Abstract
The current study examined the interactive effects of anxiety sensitivity (AS; fear of anxiety and anxiety-related sensations) and menstrual cycle phase (premenstrual phase vs. follicular phase) on panic-relevant responding (i.e., cognitive and physical panic symptoms, subjective anxiety, and skin conductance level). Women completed a baseline session and underwent a 3-minute 10% CO2-enriched air biological challenge paradigm during her premenstrual and follicular menstrual cycle phases. Participants were 55 women with no current or past history of panic disorder recruited from the general community (Mage = 26.18, SD = 8.9) who completed the biological challenge during both the premenstrual and follicular cycle phases. Results revealed that women higher on AS demonstrated increased cognitive panic symptoms in response to the challenge during the premenstrual phase as compared to the follicular phase, and as compared to women lower on AS assessed in either cycle phase. However, the interaction of AS and menstrual cycle phase did not significantly predict physical panic attack symptoms, subjective ratings of anxiety, or skin conductance level in response to the challenge. Results are discussed in the context of premenstrual exacerbations of cognitive, as opposed to physical, panic attack symptoms for high AS women, and the clinical implications of these findings.
Keywords: anxiety sensitivity, biological challenge, menstrual cycle, panic disorder, premenstrual phase
Anxiety sensitivity (AS), or the tendency to respond fearfully to anxiety symptoms (McNally 2002), is a cognitive risk factor for the development of panic disorder (PD; Donnell and McNally 1989; Maller and Reiss 1992; McNally and Lorenz 1987). First, AS is elevated among individuals with PD compared to other anxiety and mood disorders (McNally 1996; Taylor et al. 1992). Second, it is related to greater anxiety levels in response to a biological challenge (Zvolensky and Eifert 2000). Finally, it predicts the development of spontaneous panic attacks and PD in longitudinal studies above and beyond trait anxiety (Li and Zinbarg 2007; Schmidt et al. 2006) and negative affectivity (Zvolensky et al. 2005). AS is often, but not uniformly (van Beek and Griez 2003), significantly elevated among females as compared to males in university samples (Stewart et al. 1997) and among PD patients (Schmidt and Koselka 2000), particularly pertaining to physical concerns (Foot and Koszycki 2004).
The premenstrual phase of the menstrual cycle may constitute a possible sex-specific, cyclical stressor that contributes to the onset of maladaptive anxiety and panic pathology in women with a vulnerability to anxiety (i.e., high AS). A variety of psychological and bodily symptoms have been correlated with the premenstrual phase, including anxiety (Bloch et al. 1997; Chrisler and Caplan 2002; Logue and Moos 1986; Freeman 2003), and approximately 50-80% of women report that they experience at least some symptoms during this phase (Halbreich et al. 2003; Pearlstein and Stone 1998; Wittchen et al. 2002). Although only 2-8% rate these symptoms as ‘disabling’ or ‘severe’ (Asso 1983; Halbreich et al. 2003), the majority of women experience some degree of impairment (Barnard et al. 2003).
Several different areas of research implicate the menstrual cycle in the development and/or maintenance of panic psychopathology. First, greater premenstrual symptoms or a diagnosis of PMDD are associated with increased panic attack symptoms following laboratory-based panic provocation (Kent et al. 2001; Le Melledo et al. 1999; Harrison et al. 1989; Nillni et al. 2009). Second, both retrospective and prospective studies have often, but not uniformly (e.g., see Stein et al. 1989), found an increase in anxiety symptoms and frequency of panic attacks during the premenstrual phase among women with PD (Breier et al. 1986; Kaspi et al. 1994). Additionally, studies have found menstrual cycle phase differences in panic symptoms and skin conductance response frequency and magnitude following anxiety induction procedures in women with PD, but not controls (Perna et al. 1995; Sigmon et al. 2000). Third, ovarian hormones (e.g., progesterone) and their metabolites (e.g., allopregnanolone or THP) have been shown to influence GABAA receptor expression and, subsequently anxiety behavior (Follesa et al. 2000), and have been hypothesized to correspond to anxiety and premenstrual symptoms in women, but the literature is inconsistent (Halbreich et al. 1986; Schmidt et al. 1991).
To our knowledge, only one study has examined the role of AS physiological responding to anxiety-provoking stimuli during the premenstrual phase (Sigmon et al. 1996). Specifically, the authors examined the interaction between AS (high vs. low) and menstrual phase [premenstrual (Days −5 to −1) vs. intermenstrual (Days 8-22)] on psychophysiological and psychological reactivity to anxiety-provoking stimuli (i.e., listening to descriptions of anxiety-provoking vs. neutral scenarios) using a cross-sectional design. High AS women measured in their premenstrual phase displayed greater skin conductance response frequency and magnitude in response to the anxiety scenes in comparison to high AS women assessed in the intermenstrual phase and low AS women assessed in either cycle phase above and beyond the effects of baseline state anxiety and panic attack history (Sigmon et al. 1996). This study suggested that AS may play a prominent role in premenstrual panic symptoms. Additionally, past work (Sigmon et al. 1996, 2000), suggests that skin conductance responsivity increases premenstrually in reaction to anxiety-provoking stimuli, but does not fluctuate across the menstrual cycle in the absence of a stressor.
Although some evidence exists for menstrual cycle phase differences in panic attack symptoms and responding among individuals with PD, little work has examined this within at-risk samples (i.e., high AS). Additionally, to our knowledge, no prior study has examined panic-relevant responding during the premenstrual (Days −5 to −1) versus follicular (Days 6-12) phases among the same women. These two phases may be the most theoretically important comparison, particularly in the context of AS, because the premenstrual phase, a period of flux in progesterone levels, is associated with increased symptom reports, while the follicular phase, a period of stable progesterone levels, is associated with low symptom reports (Gonda et al. 2008). The current study also extends previous work by using a repeated measures design and utilizing a strict method of cycle phase confirmation as opposed to relying solely on day count.
