Abstract
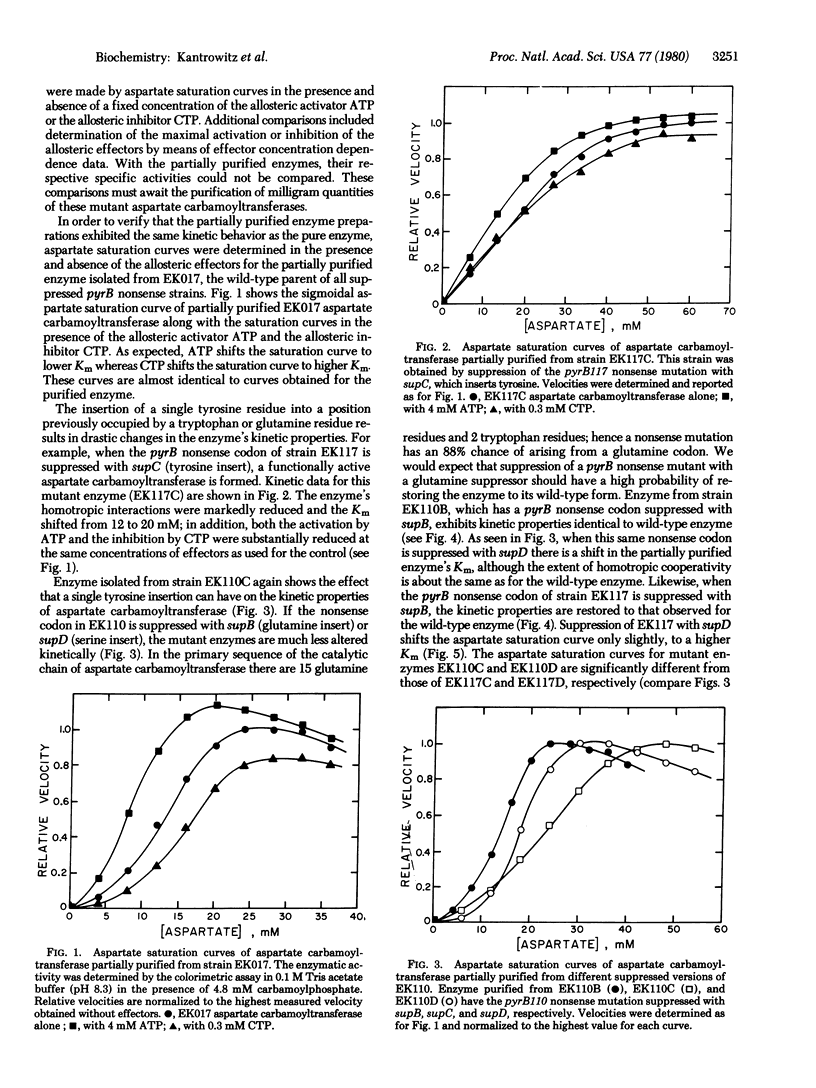
In order to isolate functional Escherichia coli aspartate carbamoyltransferase (carbamoylphosphate:L-aspartate carbamoyltransferase, EC2.1.3.2) with single amino acid replacements, a series of pyrB nonsense mutants has been isolated. These nonsense mutants were induced by 2-aminopurine mutagenesis and selected by a combination of antibiotic treatments, direct enzyme assays, and suppressibility tests. Suppression of the pyrB nonsense mutation with various suppressors, which insert different amino acids, has resulted in the formation of a series of mutant aspartate carbamoyltransferases, each differing in one amino acid from the wild-type enzyme. After partial purification, kinetic studies revealed that some of the mutant enzymes had altered homotropic and heterotropic interactions. The mutants that had a tyrosine insert showed the most pronounced changes, followed by those with a serine insert. The mutants having a glutamine insert, howevr, were indistinguishable from the wild-type enzyme, supporting the conclusion that, because of the specificity of the mutagen, the glutamine insert had regenerated the wild-type enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell M. R., Smith K. E., White J. S., Jones M. E. Carbamyl phosphate: an allosteric substrate for aspartate transcarbamylase of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1442–1449. doi: 10.1073/pnas.60.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Kantrowitz E. R., Lipscomb W. N. An essential residue at the active site of aspartate transcarbamylase. J Biol Chem. 1976 May 10;251(9):2688–2695. [PubMed] [Google Scholar]
- Kolata G. B. Protein structure: systematic alteration of amino Acid sequences. Science. 1976 Jan 30;191(4225):373–373. doi: 10.1126/science.191.4225.373. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A., Pigiet V., Schachman H. K. Hybridization of native and chemically modified enzymes. 3. The catalytic subunits of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1970 Jan;65(1):234–241. doi: 10.1073/pnas.65.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem. 1956 Aug;221(2):757–770. [PubMed] [Google Scholar]
- Pastra-Landis S. C., Evans D. R., Lipscomb W. N. The effect of pH on the cooperative behavior of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1978 Jul 10;253(13):4624–4630. [PubMed] [Google Scholar]
- Perbal B., Hervé G. Biosynthesis of Escherichia coli aspartate transcarbamylase. I. Parameters of gene expression and sequential biosynthesis of the subunits. J Mol Biol. 1972 Oct 14;70(3):511–529. doi: 10.1016/0022-2836(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]



