealed “positive” role model effects; persons who witnessed significant others’ deaths that occurred at home, were free of problems associated with end-of-life care, and where advance directives were used are more likely to make end-of-life preparations. Open-ended data showed that 19% cited others’ deaths as the main trigger for their own planning, with most citing negative factors (pain, connection to machines, coma) that they hoped to avoid.
Implications:
Practitioners should encourage patients to use conversations about others’ deaths as springboards for discussions about one’s own end-of-life care, and to engage in ACP together with family. Implications for health care reform are highlighted.
Keywords: Advance care planning, Death quality, End of life, Survey data
At the end of life, most chronically ill older adults experience physical discomfort, limited mobility, and impaired cognitive functioning (Field & Cassel, 1997). Patients who are incapacitated and have not previously stated their treatment preferences may receive unwanted, futile, and costly medical interventions or the withdrawal of treatments they may have desired (Detering, Hancock, Reade, & Silvester, 2010; Silveira, Kim, & Langa, 2010). Difficult decisions about withholding or continuing treatment often fall upon distressed family members who may not know the patient’s preferences or may disagree with one another (Breen, Abernathy, Abbott, & Tulsky, 2001).
In response to the financial and emotional costs associated with problematic end-of-life care, policy makers have established practices that enable patients to formally state their treatment preferences when they are still cognitively intact. The Patient Self-Determination Act (PSDA), passed by Congress in 1990, requires that federally funded hospitals and nursing homes give patients an opportunity to complete an advance directive, which comprises a living will and durable power of attorney for health care (DPAHC). A living will is a legal document specifying the treatments a person would like to receive if incapacitated. A DPAHC permits a person appointed by the patient to make health care decisions if the patient is incapable of doing so. Living wills and DPAHC appointments have widely recognized limitations (Fagerlin & Schneider, 2004), so health care professionals urge patients to also convey their preferences and values to significant others via informal conversations (Doukas & Hardwig, 2003).
Despite strong encouragement by health care organizations and policy makers, only one-third to one-half of all adults in the United States have advance directives (Hopp, 2000; U.S. Department of Health and Human Services, 2008). Completion rates increase with age and declining health, however; recent studies find rates of 50%–60% among samples of older adults (Carr & Khodyakov, 2007; Silveira et al., 2010), and as high as 70% among recent decedents (Teno, Gruneier, Schwartz, Nanda, & White, 2007). Mounting research investigates the factors that encourage or discourage advance care planning (ACP), with most focusing on health conditions and events (Collins, Parks, & Winter, 2006), demographic characteristics (Kwak & Haley, 2005), attitudinal factors such as death anxiety (Ditto, Hawkins, & Pizzaro, 2006; Zimmermann, 2007), and educational interventions (Briggs, Kirchhoff, Hammes, Song, & Colvin, 2004). However, I know of no studies that examine empirically whether one’s own ACP is done as a reaction to significant others’ death “quality”; individuals may use ACP as a strategy to emulate aspects of “good deaths” or avoid aspects of “bad deaths” that they witnessed among their loved ones (Byock, 1996; Carr, 2003; Emanuel & Emanuel, 1998).
The aim of this study is to explore whether aspects of significant others’ deaths trigger one’s own ACP, by serving as “positive” role models to emulate or “negative” models to avoid (e.g., Lockwood, Chasteen, & Wong, 2005). Specifically, I evaluate: (a) the extent to which five aspects of a significant other’s death quality (i.e., pain, decision-making capacity, location, problematic end-of-life care, preparation) affects one’s own formal (living will, DPAHC) and informal (discussions) preparations for end-of-life care; (b) whether these associations persist net of potential confounds, given patterns of homophily in social relationships; and (c) the specific aspects of others’ deaths to which individuals attribute their own planning. Analyses are based on a sample of older adults in New Jersey seeking care at one of two major health care centers.
Death Quality: Do We Seek to Emulate or Avoid?
Empirical and philosophical writings consistently identify factors that distinguish a “good death” from a poor quality death. The cornerstone of a “good death” (Carr, 2003; Emanuel & Emanuel, 1998) or “dying well” (Byock, 1996) is end-of-life medical treatments that minimize avoidable pain, and that match patients’ and family members’ preferences. A “good death” also encompasses social, psychological, and philosophical elements, such as maintaining close relationships with loved ones during their final days, accepting one’s impending death, dying at the end of a long and fulfilling life, being treated with dignity, and not feeling like a burden to others (Emanuel & Emanuel, 1998; Steinhauser et al., 2000).
One study of seriously ill patients, their family members, and health care providers found that the conditions consistently rated as highly important at the end of life were: pain and symptom management, preparation for death, being mentally aware, achieving a sense of completion, participating in treatment decisions, and being treated as a “whole person” (Steinhauser et al., 2000). National surveys show that more than three-quarters of adults in the United States would like to die at home, although less than a quarter actually do so (Weitzen, Teno, Fennell, & Mor, 2003). ACP is widely regarded as an essential step toward achieving a “good death,” and a necessary precondition for having one’s preferences heeded (Field & Cassel, 1997). As Rhodes and Teno (2009: 5,498) observe: “preferences are meaningless without the care plan … . in place to ensure that those wishes are respected.”
I propose that older adults, who presumably desire a “good death,” may use ACP as a strategy to either strive for good or avoid bad deaths similar to those they witnessed among their significant others. Prior research on health behaviors offers strong evidence of role model effects, where diet, exercise, alcohol consumption, physician visits, and smoking patterns spread through systems of acquaintances and family members (e.g., Christakis & Fowler, 2008). The impact of others’ behaviors may operate via positive or negative role models. Negative models are believed to frighten individuals into changing their behaviors in order to avoid a similarly undesirable outcome, whereas positive models inspire people to change their behaviors to achieve a similarly desirable outcome (Lockwood et al., 2005). Research on gain- and loss-framed messages also reveals that individuals rely on cues from others as guides for their own behaviors; loss-framed messages emphasize costs of engaging in (or avoiding) a particular health behavior, whereas gain-framed messages emphasize benefits (Rothman & Salovey, 1997).
