Abstract
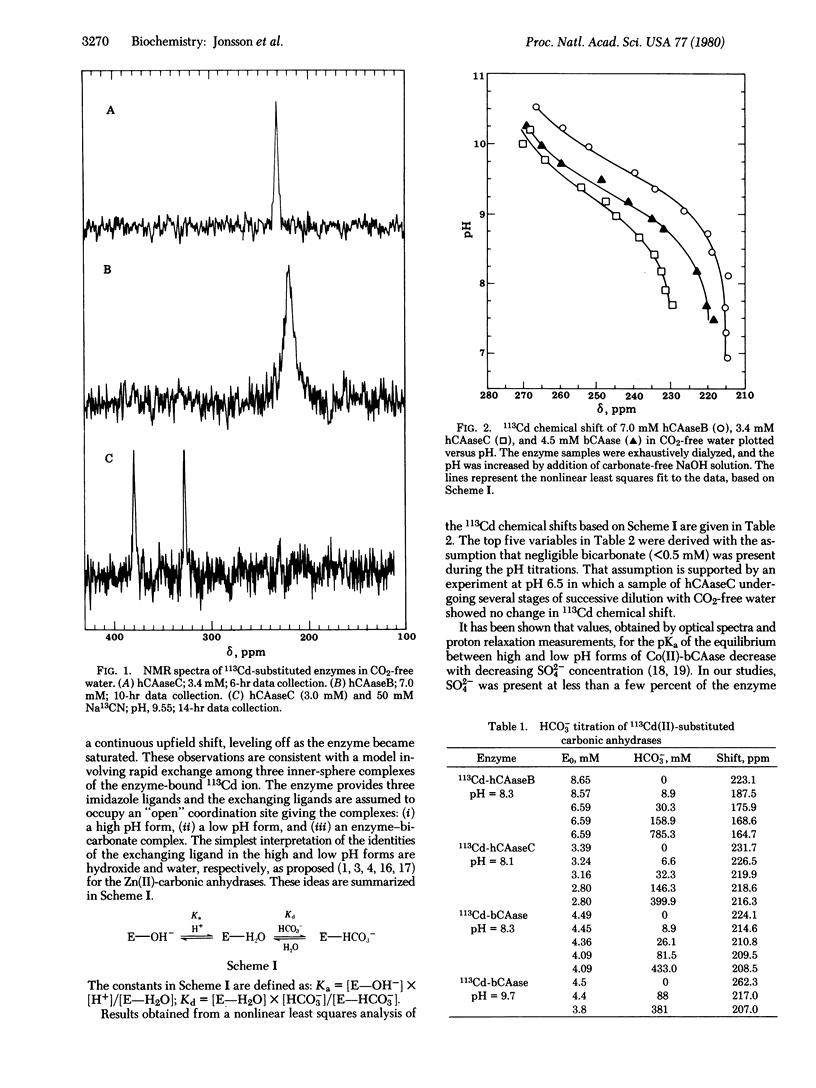
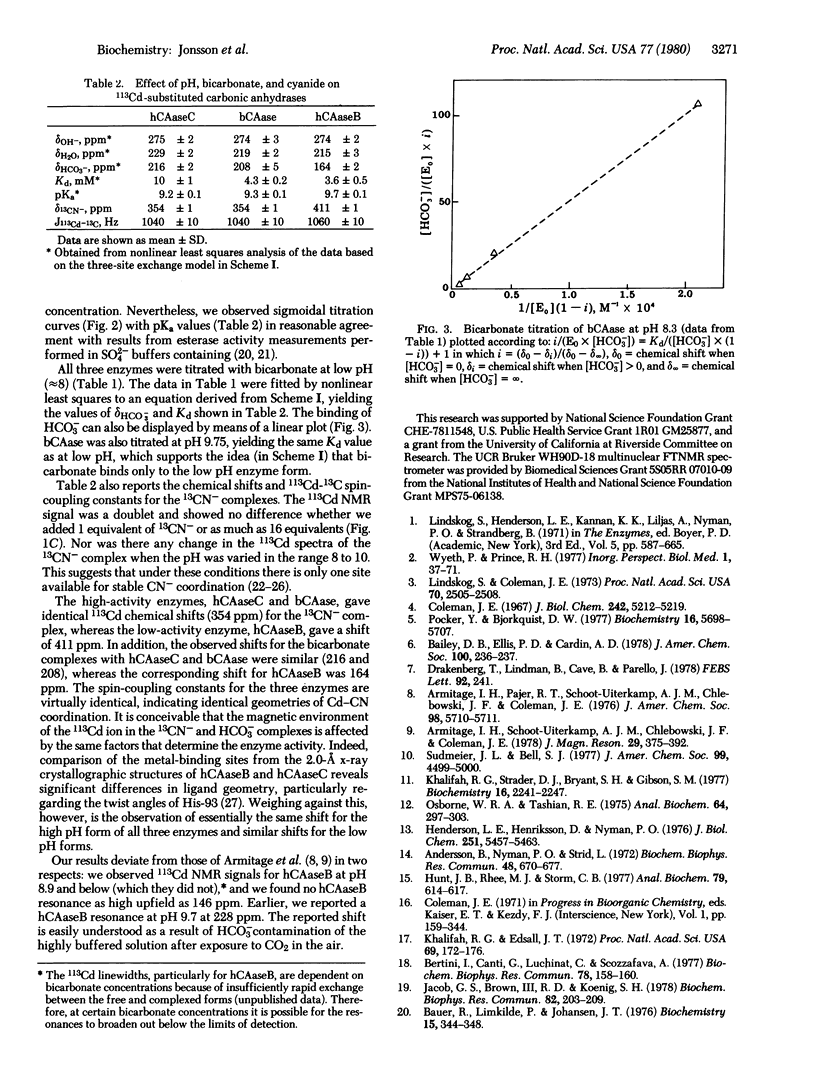
113Cd-Substituted human and bovine erythrocyte carbonic anhydrases have been studied by 113Cd NMR as a function of pH and bicarbonate concentration. Plots of chemical shift versus pH give sigmoidal titration curves in the pH range of the study, 6.9 to 10.5. The pKa values vary from 9.2 to 9.7, which correlates well with available activity profiles for the Cd-enzymes. Because the samples contain no buffers and no anions other than hydroxide, the results point to the existence of high and low pH forms of the enzymes in rapid exchange and differing in inner sphere coordination. When bicarbonate is added to the samples, upfield shifts are produced which eventually level off. Only a single CN- binds to the metal for all three enzymes. These observations are best explained by a rapid exchange among three species in which the open coordination site of the metal ion is occupied by hydroxide, water, or bicarbonate, as in the scheme: E--OH- in equilibrium or formed from E--H2O in equilibrium or formed from E--HCO-3.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Nyman P. O., Strid L. Amino acid sequence of human erythrocyte carbonic anhydrase B. Biochem Biophys Res Commun. 1972 Aug 7;48(3):670–677. doi: 10.1016/0006-291x(72)90400-7. [DOI] [PubMed] [Google Scholar]
- Armitage I. M., Pajer R. T., Uiterkamp A. J., Chleowski J. F., Coleman J. E. Letter: Cadmium-113 Fourier transform nuclear magnetic resonance of cadmium(II) carbonic anhydrases and cadmium(II) alkaline phosphatase. J Am Chem Soc. 1976 Sep 1;98(18):5710–5712. doi: 10.1021/ja00434a058. [DOI] [PubMed] [Google Scholar]
- Bertini I., Canti G., Luchinat C., Scozzafava A. Evidence of exchangeable protons in the donor groups of the acidic form of cobalt bovine carbonic anhydrase B. Biochem Biophys Res Commun. 1977 Sep 9;78(1):158–160. doi: 10.1016/0006-291x(77)91234-7. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. Mechanism of action of carbonic anhydrase. Subtrate, sulfonamide, and anion binding. J Biol Chem. 1967 Nov 25;242(22):5212–5219. [PubMed] [Google Scholar]
- Grell E., Bray R. C. Electron paramagnetic resonance spectroscopy of bovine cobalt carbonic anhydrase B. Biochim Biophys Acta. 1971 May 25;236(2):503–506. doi: 10.1016/0005-2795(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Henriksson D., Nyman P. O. Primary structure of human carbonic anhydrase C. J Biol Chem. 1976 Sep 25;251(18):5457–5463. [PubMed] [Google Scholar]
