Abstract
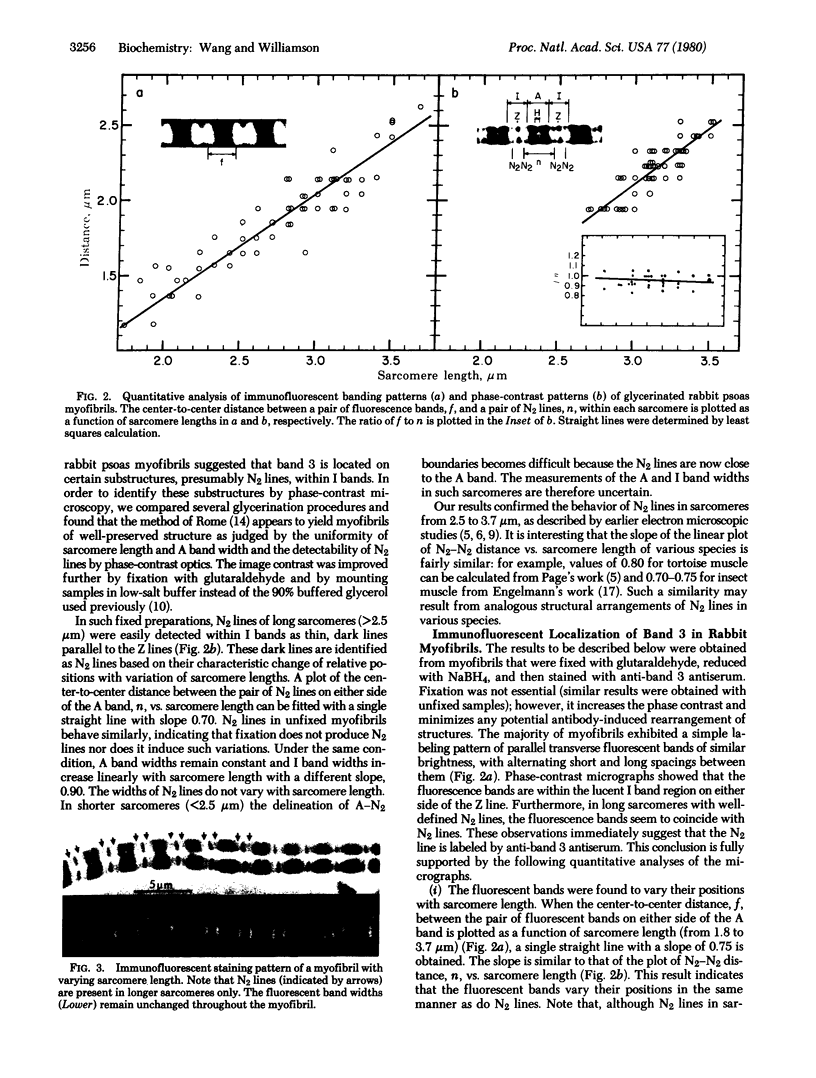
Three unusually large myofibrillar proteins (Mr, > 500,000) are present in major amounts in the striated muscle of various species. the molecular properties and localization of two of these proteins (Mr, approximately 1 X 10(6)), named "titin," have been reported recently [Wang, K., McClure, J. & Tu, A.(1979) Proc. Natl. Acad. Sci. USA 76, 3698-3702]. We have now purified the third protein (Mr, approximately 5-6 X 10(5)), designated "band 3," from rabbit psoas myofibrils. Band 3 is distinct chemically and immunochemically from titin and myosin heavy chain. Immunofluorescent localization studies of band 3 in glycerinated rabbit psoas myofibrils indicated a biphasic distribution. In sarcomeres 1.8-3.7 micrometer long, band 3 is localized predominately on the N2 line in the phase-contrast lucent region of the I band. Quantitative analysis of light micrographs confirmed the unique behavior of N2 lines described in early electron microscopic studies. As the sarcomeres lengthen, the N2 line moves away from both the Z line and the M line, maintaining the same proportional distance. In very short sarcomeres (1.5-1.8 micrometer), band 3 is found mainly in the A band, suggesting that either the N2 line moves into the A band or that band 3 has dissociated from the N2 line which remains near the Z line. We conclude that band 3 is an N2 line component of rabbit skeletal myofibrils.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT H. S., PORTER K. R. An electron microscope study of sectioned breast muscle of the domestic fowl. Am J Anat. 1953 Jul;93(1):61–105. doi: 10.1002/aja.1000930104. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Leet N. G. Histology of highly-stretched beef muscle. IV. Evidence for movement of gap filaments through the Z-line, using the N2-line and M-line as markers. J Ultrastruct Res. 1976 Jul;56(1):31–38. doi: 10.1016/s0022-5320(76)80138-4. [DOI] [PubMed] [Google Scholar]
- Page S. G. Fine structure of tortoise skeletal muscle. J Physiol. 1968 Aug;197(3):709–715. doi: 10.1113/jphysiol.1968.sp008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. Relaxation of glycerinated muscle: low-angle x-ray diffraction studies. J Mol Biol. 1972 Mar 28;65(2):331–345. doi: 10.1016/0022-2836(72)90285-9. [DOI] [PubMed] [Google Scholar]
- Squire J. M. Muscle filament structure and muscle contraction. Annu Rev Biophys Bioeng. 1975;4(00):137–163. doi: 10.1146/annurev.bb.04.060175.001033. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., McClure J., Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M. Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1820–1824. doi: 10.1073/pnas.75.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]