The extent to which positive versus negative models and messages affects one’s behavior varies over the life course, however. Recent research suggests that older adults are more likely than younger persons to be motivated by negative models (Lockwood et al., 2005). As individuals age, they are becoming increasingly aware of their physical vulnerabilities, have growing concerns about their health, and become sensitive to the need to stave off threatening health outcomes (Cross & Markus, 1991). I know of no studies that explore explicitly whether older adults engage in ACP as a strategy to avoid undesirable death attributes observed in others, although several studies offer suggestive evidence. Qualitative studies have found that older adults are more likely to prepare for end-of-life care if they know someone with severe cognitive impairment (Bravo, Dubois, & Paquet, 2003) or a serious illness or injury (Lambert et al., 2005). Quantitative studies based on a cohort of white older adults show that persons who describe a recent parental or spousal death as “painful” are more likely than others to execute living wills and DPAHC appointments (e.g., Carr & Khodyakov, 2007). Thus, I evaluate whether specific aspects of a significant other’s death predict one’s own formal and informal ACP. I also examine the specific attributions that individuals make for their own ACP, and highlight aspects of significant others’ deaths cited as prompts.
Other Influences on ACP
If the quality of a significant other’s death is associated with study participants’ end-of-life preparations, this association could be spurious rather than causal—reflecting the fact that both study participants and their significant others may share characteristics that would be associated independently with ACP practices. For instance, both the decedents and study participants might have engaged in ACP if they both possess high levels of education and access to care (Carr & Khodyakov, 2007). Research on social homophily shows that friends tend to be of similar socioeconomic standing, and the same race/ethnicity, age, and political orientations (e.g., de Klepper, Sleebos, van de Bunt, & Agneessens, 2010). Family members typically are of the same socioeconomic status (SES) and race/ethnicity, due to intergenerational transmission of social class and homogamy (i.e., marrying one who shares social characteristics; Kalmijn, 1998). Thus, all analyses are adjusted for age, race, gender, Spanish language use, marital status, parental status, education, home ownership, and income. Physical and mental health affects ACP, thus models are adjusted for self-rated health and depressive symptoms (Carr & Khodyakov, 2007).
Design and Methods
Sample
The New Jersey End of Life study is a sample of 305 noninstitutionalized adults ages 55 and older who are residing in New Jersey. To be eligible for the study, individuals had to speak English or Spanish, have no cognitive limitations, and have been diagnosed with one or more of the following health conditions: colorectal cancer, Type II diabetes, or congestive heart failure. These diseases were selected as inclusion criteria because each is chronic, with intrusive symptoms, and affects men and women in roughly equal proportions. A control group of “healthy” patients also was recruited; they have not been diagnosed with a serious chronic illness (e.g., cancer, heart disease). Recruitment was conducted over the telephone from two large university hospitals and one comprehensive cancer center in New Jersey. Human subjects’ research approval was obtained from the institutional review boards at both the principal investigator’s university and each of the study sites.
The initial sampling frame included 1,146 patients who were identified as potential study participants by the general internal medicine department at the University of Medicine and Dentistry of New Jersey. Of these, 575 respondents met the inclusion criteria in the initial sampling pool. Reasons for exclusion include: invalid contact information; died after having been identified as a study candidate; severe cognitive and physical limitation precluding participation; and not meeting the study’s age criteria. The final sample includes 305 persons who consented to participate, representing 53% of the eligible sampling frame. Reasons for nonparticipation included reluctance to participate in research, frailty, and time constraints.
Trained interviewers conducted 90-min face-to-face structured interviews using computer-assisted personal interview technology; data were collected from 2006 through 2008. The interviewers were advanced graduate students in sociology, social work, and psychology who had prior experience working as either survey interviewers or practitioners working with older adults. All study participants read and signed consent forms prior to participation. The survey obtained information on sociodemographics, health status and behaviors, end-of-life planning, attitudes toward medical treatments, religion, and social relations. Nearly all questions on the survey had fixed-choice response categories; however, for a subset of questions, responses were followed up with an open-ended question. Persons who indicated that they had done ACP were then asked “why did you do so at that time?” Responses to this question provide the data for the second part of my analysis.
Analytic Samples
The analyses presented here focus on two subsamples. First, the bivariate and multivariate analyses focus on the 253 persons (84% of total sample) who experienced the loss of a close significant other in the 10 years prior to interview. Only persons who experienced a loss were asked questions about the quality and context of the death. Sixty percent of the deaths occurred within 5 years prior to interview, and one-third occurred within 2 years of the interview. More than 75% of respondents reported that they were “very close” with the decedent. The majority of decedents were close family members: parent (22%), spouse (11%), sibling (20%), child (7%), friend (15%), and other relationship (25%). Preliminary analyses revealed that respondents who did (vs. did not) experience a loved one’s death have similar rates of ACP. Second, the descriptive analysis of open-ended data focuses on the 138 sample members who reported that they had done ACP; only those who have done planning were asked to explain the reason why they did so.
Measures
Dependent Variables.—
Sample members who have either a living will or a DPAHC appointment (or both) are coded as having done formal planning. I group these two activities together because they typically are done in tandem; 81% of study participants with a living will also have a DPAHC and 89% of those with a DPAHC also have a living will. Informal planning refers to whether one discussed their future health care plans and preferences with any one: future health care plans are defined as “plans about the types of medical treatment you want or don’t want to receive if you become seriously ill in the future.” Persons who have done formal planning are asked “why did you [do so] at that time?” Interviewers directly transcribed the responses into the study database during the face-to-face interview. The study’s principal investigator coded these brief open-ended responses into nine categories, as discussed in following.