- Hunt J. B., Rhee M. J., Storm C. B. A rapid and convenient preparation of apocarbonic anhydrase. Anal Biochem. 1977 May 1;79(1-2):614–617. doi: 10.1016/0003-2697(77)90444-4. [DOI] [PubMed] [Google Scholar]
- Jacob G. S., Brown R. D., 3rd, Koenig S. H. Relaxation of solvent protons by cobalt bovine carbonic anhydrase. Biochem Biophys Res Commun. 1978 May 15;82(1):203–209. doi: 10.1016/0006-291x(78)90596-x. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Petef M., Fridborg K., Cid-Dresdner H., Lövgren S. Structure and function of carbonic anhydrases. Imidazole binding to human carbonic anhydrase B and the mechanism of action of carbonic anhydrases. FEBS Lett. 1977 Jan 15;73(1):115–119. doi: 10.1016/0014-5793(77)80027-6. [DOI] [PubMed] [Google Scholar]
- Khalifah R. G., Edsall J. T. Carbon dioxide hydration activity of carbonic anhydrase: kinetics of alkylated anhydrases B and C from humans (metalloenzymes-isoenzymes-active sites-mechanism). Proc Natl Acad Sci U S A. 1972 Jan;69(1):172–176. doi: 10.1073/pnas.69.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifah R. G., Strader D. J., Bryant S. H., Gibson S. M. Carbon-13 nuclear magnetic resonance probe of active-site ionizations in human carbonic anhydrase B. Biochemistry. 1977 May 17;16(10):2241–2247. doi: 10.1021/bi00629a031. [DOI] [PubMed] [Google Scholar]
- Lindskog S., Coleman J. E. The catalytic mechanism of carbonic anhydrase. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2505–2508. doi: 10.1073/pnas.70.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Tashian R. E. An improved method for the purification of carbonic anhydrase isozymes by affinity chromatography. Anal Biochem. 1975 Mar;64(1):297–303. doi: 10.1016/0003-2697(75)90434-0. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Bjorkquist D. W. Comparative studies of bovine carbonic anhydrase in H2O and D2O. Stopped-flow studies of the kinetics of interconversion of CO2 and HCO3. Biochemistry. 1977 Dec 27;16(26):5698–5707. doi: 10.1021/bi00645a008. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. A stable decrease in long chain fatty acyl CoA synthetase activity after treatment of rat adipocytes with adrenaline. FEBS Lett. 1978 Aug 15;92(2):241–244. doi: 10.1016/0014-5793(78)80762-5. [DOI] [PubMed] [Google Scholar]
- Sudmeier J. L., Bell S. J. Cadmium-113 nuclear magnetic resonance studies of 113Cd(ii)-substituted human carbonic anhydrase B. J Am Chem Soc. 1977 Jun 22;99(13):4499–4500. doi: 10.1021/ja00455a049. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Coleman J. E. Electron spin resonance of metallocarbonic anhydrases. J Biol Chem. 1971 Nov 25;246(22):7058–7067. [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]


