Abstract
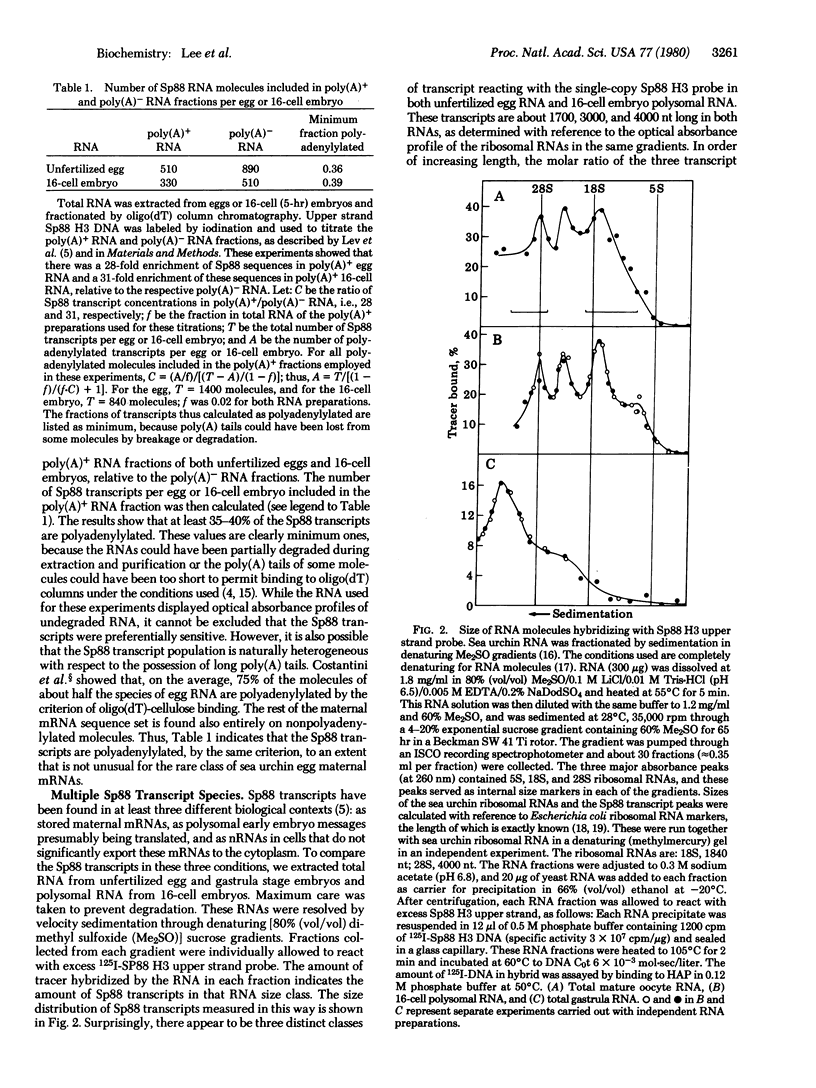
This report concerns a set of sea urchin egg and embryo transcripts complementary to a single-copy region of a cloned DNA fragment (Sp88). Three distinct 16-cell embryo polysomal RNA species were found to hybridize with this fragment. These RNAs are about 1700, 3000, and 4000 nucleotides (nt) in length, and the same species were identified in unfertilized eggs. A significant fraction of all three species of the egg and early embryo transcripts is polyadenylylated. At gastrula stage Sp88 transcripts are almost completely confined to the nucleus [Lev, Z., Thomas, T. L., Lee, A. S., Angerer, R. C., Britten, R. J. & Davidson, E. H. (1980) Dev. Biol, 75, in press]. The Sp88 transcripts of gastrulae are present as a fourth RNA species approximately 5800 nt in length. The four species share a sequence element of cloned DNA fragment that is about 1000 nt long. These RNAs constitute a set of alternative partially overlapping transcripts from the same genomic region.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. D., Scheller R. H., Britten R. J., Davidson E. H. Repetitive sequence transcripts in the mature sea urchin oocyte. Cell. 1978 Sep;15(1):173–187. doi: 10.1016/0092-8674(78)90093-4. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dubroff L. M., Nemer M. Developmental shifts in the synthesis of heterogeneous nuclear RNA classes in the sea urchin embryo. Nature. 1976 Mar 11;260(5547):120–124. doi: 10.1038/260120a0. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Wold B. J., Ernst S. G., Britten R. J., Davidson E. H. Appearance and persistence of maternal RNA sequences in sea urchin development. Dev Biol. 1977 Oct 1;60(1):258–277. doi: 10.1016/0012-1606(77)90123-3. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Gross P. R. Informational RNA sequences in early sea urchin embryos. Biochim Biophys Acta. 1972 Jan 18;259(1):104–111. doi: 10.1016/0005-2787(72)90477-7. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Britten R. J., Davidson E. H. Short-period repetitive-sequence interspersion in cloned fragments of sea urchin DNA. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1065–1076. doi: 10.1101/sqb.1978.042.01.107. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Species identification and genome mapping of cytoplasmic adenovirus type 2 RNAs synthesized late in infection. J Virol. 1977 Aug;23(2):240–249. doi: 10.1128/jvi.23.2.240-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hough B. R., Chamberlin M. E., Davidson E. H. Repetitive and non-repetitive sequence in sea urchin heterogeneous nuclear RNA. J Mol Biol. 1974 May 5;85(1):103–126. doi: 10.1016/0022-2836(74)90132-6. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Simian virus 40 transcription in productively infected and transformed cells. J Virol. 1974 Jun;13(6):1263–1273. doi: 10.1128/jvi.13.6.1263-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Wold B. J., Klein W. H., Hough-Evans B. R., Britten R. J., Davidson E. H. Sea urchin embryo mRNA sequences expressed in the nuclear RNA of adult tissues. Cell. 1978 Aug;14(4):941–950. doi: 10.1016/0092-8674(78)90348-3. [DOI] [PubMed] [Google Scholar]
- Ziff E., Fraser N. Adenovirus type 2 late mRNA's: structural evidence for 3'-coterminal species. J Virol. 1978 Mar;25(3):897–906. doi: 10.1128/jvi.25.3.897-906.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