Independent Variables.—
Significant Other’s Quality of Death. Participants were asked to think about a family member or friend who died within the past 10 years, with whom they were “very close and had frequent contact.” Pain is assessed with the question “during the last week of life, how much pain did he/she have?” Response categories are: no pain, unconscious during final week; no pain, death was sudden; no pain; slight pain, moderate pain; severe pain; and don’t know. Mental awareness is evaluated with the question “Was [he/she] able to make decisions in the last week of life?” Response categories are yes, no, and don’t know. Death at home refers to whether the person died at home (including in-home hospice) versus an institution (e.g., nursing home, hospital). Preparation refers to whether the decedent had either a living will or DPAHC.
Problems with end-of-life care refer to whether one reported at least one of four problems: medical care inconsistent with patient wishes, poor doctor–patient communication, problems with living will, and problems with DPAHC. Care inconsistent with wishes is captured with the question “during that last week, were there any medical procedures or treatments that happened to him/her that were not consistent with his/her previously stated wishes?” Poor communication refers to a negative response to the question “did the doctor or medical staff who cared for your [significant other] speak to him/her or to one of his/her close relatives about making sure his/her care was consistent with his/her wishes?” Problems with living will and DPAHC are evaluated with the questions: “what role did it (living will/DPAHC) play in the last week of life? It helped a great deal, it had no effect, it caused some problems, it caused major problems.” The latter two categories are coded as indicative of problematic care. I constructed this composite measure because few persons reported each such problem; small cell sizes may generate instable parameter estimates in logistic regression models (Long, 1997). The number (and percentage) of persons reporting each problem are 18 (7.0%), 39 (15.2%), 2 (.8%), and 6 (2.3%), respectively.
Death suddenness is controlled in all models, because death quality attributes vary based on whether the death occurred suddenly or following a chronic illness (Carr, 2003). Suddenness is evaluated with the question “Was there warning before [your significant other] passed on?”
Control Variables.—
Demographic characteristics include age, gender, race/ethnicity, Spanish as primary language, marital status, and number of children. SES indicators include education (in years), home ownership, and current household income (natural log, to adjust for skewed distribution). For the 8% of cases who did not report their income, I imputed the mean and used a dummy variable to indicate missing data.
Health Characteristics.—
Physical health is assessed with the item “how would you rate your health at the present time?” Responses of fair and poor are coded as 1; good or better is the reference group. Despite the simplicity of this measure, it is an excellent predictor of morbidity (Idler & Benyamini, 1997) and mortality (Ferraro & Farmer, 1999). Depressive symptoms (α = .80) in the past week are assessed with a subset of nine items from the Center for Epidemiologic Studies Depression scale (Radloff, 1977).
Results
Bivariate Analyses
Means (for continuous measures) and proportions (for categorical measures), by whether one has done formal and informal planning, are presented in Table 1. Planners and nonplanners do not differ significantly with respect to most death quality indicators, with two exceptions. Persons whose loved ones were capable of decision making in the last week of life are significantly more likely to have discussed their own end-of-life preferences, whereas those whose loved ones had completed living wills and named DPAHCs are more likely to do both formal and informal planning. Those who do planning have significantly more social advantages including higher levels of education, higher rates of home ownership, and higher earnings than those who do not. They are more likely to be married and have more children than nonplanners.
Table 1.
Descriptive Statistics for All Variables Used in the Analysis, by Formal and Informal End of Life Planning Strategy, New Jersey End of Life Study (NJEOL; N = 253).
| Total | Formal planning | Informal planning | |||
| Yes | No | Yes | No | ||
| Death quality attributes | |||||
| Pain | |||||
| No pain: not conscious | 6.32 | 8.4 | 4.5 | 7.2 | 4.1 |
| No pain: death was sudden | 8.30 | 6.7 | 9.7 | 7.2 | 10.9 |
| No pain | 16.2 | 19.3 | 13.4 | 17.8 | 12.3 |
| Slight pain | 8.30 | 7.6 | 8.9 | 10.0 | 4.1 |
| Moderate pain | 12.7 | 15.9 | 9.7 | 12.2 | 13.7 |
| Severe pain | 23.5 | 18.5 | 26.1 | 21.7 | 24.7 |
| DK | 25.7 | 23.5 | 27.6 | 23.9 | 30.1 |
| Mental awareness | |||||
| Yes, could make decisions | 47.1 | 50.4 | 44.0* | 52.8 | 32.9* |
| No, could not make decisions | 39.9 | 38.7 | 41.0* | 35.6 | 50.7* |
| DK | 12.0 | 10.1 | 14.2* | 10.6 | 16.4 |
| Died at home | |||||
| Yes, at home | 40.0 | 42.1 | 38.1 | 41.1 | 36.9 |
| No, at institution | 60.0 | 57.9 | 61.9 | 58.9 | 63.0 |
| At least one problem with end-of-life care | |||||
| Yes | 23.1 | 22 | 24.3 | 20.7 | 28.6 |
| No | 76.9 | 78 | 75.7 | 79.3 | 71.4 |
