Abstract
In lung cancer, platelet-derived growth factor receptor α (PDGFRα) is expressed frequently by tumor-associated stromal cells and by cancer cells in a subset of tumors. We sought to determine the effect of targeting stromal PDGFRα in preclinical lung tumor xenograft models (human tumor, mouse stroma). Effects of anti-human (IMC-3G3) and anti-mouse (1E10) PDGFRα mAbs on proliferation and PDGFRα signaling were evaluated in lung cancer cell lines and mouse fibroblasts. Therapy studies were performed using established PDGFRα-positive H1703 cells and PDGFRα-negative Calu-6, H1993, and A549 subcutaneous tumors in immunocompromised mice treated with vehicle, anti-PDGFRα mAbs, chemotherapy, or combination therapy. Tumors were analyzed for growth and levels of growth factors. IMC-3G3 inhibited PDGFRα activation and the growth of H1703 cells in vitro and tumor growth in vivo, but had no effect on PDGFRα-negative cell lines or mouse fibroblasts. 1E10 inhibited growth and PDGFRα activation of mouse fibroblasts, but had no effect on human cancer cell lines in vitro. In vivo, 1E10-targeted inhibition of murine PDGFRα reduced tumor growth as single-agent therapy in Calu-6 cells and enhanced the effect of chemotherapy in xenografts derived from A549 cells. We also identified that low expression cancer cell expression of VEGF-A and elevated expression of PDGF-AA were associated with response to stromal PDGFRα targeting. We conclude that stromal PDGFRα inhibition represents a means for enhancing control of lung cancer growth in some cases, independent of tumor cell PDGFRα expression.
Keywords: Platelet-derived growth factor receptor, mouse model, lung cancer, stroma, microenvironment, fibroblast, xenograft
Introduction
Platelet-derived growth factor receptor (PDGFR) is a transmembrane receptor tyrosine kinase. Upon binding of circulating PDGF ligand, PDGFRα and β subunits homodimerize or heterodimerize, undergo autophosphoryation, and activate downstream signal transduction molecules including phosphoinositide 3-kinase, Ras, phopholipase C-γ, Src, and signal transducer and activator of transcription1. In normal physiology, PDGFRα functions in early and late stages of embryonic development (during which it drives proliferation of undifferentiated mesenchymal populations, tissue remodeling, and cellular differentiation), wound healing, angiogenesis, and modulating interstitial fluid pressure2–3.
When expressed on cancer cells, PDGFRα has been implicated in the development and progression of several malignancies4. Co-expression of PDGF and PDGFRα has been reported in various cancer types, consistent with autocrine-mediated growth3. In lung cancer, expression of PDGF and/or PDGFRα is associated with more aggressive tumor biology and worse prognosis5. Specific genetic alterations of the PDGF-PDGFRα axis occur in gastrointestinal stromal tumors (activating mutations in the intracellular domain of PDGFRα)6, certain gliomas and NSCLCs (PDGFRα amplification)7–8, dermatofibrosarcoma protuberans (a 17;22 chromosomal translocation resulting in a fusion oncogene encoding PDGF-B)9, and hypereosinophilic syndrome (FIP1L1-PDGFRA fusion transcripts)10.
Given the encouraging results achieved with targeting epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) in patients with lung tumors harboring EGFR mutations and ALK translocations, recent PDGFRα research has focused on identifying those lung cancers with aberrant tumor cell PDGFRα activity as potential candidates for PDGFRα-directed therapy. For instance, in a recent screen of NSCLC cell lines, 1 of 103 cell lines responded in vitro to the PDGFR inhibitor sunitinib11. The sensitive cell line, H1703, was noted to have high-level PDGFRA amplification. Among the larger set of 637 human tumor-derived cell lines evaluated in the study, only one other, the PDGFRα-positive A-204 rhabdomyosarcoma cell line, responded to sunitinib, suggesting that only rare solid tumor patients might benefit from PDGFRα inhibition. However, such an approach does not account for the potential therapeutic effects of stromal PDGFRα inhibition. In tumor stroma, the PDGF-PDGFRα axis functions in fibroblast activation, aberrant epithelial-stromal interactions, modulation of tumor interstitial pressure, and production and secretion of vascular endothelial growth factor (VEGF)12–14. In studies with VEGF-null tumorigenic cells, PDGF-AA was identified as the major stromal fibroblast chemotactic factor produced by the tumor cells that lead to recruitment of VEGF-producing stromal fibroblasts for tumor angiogenesis and growth14.
To determine the effects of stromal PDGFRα inhibition, we capitalized on xenograft modeling of cancer (human tumor cells, mouse stromal cells) and the availability of species-specific anti-PDGFRα monoclonal antibodies (mAbs). In this model, IMC-3G3, a fully human anti-human PDGFRα mAb, targets tumor cell PDGFRα15, whereas 1E10, a fully human anti-mouse PDGFRα mAb, targets stromal PDGFRα. Our studies demonstrate that targeting stromal PDGFRα has single-agent anti-tumor activity and the potential to enhance the effect of chemotherapy in murine models of lung cancer. The cell lines of xenografts sensitive to targeting stromal PDGFRα expressed a high PDGF-AA/VEGF-A ratio relative to a resistant xenograft line, thus suggesting a potential selection strategy for therapy.