| Timing | |||||
| Sudden | 29.6 | 24.4 | 34.3† | 27.8 | 34.2 |
| Forewarned | 67.2 | 73.9 | 61.2† | 68.9 | 63.0 |
| DK | 3.2 | 1.7 | 4.3 | 3.3 | 2.7 |
| Decedent’s preparation | |||||
| Formal preparation | |||||
| Yes, did any ACP | 38.4 | 58.0 | 35.8*** | 17.8 | 37.0*** |
| No, did not do ACP | 36.4 | 11.8 | 33.6*** | 52.2 | 31.5*** |
| DK | 25.2 | 30.3 | 30.6 | 30.0 | 315 |
| Living will | |||||
| Yes | 35.6 | 51.3 | 21.6*** | 43.9 | 15.1*** |
| No | 33.9 | 18.5 | 47.8*** | 26.1 | 53.4*** |
| DK | 30.5 | 30.3 | 30.6 | 30.0 | 31.5 |
| Durable power of attorney for health care | |||||
| Yes | 45.1 | 57.1 | 34.3*** | 51.7 | 28.8*** |
| No | 26.1 | 12.6 | 38.1*** | 18.9 | 43.8*** |
| DK | 28.8 | 30.3 | 26.9 | 28.9 | 27.4 |
| Demographic characteristics | |||||
| Age | 69.44 (8.93) | 71.43 (8.88) | 67.66*** (8.62) | 70.15 (8.91) | 67.69*** (8.81) |
| Female | 64.0 | 56.3 | 71.9* | 63.3 | 65.8 |
| White | 51.2 | 76.3 | 29.1*** | 62.6 | 23.3*** |
| Black | 25.3 | 15.9 | 33.6*** | 22.8 | 31.5 |
| Hispanic | 18.7 | 4.2 | 32.0*** | 10.6 | 38.9*** |
| Other race/ethnicity | 4.4 | 3.4 | 5.3 | 3.9 | 5.6 |
| Spanish language | 10.3 | 1.7 | 18.0*** | 3.3 | 27.4*** |
| Family characteristics | |||||
| Married | 49.6 | 58.0 | 42.1* | 53.6 | 39.7*** |
| Widowed | 26.6 | 24.4 | 28.6* | 26.3 | 27.4 |
| Separated/divorced | 14.7 | 10.1 | 18.8 | 11.7 | 21.9* |
| Never married | 9.1 | 7.6 | 10.5 | 8.4 | 10.9 |
| Number of children | 3.25 (2.44) | 2.81 (2.47) | 3.65** (2.35) | 3.10 (2.45) | 3.62 (2.38) |
| Socioeconomic Status | |||||
| Education (in years) | 13.71 (4.49) | 15.2 (4.08) | 12.38*** (4.42) | 14.53 (3.87) | 11.74*** (5.23) |
| Owns home | 56.6 | 78.2 | 37.3*** | 63.9 | 38.4 |
| Income | 49,792 (44,517) | 63,781 46,005) | 37,369*** (39.326) | 57,319 (46,371) | 31,232*** (33,156) |
| Income data missing | 9.1 | 10.1 | 8.2 | 8.3 | 10.9 |
| Health characteristics | |||||
| Self/rated health, fair/poor | 46.3 | 41.2 | 50.8 | 41.7 | 57.5* |
| Depressive symptoms (CES-D) | 1.23 (1.26) | 1.14 (1.18) | 1.32 (1.34) | 1.13 (1.16) | 1.49* (1.47) |
| N | 253 | 119 | 134 | 73 | 180 |
| % | 100 | 47 | 53 | 39 | 71 |
Notes: Means (and SDs) are shown for continuous variables, percentages are shown for categorical variables. Significant subgroup differences are evaluated with t tests (continuous measures) and chi-square tests (categorical measures). Statistically significant differences are signified as †p < .15. *p < .05. **p < .01. ***p < .001.
ACP = advance care planning; DK = don’t know
Multivariate Analyses
I next evaluate the extent to which significant others’ deaths affect one’s own formal and informal planning, after potential confounds are controlled. I estimated a series of nested multivariate logistic regression models separately for each death attribute, and sequentially added in blocks of potential explanatory variables; results are summarized in Table 2. Model 1 adjusts for demographic, family, and SES characteristics, and whether the death was sudden (except for pain model in top panel, because suddenness is a category). Model 2 also adjusts for physical and mental health. Model 3 incorporates a control for whether the decedent did any ACP. (For the models presented in the bottom two panels, where the decedent’s living will and DPAHC are the “key” predictors, a third model is not estimated).
Table 2.
Summary of Logistic Regression Models Predicting Formal and Informal End of Life Planning, by Significant Other’s Death Quality Attributes (N = 253)
| Formal advance care planning | Informal discussions | |||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| [No pain] | ||||||
| No pain: not conscious | .96 | .94 | 1.07 | 1.11 | 1.09 | 1.12 |
| No pain: death was sudden | .33† | .27* | .29† | .42 | .42 | .44 |
| Slight pain | .29* | .29* | .25* | 1.6 | 1.6 | 1.54 |
| Moderate pain | .46 | .47 | .51 | .26* | .26* | .27* |
| Severe pain | .53 | .47 | .52 | .92 | .89 | .95 |
| DK | .50 | .46 | .44 | .77 | .65 | .77 |
| [Decedent aware, could make decisions] | ||||||
| Not aware, could not make decisions | .89 | .91 | .90 | .44* | .45* | .45* |
| DK if decedent could make decisions | 1.97 | 2.29 | 2.89 | .70 | .71 | .79 |
| [Died at home] | ||||||
| Died in institution | .47* | .50* | .51* | .53† | .52† | .54† |
| [No problems reported] | ||||||
| At least one problems with end-of-life care | .98 | .91 | 1.04 | .50† | .49† | .51† |
| [Had living will] | ||||||
| Did not have living will | .25*** | .24*** | .33* | .32* | ||
| DK if decedent had living will | .46* | .44* | .51 | .50 | ||
| [Had DPAHC] | ||||||
| Did not have DPAHC | .32** | .27** | .53† | .51† | ||
| DK if decedent had DPAHC | .99 | .84 | .92 | .77 | ||
Notes: Odds ratios presented; omitted category noted in brackets. Each set of death characteristics comprises a separate set of models. Model 1 adjusts for demographic, family, and SES characteristics, and whether the death was sudden (except for pain model, because suddenness is a category). Model 2 also adjusts for physical and mental health. Model 3 incorporates a control for whether the decedent did any advance care planning. (For the models presented in the bottom two panels, the decedent’s living will and DPAHC are the “key” predictor thus a third model is not estimated). Significance levels are denoted as: *p < .05. **p < .01. *** p < .001.
DPHAC = durable power of attorney for health care; DK = don’t know
The results show that one’s own end-of-life planning mainly reflects efforts to replicate those aspects of others’ deaths that are presumably desirable. However, these effects are evidenced more strongly for the outcome of discussions than for formal ACP. Persons whose loved one was able to make decisions at the end of life are more than twice as likely to discuss their own treatment preferences (1/0.45 = 2.2), and this effect persisted even when the decedent’s ACP was controlled. Loss of awareness and decision-making capacity is considered one of the least desirable aspects of the dying process (Steinhauser et al., 2000).
As noted earlier, few study participants reported that their loved one experienced major problems regarding end-of-life care; just one in five reported care inconsistent with patient wishes, lack of doctor-patient communication, or problems with the decedent’s advance directive. However, persons who reported any of these four problems are half as likely as those not reporting such a problem to do ACP. That is, those who witnessed relatively problem-free deaths are twice as likely to discuss their own end-of-life treatment preferences. Persons whose loved one died at home, widely considered the most desirable place to die, were roughly twice as likely as those who saw their loved one die in an institution to do formal ACP and discuss their treatment preferences. These effects persisted net of the decedent’s own ACP.
One of the most powerful findings is that individuals whose loved one engaged in ACP are significantly more likely than those who did not to also do ACP. Persons whose significant other had a living will are four times as likely as those who did not to do formal ACP and roughly three times as likely to discuss their preferences. Similarly, persons whose significant others had a DPAHC are about three times as likely to do formal planning and twice as likely to have discussions. As the open-ended data in the following section will show, some individuals learn to do ACP or are prompted to do so only when a dying loved one is going through a similar experience.
Although the results thus far show that study participants model positive aspects of their significant others’ deaths, less clear-cut findings emerged for the pain measures. The effects of decedent pain on one’s own ACP are inconsistent and weak. Relative to those reporting that their loved one’s death was pain free, those who evaluated the death as either sudden or slightly painful were one-third as likely to do formal ACP. Those who rated the death as moderately painful were 27% as likely as those witnessing pain-free deaths to discuss their own preferences.
Open-Ended Responses
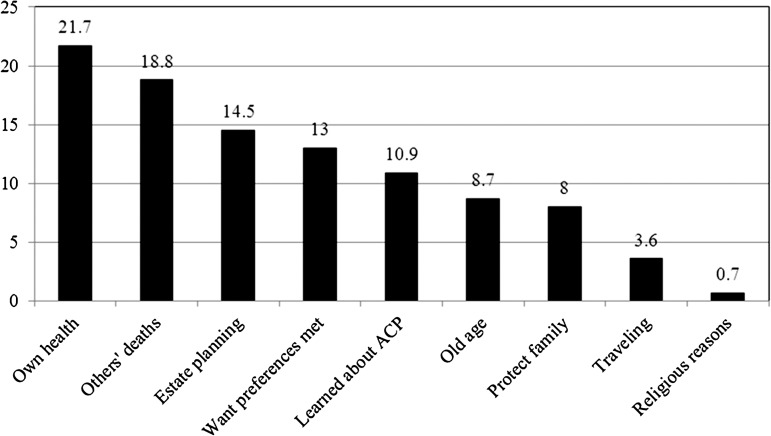
Finally, I describe the personal explanations given by those who engaged in formal planning (n = 138). Respondents were asked “why did you complete a living will/appoint a DPAHC at that time?” I coded the brief open-ended responses into nine mutually exclusive categories: ill health/surgery (e.g., “I was having heart surgery”); others’ experiences (e.g., “my mother had been in a coma”); estate planning (e.g., “I was making out a regular will and my lawyer suggested it”); to protect family members (e.g., “I didn’t want my children disputing about such things”); old age (e.g., “I’m just getting to that age when I need to make these kinds of decisions”); travel (e.g., “my husband and I were taking a trip to Italy, and were worried something would happen”); learned about it from others (e.g. “the social worker at the hospital said I should do it”); want preferences met (e.g., “I don’t want to be kept alive on machines”); and religious reasons (e.g., “my rabbi said it wasn’t in violation of [Orthodox] Jewish law”). Frequencies are plotted in Figure 1. The most frequently named reason was own health problems (n = 30; 21.7%), followed by experiences of a loved one (n = 26; 18.8%), estate planning (n = 20, 14.5%), want preferences met (n = 18, 13%), and learned about it from others (n = 15, 11%). Fewer than 10% cited old age, a desire to minimize family distress, travel, and religious reasons. I present illustrative quotes, to capture the key themes that emerged.
Figure 1.
Of those doing advance care planning (n = 138), percentage reporting main reason why done at that time.
Of the 26 who said others’ deaths triggered their own ACP, the specific conditions cited mesh closely with prior writings on the “good death.” Respondents referred to pain, prolongation, mental incapacitation, and an undesirable reliance on machines. Nearly all respondents conveyed that a loved one’s life quality had been compromised in exchange for (unwanted) life extension. Although the multivariate analyses showed that positive aspects of others’ deaths were associated with one’s own ACP, the vast majority of open-ended responses cited aspects of poor quality deaths that the respondent hoped to avoid.
Those who felt that their loved one’s life was extended unnecessarily almost always mentioned the use of technologies. One respondent recalled the deaths of her older siblings: “just seeing how they [doctors] prolonged their life even when their organs were already deteriorating. I wouldn’t want that for myself.” Another man noted that his brother had received treatments he would not have wanted: “my brother had a stroke…and now he has a feeding tube and catheter and normally he wouldn’t want that.”