Materials and Methods
Cell Culture
With the exception of A549 which was purchased from the American Type Culture Collection (ATCC), all tumor cell lines were provided by Dr. John Minna (UT Southwestern)16. Cell lines were authenticated. Specifically, the identity of each cell line was confirmed by DNA fingerprinting via short tandem repeats using the PowerPlex 1.2 kit (Promega, Madison, WI). Fingerprinting results were compared to reference fingerprints. All cancer cell lines were grown in a humidified atmosphere with 5% CO2, at 37°C in RPMI-1640 medium (Life Technologies Inc., Rockville, MD) supplemented with 5% fetal bovine serum. Each cell line was DNA fingerprinted for provenance using the PowerPlex 1.2kit (Promega) and confirmed to be the same as the DNA fingerprint library maintained by ATCC and the Minna/Gazdar lab (the primary source of the lines), and confirmed to be free of mycoplasma by e-Myco kit (Boca Scientific).
Using previously generated genomic microarray data (Affymetrix, Inc., Santa Clara, Calif. and Illumina, Inc., San Diego, Calif.), 29 NSCLC cell lines with varying expression of PDGFRα were identified. To confirm microarray findings, selected cells were grown in media, harvested at 70–80% confluency, and lysates extracted. Equal amounts of protein were subjected to SDS-PAGE followed by Western blot analysis with anti-PDGFRα antibodies and detected by chemiluminescence. Additionally, confirmation by quantitative PCR was performed. RNA was prepared using TRIzol (Invitrogen) according to the manufacturer’s instructions. The quality of RNA was evaluated using spectrophotometry. The cDNA used for subsequent for PCR was made using iScript (Bio-Rad Laboratories, Hercules, CA). The expression of human PDGFRα was analyzed by quantitative real-time RT-PCR using an assay on demand (Mm00440111_m1) from Applied Biosystems. Mouse GAPDH and human RPLPO (Applied Biosystems assay-on-demand) were used as internal reference genes to normalize input cDNA. Quantitative real-time RT-PCR was performed in a reaction volume of 25 μl including 5 μl of cDNA, and each reaction was performed in triplicate. The comparative Ct method was used to compute relative expression values17.
Anti-PDGFRα Antibody Generation and Characterization
Anti-mouse PDGFRα antibody 1E10 was obtained by selection of a human naïve display Fab library18 on recombinant mouse PDGFRα extracellular domain (ECD) protein following a protocol described previously19. After being converted into full-length IgG format, 1E10 was expressed in NS0 cells as described20. Full-length IgG1 antibody was purified by protein A affinity chromatography (Poros A, PerSeptive Biosystems, Inc., Foster City, CA). IMC-3G3, a fully human anti-human PDGFRα IgG1 mAb, was developed as reported previously15.
Antibody species specificity was confirmed in a solid-phase blocking assay and cell-based signaling assays. In the solid-phase blocking assay, antibodies were first mixed with a fixed amount of mouse PDGFRα/Fc (50 ng at 0.5 μg/ml, R&D Systems, Minneapolis, MN). The mixture was transferred to 96-well plates precoated with PDGF-AA (100 ng/well), followed by incubation with a goat anti-human Fc antibody-HRP conjugate (Jackson ImmunoResearch). Plates were read at A450 nmusing a microplate reader (Molecular Devices, Sunnyvale, CA).
Growth Factor Quantitation
Cells were seeded into 100 mm tissue culture dishes and maintained in 15 ml of media for 96 hrs at 5% CO2 and 37°C. At the 96 hr time point, cell monolayers were approximately 85–90% confluent. The media was collected and spun three times at 1,000 × g to remove particulate matter. The concentration of PDGF-AA and VEGF-A in the media was measured by ELISA (Quantikine kits from R&D systems; cat. # DAA00B and DVE00). The cells in the dishes were washed with PBS, removed by trypsin and counted for normalization of growth factor concentration to cell number.
Cell-Signaling Assays
Receptor and downstream signaling molecule phosphorylation assays were performed as described15. Cell lysates were prepared in lysis buffer a (150 mM NaCl, 50 mM Tris pH 7.4, 1% Triton X-100, 1 mM EDTA, 10 mM NaPPi, 50 mM NaF, 1 mM Na3VO4) with protease inhibitors (Roche, cat. no. 04 693 124 001) and PhosSTOP phosphatase inhibitor (Roche, cat no. 04 906 845 001). Lysates were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were probed with the following antibodies: anti-PDGFRα (Cell Signaling Technology, #3174), anti-PDGFRα (Neomarkers, RB-9027), phospho-PDGFRα (Santa Cruz Biotechnology, sc-12910), anti-β-actin (Santa Cruz, sc-8432), anti-phospho-PLCγ (Cell Signaling Technology, #2821), anti-PARP (Cell Signaling, #9542), and anti-PDGF-C (Santa Cruz Biotechnology, sc-18228). Antibody reactivity was detected using the appropriate peroxidase conjugated secondary antibody (Jackson ImmunoResearch) and subsequent chemiluminescence substrate (Pierce).