Two respondents spoke in detail about their friends who were kept alive, despite very low levels of functioning: one man noted, “we have seen how people were put on feeding tubes and everything was gone, and they just ‘existed’ which we were very much opposed to. If you’re going to die, just let nature take over and die.” Another woman recalled “we have had friends who were tied up to machines for months and months. We did not want that.” One respondent noted that he was motivated by the suffering of persons he did not know, pointing to television news coverage of cases like Terri Schaivo: “We had seen TV stories over the years about people in comas and the arguments about whether to keep them alive.”
Cognitive impairment and an inability to convey one’s preferences were cited by several respondents as a fate they hoped to avoid. One woman recalled: “my husband’s mom had Alzheimer’s and that initiated our planning. I saw what she was going through. She didn’t have a living will and was in such a bad state … .” Similarly, another woman said “my mother had been in a coma for three weeks before passing. I did it [ACP] at that time because of my husband’s declining health and my mother’s circumstances helped to inform my preferences.”
Although the majority viewed ACP as a way to avoid the undesirable fate that had befallen their loved one, 4 of the 26 respondents noted that others served as positive role models. That is, family members’ ACP motivated them to do their own. One woman recalled “my sister, brother-in-law, and husband all did it together and witnessed each other’s living wills. This was because my sister found out she had breast cancer.” Another mentioned that “my mother was doing her living will with her doctor, so I completed mine at the same time.” Although respondents mentioned negative role models more frequently than positive ones, both groups underscored that others’ illnesses and deaths triggered their own planning.
Discussion
This study showed that significant others’ deaths may serve as both positive and negative role models that influence one’s own end-of-life preparations. These effects were more powerful for informal discussions rather than formal ACP. Although the multivariate analyses offered evidence of positive role models or “gain-framed” messages, the open-ended data showed that respondents overwhelmingly named distressing conditions (i.e., negative models) they hoped to avoid.
The multivariate analyses showed that deaths marked by patient’s mental awareness were associated with increased chances of discussing one’s own treatment preferences, relative to deaths where the decedent was not capable of making decisions. Similarly, persons who reported no problems regarding their significant other’s end-of-life care, including care inconsistent with the patient’s wishes, doctor’s failure to discuss the patient’s wishes, or problems generated from ACP, were twice as likely as those reporting problems to discuss their treatment preferences. Deaths occurring at a patient’s home also were associated with a respondent’s greater likelihood of discussing his or her preferences and doing formal ACP.
Perhaps the strongest evidence of positive role model effects is the finding that a significant other’s ACP was associated with a two to fourfold increase in the odds of doing one’s own formal and informal planning. This pattern may reflect either positive role modeling, or family-level ACP. The open-ended responses showed that several individuals did ACP along with their unhealthy relatives, treating the process as a family-level behavior. In this way, a decedent’s planning may directly trigger one’s own planning.
I found no support for negative role model effects in the multivariate analysis; nearly all measures associated with a “bad” death predicted significantly lower odds of one’s formal or informal ACP than did the “good” death category (i.e., reference category). By contrast, nearly all the open-ended responses cited negative aspects of significant others’ deaths that one hoped to avoid, including cognitive impairment, reliance on machines, and unnecessary prolongation. This recollection of problematic conditions may reflect “negativity bias,” where negative events are recalled more easily than positive events (Cacioppo & Berntson, 1994). These processes also may reflect the fact that older adults become increasingly cognizant of their own vulnerabilities and become acutely aware of the need to stave off potentially distressing health conditions and treatments, thus they attend more strongly to negative rather than positive images of aging and health decline (e.g., Cross & Markus, 1991).
One surprising finding was that pain did not have consistent effects on ACP; neither very painful nor pain-free deaths were associated with one’s own ACP. However, persons whose loved one died suddenly or with slight pain were one-third as likely as those witnessing pain-free deaths to do formal planning. This may reflect a key theme of research on loss-framed messages or “fear appeals” (Janis & Feshbach, 1953); individuals will engage in a particular health behavior only if they perceive it to have the desired and anticipated impact. Persons who witnessed sudden deaths may feel that ACP is not valuable, given that sudden deaths may not require treatment decisions. Similarly, some may view slight or moderate pain as an unavoidable aspect of the dying process, regardless of whether one has done ACP (Berry & Ward, 1995).
Overall, the multivariate analyses revealed more consistent effects for discussions than for formal ACP. Informal discussions are volitional and may be more strongly affected by personal or idiosyncratic experiences. Formal ACP, by contrast, often is instigated by contact with health care providers, given the passage of the PSDA (1990). By design, all study participants were seeking care at federally funded health centers, and thus were exposed to information on formal ACP, although this information may not necessarily be accompanied by (or serve as a trigger to) meaningful discussions (Fagerlin & Schneider, 2004).
In sum, the results suggest that observations of significant other’s good-(or poor-) quality deaths may be an important influence on one’s own ACP. However, it is important to underscore that only one-fifth of persons who did ACP attributed their behavior to others’ deaths, and the multivariate models assessing the effects of death quality on one’s ACP had limited explanatory power; the death quality indicators alone explained roughly less than 10% of the variance in the study outcomes. Thus, it is important that researchers continue to identify the diverse range of factors that may shape older adults’ ACP.
Limitations
This study has several limitations. First, I cannot ascertain whether respondents’ assessments of their significant others’ deaths are accurate, as I do not have chart data on those deaths. However, for the purposes of this study, I argue that it is one’s perception of the death quality that matters for the respondent’s behaviors.