MTS Assay
Assays were performed as described previously21. Briefly, 500–4,000 cells were allowed to seed for 24 hrs before four-fold serial dilutions of drug were added. The maximum dose was 500 μg/ml (~3300 nM) for IMC-3G3 and 1E10. Cells were incubated for four days then relative cell number was determined by the addition of MTS (Promega, Madison, WI, final concentration 333 μg/ml), incubating for 1 to 3 hours at 37°C and reading absorbance at 490 nm in a plate reader (Spectra Max 190, Molecular Devices, Downington, PA). Each experiment contained eight replicates per concentration and the entire assay was performed in multiple replicates. Drug sensitivity curves and IC50 values were calculated using in-house software (DIVISA).
Animal Studies
Animal studies were conducted according to U.S. Department of Agriculture and NIH guidelines and approved by the respective Institutional Animal Care and Use Committees. Female athymic nude (Crl:NU/NU-nuBR, Charles River Laboratories, Wilmington, MA) or SCID mice were implanted subcutaneously with 5–20 × 106 cells from selected NSCLC cell lines, as previously described15. For the H1703 monotherapy study, mice were randomized into two groups (n = 12) and treated with human IgG (40 mg/kg) or IMC-3G3 (40 mg/kg) three times weekly. There is a precedent in animal studies for antibody treatment at 40 mg/kg to achieve circulating antibody trough levels consistent with those achieved in man. For example, the target serum concentration for IMC-3G3 is hypothesized to be one that maintains concentrations above levels associated with inhibition of the growth of human tumor growth xenograft models in mice (155–258 μg/ml). Results of the PK data from patients in the IMC-3G3 Phase 1 study demonstrate that IMC-3G3 dosing yielded serum concentrations at or above the target trough concentrations22.
In the 1E10 plus chemotherapy combination studies in A549 and H1993 xenografts, cohorts of animals with established tumors were randomized into four groups (n = 8) and treated with 1) vehicle, 2) chemotherapy (cisplatin, 1 mg/kg 1x/week; gemcitabine 25 mg/kg, 2x/week), 3) 1E10, or 4) chemotherapy plus 1E10. Mice were treated by i.p. injection twice weekly for the duration of the study. In each group, on the day of dosing, the first treatment listed above was administered first, followed by the second treatment 30 minutes later. Tumor volumes were evaluated twice weekly. In all xenograft studies, animals were assessed for toxicity (weight loss, death, cage side observations) and tumors were measured twice weekly. Tumor volume was calculated according to the formula π/6 × longest length × perpendicular width2. Tumor growth in the treatment groups was compared with a repeated-measures ANOVA.
Tumors were harvested for analysis of growth factors by ELISA and Western blotting. Tumors were homogenized in lysis buffer b (Cell Signaling, cat. no. 9803) supplemented with protease inhibitors (Roche) and PhosStop phosphatase inhibitor (Roche). The lysates were centrifuged twice at 14,000 rpm and the protein concentration for the collected supernatant was determined (Bio-Rad, cat. no. 500–0116).
Statistical Analysis
For cell proliferation assays, sigmoidal dose response curves were fitted and the inhibitory concentrations at 50% (IC50) were calculated using the Weibull model. Tumor volumes and weights were compared using the Student’s t test. For IHC studies, tumors were analyzed and compared by One Way ANOVA, followed by Fisher’s LSD PostHoc test for multiple comparisons. The number of samples in each group was always taken as the number of tissues used in the analysis. For PDGF-CC analysis, Adobe Photoshop 7 was used to determine the mean luminosity of the Western blot protein bands. The mean luminosity of each PDGF-C GFD band was normalized by the mean luminosity of the respective β-actin band. These values were plotted in GraphPad Prism 5, and an unpaired t-test was used to determine significance. All calculated P values are two sided. For all tests, P < 0.05 was considered significant.
Results
NSCLC cell line PDGFRα expression and response to inhibition
PDGFRα expression was evaluated by microarray analysis in a panel of NSCLC cell lines (n =119) (Fig. 1a). A subset of these lines (n = 32) were screened for protein expression by Western blot analysis (Supplementary Fig. S1). PDGFRα expression was demonstrated in human H1703 NSCLC cells, but not in A549 or H1993 cells or in mouse fibroblasts (3T3 cells and MEFs) (Fig. 1b). 29 NSCLC cell lines with varying PDGFRα expression by microarray analysis were selected and treated in vitro with escalating concentrations of IMC-3G3. A single cell line, H1703, expressed PDGFRα robustly at the protein level and demonstrated sensitivity to IMC-3G3 with an IC50 of 225 nM (34 μg/ml). All other cell lines (including A549, H1993, and Calu-6) demonstrated resistance to IMC-3G3, with IC50 greater than 3300 nM (500 μg/ml)(see Table 1). The PDGFRα-positive H1703 lung cancer line contains both PDGFRα and PDGF-CC gene amplifications and shows a co-dependency on this axis for proliferation11. Co-dependence of this kind is a rare event in human lung cancer cell lines, suggesting that only a limited number of lung tumor patients might benefit from tumor cell PDGFRα inhibition. By contrast, IMC-3G3 did not inhibit proliferation of H1792 cells (IC50 > 500 μg/mL). These cells overexpress PDGFRα (see Supplementary Fig. S1) but not PDGF ligand11. This observation suggests that, in the absence of PDGFRα mutation or amplification, overexpression of both ligand and receptor are required for cell autonomous growth.
Figure 1.
PDGFRα is expressed by the non-small cell lung cancer cell line H1703. (a) Microarray expression of PDGF ligand isoforms and PDGFRs. Of 119 cell lines analyzed, 23 are displayed; cell lines selected for in vitro ± in vivo studies are highlighted in yellow. Microarray analysis was performed using the Illumina v3 platform with the following probes: PDGFRA (NM_006206), PDGFRB (NM_002609), PDGFA (NM_033023), PDGFB (NM_033016), PDGFC (NM_016205), PDGFD (NM_025208). (b) Quantitative PCR for human PDGFRα in mouse fibroblasts (3T3 cells and MEFs) and human NSCLC cell lines (A549, H1993, H1703).
TABLE 1.
Expression of PDGF-AA and VEGF-A and response to PDGFRα inhibition in lung cancer cell lines grown in culture and in vivo.
| Cell Line | Avg PDGF-AA (ng/ml/105 cells± SD) | Avg VEGF-A (ng/ml/105 cells± SD) | Ratio PDGF- AA/VEGF-A | Ratio VEGF- A/PDGF-AA | In vitro anti- human PDGFRα mAb IC50 (μg/mL)† | In vivo anti- mouse PDGFRα mAb tumor/control volume |
|---|---|---|---|---|---|---|
| Calu-6 | 0.0708± 0.0350 | 0.1388± 0.0769 | 0.510 | 1.960 | >500 | 0.51 |
| A549 | 0.07833± 0.0232 | 0.8518± 0.255 | 0.092 | 10.874 | >500 | 0.66 |
| H1993 | 0.0235± 0.0148* | 1.5476± 0.178 | 0.015 | 65.855 | >500 | 1.21 |
| H2073 | 0.0086 ±0.0010* | 0.5224± 0.131 | 0.016 | 60.742 | >500 | N/A |
| H1703 | bld | 2.0254± 0.0784 | N/A | N/A | 34 | N/A |
Extrapolated values
With the exception of H1703, all tested NSCLC cell lines (N=29) were resistant to anti-PDGFRα antibody therapy in vitro (IC50 > 500 μg/mL)
Bld, below limits of detection; N/A, not applicable; SD, standard deviation
Cells were grown for 96 hours at 37oC and5% CO2. Supernatants were collected and analyzed by ELISAs to quantitate secreted VEGF-A and PDGF-AA.
Characterization of IMC-3G3 and 1E10
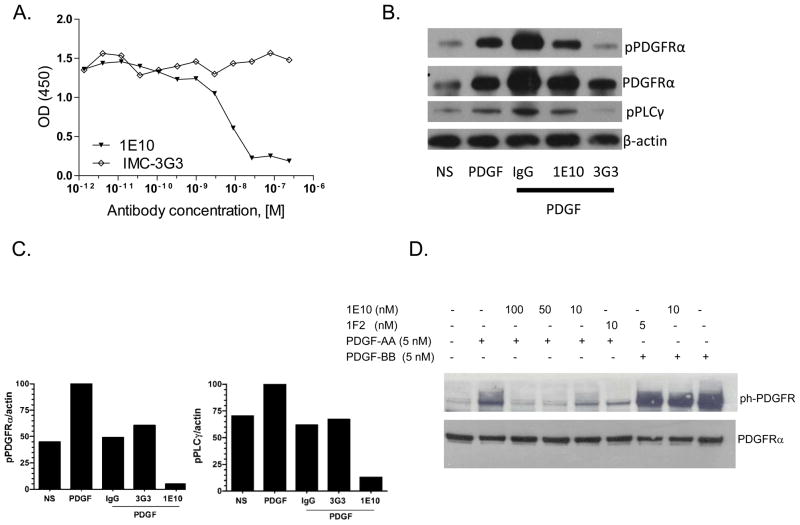
We capitalized on the xenograft model (human cancer cells, mouse stromal cells) and species-specific monoclonal antibodies to evaluate the effects of inhibiting tumor cell and stromal PDGFRα Species-specific targeting was demonstrated through binding assays, Western blot analysis, and effects on receptor activation and signaling. The anti-human PDGFRα mAb IMC-3G3 has been previously described15. To target stromal PDGFRα, an anti-mouse PDGFRα mAb (1E10) was generated. 1E10 was shown to inhibit PDGF-AA from binding to murine PDGFRα with an IC50 of 8.51 × 10−9 M (Fig. 2a). IMC-3G3 had no blocking effect in this assay. Antibody 1E10 demonstrated immunoreactivity with PDGFRα-positive mouse fibroblasts but not with PDGFRα-positive human H1703 NSCLC cells (Supplementary Fig. S2). Conversely, IMC-3G3 had immunoreactivity with PDGFRα-positive human H1703 NSCLC cells but not with PDGFRα-positive mouse fibroblasts or with PDGFRα-negative human A549 NSCLC cells (Supplementary Fig. S2). Therefore, the use of IMC-3G3 in subcutaneous human xenograft models employing tumors lines that are PDGFRα-negative would be ineffective and would not discern the therapeutic effects of stromal PDGFRα inhibition. PDGF-AA-mediated phosphorylation of PDGFRα and PLCγ in H1703 cells was inhibited by IMC-3G3 but not by 1E10 (Fig. 2b). In contrast, 1E10 but not IMC-3G3 inhibited activation of PDGFRα and the signal transduction mediator PLCγ in PDGF-stimulated mouse fibroblasts in vitro (Fig. 2c). 1E10 inhibited PDGF-AA-induced phosphorylation of mouse PDGFRα but not PDGF-BB-induced phosphorylation of mouse PDGFRβ (Fig. 2d).