Second, a sizeable minority (20%–25%) said they didn’t know whether the respondent was in pain; this may partly account for the inconsistent effects of the pain measures on ACP. These responses were not followed up with further probes, thus it is impossible to discern why they did not know (e.g., they were not with decedent at moment of death, were not told such information, etc.). In order to assess the possibility that one didn’t know because they were not close with the decedent, I evaluated whether the effect of each death quality attribute differed significantly based on whether one was “very close” versus “somewhat” or less close with the decedent. The interaction terms were not statistically significant, perhaps reflecting the fact that more than three quarters said they were very close. I could not run interaction terms assessing whether the impact of the death attributes varied based on one’s relationship to the deceased, given the small sample size. However, the respondents’ relationship to the decedent was not significantly associated with their own end-of-life planning.
Further, nearly all decedents were either contemporaries of or older than the study respondents, thus few were likely non-normative or premature deaths. Only 7% of respondents reported on the death of a child; all others reported on the death of a parent, spouse, sibling, or close friend. Still, future studies should examine whether efforts to emulate positive (or avoid negative) aspects of a significant other’s death vary based on one’s closeness and perceived similarity to that decedent (Bandura, 1969).
Third, sample size precluded a more fine-grained examination of combined aspects of death attributes; clusters of death quality characteristics may be more meaningful than individual attributes (Carr, 2003). Finally, sample size prevented me from exploring whether the effects of specific death quality attributes on one’s own end-of-life planning differ across demographic subgroups. I encourage future studies, based on larger data sets, to investigate whether others’ death experiences affect ACP based on multiple characteristics of the death and a range of demographic and SES characteristics.
Conclusion
ACP is an essential step toward the receipt of patient-centered cost-effective care, yet rates of doing so remain modest—even among older adults and the terminally ill (Silveira et al., 2010; Teno et al., 2007). Practitioners’ and policy makers’ goal of increasing rates of formal ACP and effective end-of-life discussions among older adults was derailed in January 2011, however. Congressional Democrats’ proposal for Medicare coverage of one doctor–patient ACP consultation session was deleted from the proposed Patient Protection and Affordable Care Act due in part to impassioned (and unsubstantiated) claims that it would encourage euthanasia and “death panels” (Pear, 2011). Reinstating this benefit would potentially increase rates of ACP and would give older patients the opportunity to seek further information on and discuss the range of treatments available to them at the end of life.
Practitioners who work with terminally ill older adults should discuss with them those aspects of their significant others’ deaths that they found to be distressing versus comforting for the decedent and his/her family members. Further, it is essential that practitioners encourage families to engage in ACP together, and to discuss with one another the reasons behind their specific treatment preferences. By focusing on concrete experiences and recollections, rather than abstract scenarios, vague projections of one’s own end of life, and discussions of specific interventions (e.g., feeding tubes, ventilators) that the patient may not know about or understand, patients may have more effective discussions with their physicians and ultimately better quality deaths. These discussions may also prove therapeutic for older adults; given that most older adults have experienced a relatively recent death of a friend, spouse, parent, sibling, or peer, holding discussions about a significant other’s death may both help them to make sense of the loss and to develop a strategy for thinking about and preparing for their own end-of-life health care needs. Although medical care and end-of-life decision-making is typically approached with a focus on the individual patient, my study suggests that ACP takes place at the family level; as such, it is essential that practitioners involve spouses, children, or other kin, where available, in conversations regarding end-of-life care.
Funding
This work was supported by the National Institutes of Health (AG023958).
Acknowledgments
Susan Bodnar-Deren, Carmelen Chiusano, and Howard Leventhal provide invaluable assistance with data collection.
References
- Bandura A. Social-learning theory of identificatory processes. In: Goslin DA, editor. Handbook of socialization theory and research. Chicago, IL: Rand McNally; 1969. pp. 213–262. [Google Scholar]
- Berry PE, Ward SE. Barriers to pain management in hospice: A study of family caregivers. Hospice Journal. 1995;10:19–33. doi: 10.1080/0742-969x.1995.11882805. doi:10.1300/J011v10n02_04. [DOI] [PubMed] [Google Scholar]
- Bravo G, Dubois MF, Paquet M. Advance directives for health care and research: Prevalence and correlates. Alzheimer’s Disease & Associative Disorders. 2003;17:215–222. doi: 10.1097/00002093-200310000-00004. doi:10.109/2F00002093-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Breen CM, Abernathy AP, Abbott KH, Tulsky JA. Conflict associated with decisions to limit life-sustaining treatment in intensive-care units. Journal of General Internal Medicine. 2001;16:283–289. doi: 10.1046/j.1525-1497.2001.00419.x. doi:10.1046/j.1525-1497.2001.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs LA, Kirchhoff KT, Hammes BJ, Song M, Colvin ER. Patient-centered advance care planning in special patient populations: A pilot study. Journal of Professional Nursing. 2004;20:47–58. doi: 10.1016/j.profnurs.2003.12.001. doi:10.1016/j.profnurs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Byock IR. The nature of suffering and the nature of opportunity at the end of life. Clinics in Geriatric Medicine. 1996;12:237–252. [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. doi:10.1037/0033-2909.115.3.401. [Google Scholar]