Figure 2.
Species-specific activity of IMC-3G3 and 1E10. (a) Inhibition of mouse PDGFRα binding to immobilized PDGF-AA by 1E10 but not IMC-3G3. Antibodies were pre-incubated with a fixed amount of Fc-tagged-mouse PDGFRα and then this mixture was transferred to a plate precoated with PDGF-AA. Plate-bound mouse PDGFRα was detected with a goat-anti-human Fc antibody-HRP conjugate.(b) PDGF-AA-mediated phosphorylation of PDGFRα and PLCγ in H1703 cells is inhibited by IMC-3G3 but not by 1E10. H1703 cells were non-stimulated (NS) or stimulated with PDGF-AA 50 ng/ml (1.7 nM) for 5 minutes and treated with antibodies. 1E10 and IMC-3G3 were used at concentrations of 132 μg/ml (880 nM). Lysates were prepared 10 minutes after incubation and probed by Western blot for the targets indicated. (c) In vitro activity of 1E10 in mouse fibroblasts. PDGF-AA-mediated phosphorylation of PDGFRα and PLCγ in mouse fibroblasts is inhibited by IMC-1E10 but not by 3G3. Mouse fibroblasts were non-stimulated (NS) or stimulated with PDGF-AA and treated with antibodies. Lysates were prepared 10 minutes after incubation and probed by Western blot for the targets indicated. (d) Specific inhibition of mouse PDGFRα phosphorylation by 1E10. NIH3T3 mouse fibroblasts were rendered quiescent, treated with mAbs, and then stimulated with either PDGF-AA or PDGF-BB. Afterward, cell lysates were analyzed by SDS-PAGE and Western blotting with an anti-phosphotyrosine antibody. 1E10 inhibited PDGF-AA-induced phosphorylation of PDGFRα but not PDGF-BB-induced phosphorylation of PDGFRβ.
In vitro and in vivo activity of IMC-3G3 on PDGFRα–positive NSCLC
In vitro, human H1703 cells were sensitive to IMC-3G3in MTS assay (IC50 = 10 μg/mL), but were resistant to 1E10 (IC50 > 1000 μg/mL) (Fig. 3a). Additionally, IMC-3G3 significantly inhibited growth of H1703 xenografts (P = 0.0003, Fig. 3b). Tumors in mice receiving IMC-3G3 (mean final volume 433 ± 18 mm3) were significantly smaller than in mice receiving control IgG (mean final volume 1233 ± 211 mm3), corresponding to a 65% reduction in final tumor volume.
Figure 3.
In vitro and in vivo activity of IMC-3G3. (a) Proliferation of human H1703 cells in vitro is inhibited by anti-human PDGFRα monoclonal antibody IMC-3G3 but not by anti-mouse PDGFRα monoclonal antibody1E10. Cells were allowed to seed for 24 hrs before serial dilutions of drug were added. Cells were incubated for four days then relative cell number was determined by MTS assay. (b) Growth inhibition of H1703 tumor xenografts in nude mice treated with 40 mg/kg of IMC-3G3 on a three times per week schedule.
In vivo anti-tumor activity of 1E10 on PDGFRα-negative xenografts with high PDGF-AA/VEGF-A ratio
Given that PDGF-AA has been identified as a major stromal fibroblast chemotactic factor produced by tumor cells14, it may be assumed that xenograft lines that express high PDGF-AA would have a greater dependence on stromal activation for growth. Therefore, thirteen lung cancer cell lines were screened for PDGF-AA expression to identify lines for in vivo anti-tumor studies targeting stromal PDGFRα. Specifically, the cell lines were grown in vitro and expressed PDGF-AA was measured from the supernatants (Table 1). Two lines expressing the highest PDGF-AA were chosen for in vivo therapeutic studies of 1E10. In A549 and Calu-6 xenografts, 1E10 treatment attenuated tumor growth, demonstrating a significant therapeutic effect of stromal targeting (Fig. 4). In Calu-6 xenografts, average tumor size on Day 32 was 1273 ± 187 mm3 with 1E10 treatment, compared to 2483 ± 302 mm3 with control IgG (P=0.002) (Fig. 4a). In A549 xenografts, average tumor size on Day 36 was 632 ± 65 mm3 with 1E10 treatment, compared to 960 ± 93 mm3 with control IgG(P=0.01)(Fig. 4b). 1E10also enhanced the effect of cisplatin-gemcitabine chemotherapy in A549 xenografts (Fig. 4c). Final tumor weights were as follows: control IgG 1.05 ± 0.20 g, 1E10 0.74 ± 0.08 g, cisplatin-gemcitabine 0.91 ± 0.15 g, cisplatin-gemcitabine plus 1E10 0.56 ± 0.05 g (P = 0.04 for cisplatin-gemcitabine plus 1E10 compared to cisplatin-gemcitabine). Of note, these sensitive cell lines had relatively low in vitro expression of VEGF-A compared to PDGF-AA (VEGF-A/PDGF-AA ratio 1.96 in Calu-6 cells and 10.87 in A549 cells) (Table 1).
Figure 4.