- Carr D. A “good death” for whom? Quality of spouse's death and psychological distress among older widowed persons. Journal of Health and Social Behavior. 2003;44:215–232. doi:10.2307/1519809. [PubMed] [Google Scholar]
- Carr D, Khodyakov D. End-of-life health care planning among young-old adults: An assessment of psychosocial influences. Journals of Gerontology: Social Sciences. 2007;62:S135–S141. doi: 10.1093/geronb/62.2.s135. doi:10.1093/geronb/62.2.S135. [DOI] [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. New England Journal of Medicine. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. doi:10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LG, Parks SM, Winter L. The state of advance care planning: One decade after SUPPORT. American Journal of Hospice & Palliative Medicine. 2006;23:378–384. doi: 10.1177/1049909106292171. doi:10.1177/1049909106292171. [DOI] [PubMed] [Google Scholar]
- Cross S, Markus H. Possible selves across the life span. Human Development. 1991;34:230–255. doi:10.1159/000277058. [Google Scholar]
- de Klepper M, Sleebos E, van de Bunt G, Agneessens F. Similarity in friendship networks: Selection or influence? The effect of constraining contexts and non-visible individual attributes. Social Networks. 2010;32:82–90. doi:10.1016/j.socnet.2009.06.003. [Google Scholar]
- Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. British Medical Journal (Clinical Research Ed.) 2010;340:1345. doi: 10.1136/bmj.c1345. doi:10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditto PH, Hawkins NA, Pizzaro DA. Imagining the end of life: On the psychology of advance decision making. Motivation and Emotion. 2006;27:481–502. doi:10.1007/s11031-006-9017-x. [Google Scholar]
- Doukas DJ, Hardwig J. Using the family covenant in planning end-of-life care: Obligations and promises of patients, families, and physicians. Journal of the American Geriatrics Society. 2003;51:1155–1158. doi: 10.1046/j.1532-5415.2003.51383.x. doi:10.1046/j.1532-5415.2003.51383.x. [DOI] [PubMed] [Google Scholar]
- Emanuel EJ, Emanuel L. The promise of a good death. The Lancet. 1998;251:21–29. doi: 10.1016/s0140-6736(98)90329-4. doi:10.1016/S0140-6736(98)90329-4. [DOI] [PubMed] [Google Scholar]
- Fagerlin A, Schneider C. Enough: the failure of the living will. Hastings Center Report, (March–April) 2004;34:30–42. doi:10.2307/3527683. [PubMed] [Google Scholar]
- Ferraro KF, Farmer MM. Utility of health data from social surveys: Is there a gold standard for measuring morbidity? American Sociological Review. 1999;64:303–315. doi:10.2307/2657534. [Google Scholar]
- Field M, Cassel C. Approaching death. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- Hopp F. Preferences for surrogate decision makers, informal communication and advance directives among community-dwelling elders: Results from a national study. The Gerontologist. 2000;40:449–457. doi: 10.1093/geront/40.4.449. doi:10.1093/geront/40.4.449. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. doi:10.2307/2955359. [PubMed] [Google Scholar]
- Janis IL, Feshbach S. Effects of fear-arousing communications. Journal of Abnormal and Social Psychology. 1953;48:78–92. doi: 10.1037/h0060732. doi:10.1037/h0060732. [DOI] [PubMed] [Google Scholar]
- Kalmijn M. Intermarriage and homogamy: Causes, patterns, trends. Annual Review of Sociology. 1998;24:395–421. doi: 10.1146/annurev.soc.24.1.395. doi:10.1146/annurev.soc.24.1.395. [DOI] [PubMed] [Google Scholar]
- Kwak J, Haley WE. Current research findings on end of life decision making among racially or ethnically diverse groups. The Gerontologist. 2005;45:634–641. doi: 10.1093/geront/45.5.634. doi:10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- Lambert HC, McColl MA, Gilbert J, Wong J, Murray G, Shortt SED. Factors affecting long-term care residents’ decision-making processes as they formulate advance directives. The Gerontologist. 2005;45:626–633. doi: 10.1093/geront/45.5.626. doi:10.1093/geront/45.5.626. [DOI] [PubMed] [Google Scholar]
- Lockwood P, Chasteen A, Wong C. Age and regulatory focus determine preferences for health-related role models. Psychology and Aging. 2005;20:376–389. doi: 10.1037/0882-7974.20.3.376. doi:10.1037/0882-7974.20.3.376. [DOI] [PubMed] [Google Scholar]
- Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- (1990). Patient Self-Determination Act, 42 U.S.C. § 1395. [Google Scholar]
- Pear R. U.S. alters rule on paying for end-of-life planning. New York Times (January 4) 2011 Retrieved from http://www.nytimes.com/2011/01/05/health/policy/05health.html. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;106:203–214. doi:10.1177/014662167700100306. [Google Scholar]
- Rhodes R, Teno JM. What’s race got to do with it? Journal of Clinical Oncology. 2009;24:5496–5498. doi: 10.1200/JCO.2009.24.2206. doi:10.1200/JCO.2009.24.2206. [DOI] [PubMed] [Google Scholar]
- Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: The role of message framing. Psychological Bulletin. 1997;121:3–19. doi: 10.1037/0033-2909.121.1.3. doi:10.1037//0033-2909.121.1.3. [DOI] [PubMed] [Google Scholar]
- Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. New England Journal of Medicine. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. doi:10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. Journal of the American Medical Association. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. doi:10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- Teno JM, Gruneir A, Schwartz Z, Nanda A, Wetle T. Association between advance directives and quality of end-of-life care: A national study. Journal of the American Geriatrics Society. 2007;55:189–194. doi: 10.1111/j.1532-5415.2007.01045.x. doi:10.1111/j.1532-5415.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Advance directives and advance care planning: Report to Congress [online report] 2008. Retrieved from http://aspe.hhs.gov/daltcp/reports/2008/ADCongRpt.htm. [Google Scholar]
- Weitzen S, Teno JM, Fennell M, Mor V. Factors associated with site of death: A national study of where people die. Medical Care. 2003;41:323–335. doi: 10.1097/01.MLR.0000044913.37084.27. doi:10.1097/01.MLR.0000044913.37084.27. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. Death denial: Obstacle or instrument for palliative care? Sociology of Health & Illness. 2007;29:297–314. doi: 10.1111/j.1467-9566.2007.00495.x. doi:10.1111/j.1467-9566.2007.00495.x. [DOI] [PubMed] [Google Scholar]