In vivo activity of 1E10. (a) 1E10 inhibited Calu-6 xenograft tumor growth with a T/C value of 51%. This effect was statistically significant (P = 0.002). (b) 1E10 inhibited A549 xenograft tumor growth with a T/C% of 67%. This effect was statistically significant (P = 0.01). (c and d) In vivo activity of 1E10 in combination with chemotherapy. Tumor-bearing mice were treated with chemotherapy (cisplatin, 1 mg/kg 1x/week; gemcitabine 25 mg/kg 2x/week),1E10 twice weekly, or the combination for 3 weeks. (c) 1E10 enhanced the antitumor activity of chemotherapy in A549 NSCLC xenografts. (d) 1E10 did not enhance the antitumor activity of chemotherapy in H1993 NSCLC xenografts.
Targeting of stromal PDGFRα with 1E10 had no activity as a monotherapy on the H1993 xenograft (Fig. 4d). In addition, 1E10 did not enhance the effect of cisplatin-gemcitabine chemotherapy in H1993 xenografts(final weights: control IgG 0.79 ± 0.09 g, 1E10 0.78 ± 0.06 g, cisplatin-gemcitabine 0.28 ± 0.05 g, cisplatin-gemcitabine plus 1E10 0.26 ± 0.07 g) (Fig. 4d). This cell line resistant to stromal PDGFRα inhibition had an elevated VEGF-A/PDGF-AA ratio of 65.86 (Table 1). Also of note, in the H1993 xenografts, chemotherapy alone inhibited tumor growth, but A549 xenografts were resistant to the same regimen.
Discussion
High-throughput in vitro screens offer a means to test anti-tumor agents against multiple cancer cell lines relatively quickly and inexpensively. While such features have rendered this a preferred approach to early-stage preclinical drug development, these studies provide no insight into tumor stromal effects. For inhibitors of PDGFRα, a target expressed on cancer cells and stromal fibroblasts, in vivo testing would facilitate demonstrating the full spectrum of therapeutic effects. In agreement with previous studies11, this report shows that, in vitro, PDGFRα-directed therapy has efficacy in a small proportion of cell lines with PDGFRα over-expression.
However, in addition, stromal PDGFRα targeting in vivo reduced tumor growth and enhanced the effect of cytotoxic chemotherapy for two xenografts, independent of tumor cell PDGFRα expression. The results are consistent with paracrine effects of the PDGF-PDGFRα axis, in which stromal fibroblasts express PDGFRα and malignant cells secrete PDGFs23–24. These results echo those of earlier reports suggesting that therapeutic stromal effects, which become apparent in animal models, might otherwise go unrecognized in cell cultureassays25–26.
Potential explanations for the therapeutic outcome of stromal-PDGFR targeting include effects on fibroblast activation and angiogenesis12,14,27. In a previous report, PDGF-AA, which binds PDGFRα but not PDGFRβ, was demonstrated to be the major stromal fibroblast chemotactic factor produced by VEGF-null tumorigenic cells14. In that system it was concluded that PDGFRα signaling is required for the recruitment of VEGF-producing stromal fibroblasts for tumor angiogenesis and growth. In the present study, tumor cell PDGF-AA and VEGF expression levels suggested an explanation as to why only certain lines responded to stromal PDGFRα inhibition. Elevated cancer cell expression of PDGF-AA and low expression of VEGF were associated with response to stromal PDGFRα targeting. Specifically, sensitive cell lines had relatively low VEGF-A expression (VEGF-A/PDGF-AA ratio 1.96 in Calu-6 cells and 10.87 in A549 cells), while the resistant cell line H1993 had elevated VEGF-A (VEGF-A/PDGF-AA ratio 65.86) when grown in cell culture. This difference may suggest that the 1E10-sensitive tumors have a relatively greater dependence on PDGF-AA-induced production of stromal VEGF. Notably, we have previously demonstrated the converse for anti-VEGF monoclonal antibody (bevacizumab) sensitivity, with H1993 xenografts responding to therapy but A549 and Calu-6 exhibiting a poor response28. If the predictive value of PDGF-AA/VEGF ratio is confirmed preclinically, it would be reasonable to test this biomarker in the clinical setting.
Potential explanations for the effects of stromal PDGFR targeting include effects on angiogenesis, fibroblast activation, and tumor interstitial pressure12. Preclinical studies have demonstrated that imatinib, a multitargeted tyrosine kinase inhibitor that inhibits PDGFR, reduces interstitial fluid pressure and increases intratumoral concentration of concurrently administered cytotoxic chemotherapy29–30. That 1E10 significantly improved the effect of cisplatin-gemcitabine in the A549 xenografts might suggest reduction of tumor interstitial pressure and enhanced chemotherapy delivery. PDGF-activated stromal fibroblasts synthesize collagen, express matrix metalloproteinases, and form a connective tissue network, all of which promote tumor development31–33. Although the present experiments do not elucidate a therapeutic mechanism of stromal PDGFRα inhibition, it is unlikely that the observed in vivo effects represent a non-stromal mechanism. The possibility that PDGFRα-negative cell lines adapt to express PDGFRα in vivo was considered. However, no human PDGFRα was detected in tumor lysates, and the anti-human PDGFRα antibody IMC-3G3had no effect on A549 xenograft tumor growth(data not shown).
While a number of PDGFR inhibitors are currently FDA-approved for cancer treatment, these drugs are all relatively non-specific small molecule kinase inhibitors. This lack of specificity has therapeutic as well as experimental consequences. For instance, due to cross-reactivity with c-KIT, imatinib is associated with hematologic toxicity precluding its concomitant administration with cytotoxic chemotherapy12. PDGFR kinase inhibitors also inhibit both PDGFR subunits. Inhibition of PDGFRβ, which is expressed predominantly on vessel pericytes and modulates vascular permeability, may result in clinically significant fluid retention, pleural and pericardial effusions, peripheral edema, and weight gain34. By contrast, the role of PDGFRα in normal adult physiology appears limited to wound healing3. Consequently, PDGFRα may be a less toxic and more therapeutically potent target. In a recently completed phase I monotherapy study, no specific adverse events were consistently or conclusively associated with IMC-3G322,35. Importantly, it has been noted that in cancer cells expressing both PDGF and PDGFR, PDGF forms a complex with PDGFR within the endoplasmic reticulum36, an intracellular sanctuary site not available to antibody-based therapeutics. However, the PDGF-PDGFR complex generates mitogenic signals only after migration to the cell membrane, where it is accessible to neutralizing antibodies37.
Partly for reasons described above, clinical trials of PDGFR inhibitors combined with chemotherapy for lung cancer therapy have been negative to date, and some have demonstrated a substantial increase in toxicity38–39. However, the results seen with tyrosine kinase inhibitors targeting PDGFR cannot be extrapolated to a highly specific monoclonal antibody such as IMC-3G3. With EGFR inhibitors and antiangiogenic agents, no benefit was seen when tyrosine kinase inhibitors were combined with chemotherapy, but the addition of a monoclonal antibody to chemotherapy improved overall survival40–44.
The implication that stromal PDGFR targeting could add benefit to NSCLC therapy is substantial. The overwhelming majority of NSCLCs feature stromal PDGFR expression5,45–46. Our findings support the possibility that the therapeutic effects of stromal PDGFRα inhibition may reach beyond the rare cancers sensitive to cancer-cell PDGFRα targeting. In general, stromal targets have a number of favorable properties. They are less prone to mutation than are cancer cell targets. As shown by the broad clinical use of anti-VEGF antibodies, stromal targeting may be relevant to multiple cancer types. Finally, stromal-directed therapies have been combined effectively and safely with cancer cell-directed treatments, including cytotoxic chemotherapy. As shown in our experiments, for those cancers without tumor cell PDGFRα expression, an anti-PDGFRα antibody may be most effective when combined with conventional chemotherapy or other targeted agents. Accordingly, the results of ongoing phase 2 trials incorporating anti-PDGFRα monoclonal antibodies into combination therapy for various malignancies are awaited to evaluate this strategy in the clinical setting.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS/GRANT SUPPORT
Supported by a National Institutes of Health UL1 RR024982 and KL2 RR024938-03 “North and Central Texas Clinical and Translational Science Initiative (NCTCTSI)” grants (to D.E. Gerber), a sponsored research agreement from Imclone Systems (D.E. Gerber and R.A. Brekken) and The Effie Marie Cain Scholarship in Angiogenesis Research (to R.A. Brekken). P. Gupta was supported by NCI training grant T32CA009640.
The authors acknowledge the assistance of the Biostatistics and Microarray Shared Resources at the Harold C. Simmons Cancer Center, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-01, and the support of Drs. John Minna and Joan Schiller.
The authors thank Ilse Valencia for assistance with PDGFRα Western Blot analysis and PCR in NSCLC cell lines and immortalized human bronchial epithelial cells. The authors also thank Marie Prewett for help with statistical calculations.
Footnotes
Presented in part at the Cancer Prevention and Research Institute of Texas (CPRIT) Innovations in Cancer Prevention and Research Conference, Austin, Texas, November 17–29, 2010 and at the 47th annual meeting of the American Society of Clinical Oncology, Chicago, Illinois, June 3–7, 2011.
DISCLOSURES/CONFLICTS:
D.E.G. and R.A.B. receive research funding from ImClone Systems, a wholly owned subsidiary of Eli Lilly and Company, New York, NY.
I.D., J.M., T.B., C.B., and N.L. are employed by ImClone Systems, a wholly owned subsidiary of Eli Lilly and Company, New York, NY.
References
- 1.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 2.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A, Heldin CH. Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv Cancer Res. 2001;80:1–38. doi: 10.1016/s0065-230x(01)80010-5. [DOI] [PubMed] [Google Scholar]
- 4.Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 5.Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008;3:963–70. doi: 10.1097/JTO.0b013e3181834f52. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 7.Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–3. [PubMed] [Google Scholar]
- 8.Ramos AH, Dutt A, Mermel C, Perner S, Cho J, Lafargue CJ, et al. Amplification of chromosomal segment 4q12 in non-small cell lung cancer. Cancer Biol Ther. 2009;8:2042–50. doi: 10.4161/cbt.8.21.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon MP, Navarro M, Roux D, Pouyssegur J. Structural and functional analysis of a chimeric protein COL1A1-PDGFB generated by the translocation t(17;22)(q22;q13.1) in Dermatofibrosarcoma protuberans (DP) Oncogene. 2001;20:2965–75. doi: 10.1038/sj.onc.1204426. [DOI] [PubMed] [Google Scholar]
- 10.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 11.McDermott U, Ames RY, Iafrate AJ, Maheswaran S, Stubbs H, Greninger P, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–46. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauman JE, Eaton KD, Martins RG. Antagonism of platelet-derived growth factor receptor in non small cell lung cancer: rationale and investigations. Clin Cancer Res. 2007;13:s4632–6. doi: 10.1158/1078-0432.CCR-07-0212. [DOI] [PubMed] [Google Scholar]
- 13.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–10. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–79. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 16.Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. J Natl Cancer Inst. 2010;102:1310–21. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlen Y, McNair A, Perseguers S, Mazza C, Mermod N. Statistical significance of quantitative PCR. BMC Bioinformatics. 2007;8:131. doi: 10.1186/1471-2105-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–30. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393–9. doi: 10.1002/ijc.1634. [DOI] [PubMed] [Google Scholar]
- 20.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–21. [PubMed] [Google Scholar]
- 21.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70:2852–61. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youssoufian H, Amato RJ, Sweeney C, Chiorean EG, Fox F, Katz T, et al. Phase 1 study of IMC-3G3, an IgG1 monoclonal antibody targeting PFGDRa in patients with advanced solid malignancies. J Clin Oncol 2008. 2008 May 26;20(suppl):abstract 14617. [Google Scholar]
- 23.Peres R, Betsholtz C, Westermark B, Heldin CH. Frequent expression of growth factors for mesenchymal cells in human mammary carcinoma cell lines. Cancer Res. 1987;47:3425–9. [PubMed] [Google Scholar]
- 24.Vignaud JM, Marie B, Klein N, Plenat F, Pech M, Borrelly J, et al. The role of platelet-derived growth factor production by tumor-associated macrophages in tumor stroma formation in lung cancer. Cancer Res. 1994;54:5455–63. [PubMed] [Google Scholar]
- 25.Ichihara E, Ohashi K, Takigawa N, Osawa M, Ogino A, Tanimoto M, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. 2009;69:5091–8. doi: 10.1158/0008-5472.CAN-08-4204. [DOI] [PubMed] [Google Scholar]
- 26.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169:2054–65. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Liu XW, Kim HR. Platelet-derived growth factor (PDGF) receptor-alpha-activated c-Jun NH2-terminal kinase-1 is critical for PDGF-induced p21WAF1/CIP1 promoter activity independent of p53. J Biol Chem. 2003;278:49582–8. doi: 10.1074/jbc.M309986200. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan LA, Carbon JG, Roland CL, Toombs JE, Nyquist-Andersen M, Kavlie A, et al. r84, a novel therapeutic antibody against mouse and human VEGF with potent anti-tumor activity and limited toxicity induction. PLoS One. 2010;5:e12031. doi: 10.1371/journal.pone.0012031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–34. [PubMed] [Google Scholar]
- 30.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–84. [PubMed] [Google Scholar]
- 31.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–45. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 32.Alvares O, Klebe R, Grant G, Cochran DL. Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J Periodontol. 1995;66:552–8. doi: 10.1902/jop.1995.66.7.552. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg K, Valyi-Nagy I, Heldin CH, Herlyn M, Westermark B. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci U S A. 1993;90:393–7. doi: 10.1073/pnas.90.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauro MJ, Deininger MW. Management of drug toxicities in chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22:409–29. doi: 10.1016/j.beha.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116:1018–26. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- 36.Keating MT, Williams LT. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988;239:914–6. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- 37.Fleming TP, Matsui T, Molloy CJ, Robbins KC, Aaronson SA. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989;86:8063–7. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsao AS, Liu S, Fujimoto J, Wistuba II, Lee JJ, Marom EM, et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J Thorac Oncol. 2011;6:2104–11. doi: 10.1097/JTO.0b013e31822e7256. [DOI] [PubMed] [Google Scholar]
- 39.Huang CH, Williamson SK, Van Veldhuizen PJ, Hsueh CT, Allen A, Tawfik O, et al. Potential role of platelet-derived growth factor receptor inhibition using imatinib in combination with docetaxel in the treatment of recurrent non-small cell lung cancer. J Thorac Oncol. 2011;6:372–7. doi: 10.1097/JTO.0b013e318200f9ad. [DOI] [PubMed] [Google Scholar]
- 40.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 41.Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Scagliotti G, Von Pawel J, Reck M, Cupit L, Cihon F, DiMatteo J, et al. Sorefenib plus carboplatin/paclitaxel in chemonaive patients with stage IIIB-IV non-small cell lung cancer (NSCLC): interim analysis results from the phase III, randomized controlled ESCAPE (Evaluation of Sorafenic, Carboplatin, and Paclitaxel Efficacy in NSCLC) Trial. Journal of Thoracic Oncology. 2008;3(4, Supplement 1):S97. Late Breaking Abstract 275. [Google Scholar]
- 43.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 44.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 45.Kawai T, Hiroi S, Torikata C. Expression in lung carcinomas of platelet-derived growth factor and its receptors. Lab Invest. 1997;77:431–6. [PubMed] [Google Scholar]
- 46.Donnem T, Al-Shibli K, Al-Saad S, Busund LT, Bremnes RM. Prognostic impact of fibroblast growth factor 2 in non-small cell lung cancer: coexpression with VEGFR-3and PDGF-B predicts poor survival. J Thorac Oncol. 2009;4:578–85. doi: 10.1097/JTO.0b013e31819f2e38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.