Together, the current study examined panic-relevant responding to a CO2 biological challenge in women varying in AS across the premenstrual and follicular phases of the menstrual cycle. It was hypothesized that women higher on AS would report greater post-challenge panic and anxiety sensations and exhibit heightened skin conductance level during the biological challenge in their premenstrual phase as compared to their follicular phase and as compared to women lower on AS assessed in either cycle phase. We examined the severity of total panic symptoms, as well as the two key facets of panic symptoms (physical and cognitive symptoms) separately. Although a panic attack does not necessarily necessitate the presence of cognitive symptoms (e.g., fear of dying), the presence of these catastrophic misinterpretations of physical symptoms (e.g., rapid heart beat) during a panic attack have been shown to differentiate individuals with PD from nonclinical panickers or other anxiety disorder groups (Barlow, Brown, & Craske, 1994). Moreover, inclusion of the cognitive component of panic attacks has been recommended in research on anxiety psychopathology (Barlow et al., 1994). Based on the hormonal-stress vulnerability model, this study posits that when AS is elevated in women, it serves as an underlying cognitive vulnerability that interacts with both a naturally-occurring internal stressor (premenstrual phase) and an external, anxiety-provoking stressor to elicit panic-relevant responding in women (Nillni et al. 2011).
Materials and Method
Participants
Participants were normally menstruating (i.e., average cycle length of 25-35 days that did not regularly vary in length month-to-month by ≥ 7 days) community women who responded to advertisements for a “study on women’s health.” Data collection took place between June 2009 to October 2010.
Exclusion criteria included: a) hormonal birth control methods; b) postmenopausal or perimenopausal (e.g., hot flashes, irregular periods) status; c) pregnancy or trying to become pregnant; d) current or past PD with or without Agoraphobia, e) current Generalized Anxiety Disorder, Specific Phobia, Post Traumatic Stress Disorder, Social Anxiety Disorder, Obsessive Compulsive Disorder, Alcohol or Substance Dependence, or psychosis; f) current serious suicidal intent; g) contraindicated medical conditions (e.g., cardiovascular or seizure disorder, asthma); and h) current use of anxiety medication (e.g., Beta blockers, anxiolytics).
Pre-Challenge Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders–Non Patient Version (SCID-NP; First et al. 1994) is a structured diagnostic interview that was administered to assess for the presence of current and lifetime Axis I diagnoses and current suicidal ideation. 10% of the interviews were coded for diagnostic reliability by an independent rater. There were no disagreements between raters.
The Medical Screening Questionnaire was administered to assess exclusionary criteria pertaining to medical conditions (e.g., asthma, cardiovascular disorders, seizure disorder), psychotropic medications (e.g., Beta blockers, anxiolytics), and irregular menstrual cycles.
Provisional Premenstrual Dysphoric Disorder (PMDD) Assessment
A provisional PMDD assessment using DSM-IV-TR PMDD criteria, excluding prospective recording of symptoms across two menstrual cycles, was conducted at the screening visit in order to potentially control for PMDD diagnosis given its relevance to CO2 challenge responding (e.g., Le Melledo et al., 1999). In accordance with DSM-IV-TR criteria, daily data on the Daily Record of Severity of Problems (DRSP, described below) was utilized to confirm the provisional PMDD diagnosis.
Daily Record Severity of Problems (DRSP)
The DRSP (Endicott, Nee, & Harrison, 2006) is a 14-item daily questionnaire that measures severity of symptoms (e.g., “felt angry, irritable”) on a 6-point Likert scale (1 = “Not at all” to 6 = “Extreme”) across the menstrual cycle, including 11 psychological and physical symptom items and 3 impairment items. The DRSP has been shown to be a reliable and valid measure for identification of premenstrual symptoms and impairment (Endicott et al., 2006). Participants completed this questionnaire daily throughout the study duration (≥ one full menstrual cycle). Following the method described in Borenstein, Dean, Leifke, Korner, and Yonkers (2007), a diagnosis of PMDD required DRSP data indicating: 1) 5 symptoms, including one emotional symptom (i.e., depressed, anxious, affect lability, and irritability), with a rating as > 3 during the premenstrual phase (Days −5 to −1), 2) an average daily score of < 3 in the follicular phase (Days 6-12), and 3) at least a 30% change from follicular to premenstrual phase.
The Anxiety Sensitivity Index (ASI; Reiss and McNally 1985; Reiss et al. 1986) is a 16-item, self-report assessment that measures fear of bodily sensations related to anxious arousal on a 5-point Likert scale (0 = very little to 4 = very much). Prior research has demonstrated acceptable test-retest reliability (ranging from .71 to .75) and validity (Reiss et al. 1986), good internal reliability (α = .88; Peterson and Heilbronner 1987), and has been shown to be a good representation of a single factor structure (Reiss et al. 1986). The ASI total score was used in the present study. Internal consistency of the ASI total score in the current sample was good (α = .78).
The Positive and Negative Affect Schedule – Negative Affect Scale (N-PANAS; Watson et al. 1988) is a 10-item self-report scale that assesses negative affectivity and has demonstrated sound psychometric properties (Crawford and Henry 2004; Watson et al. 1988). The N-PANAS was used to control for negative affectivity and internal consistency of N-PANAS total score in the current sample was excellent (α = .89).
Challenge Measures
During the CO2 challenge procedure, skin conductance level (SCL) was measured using a J&J Engineering I-330-C2 system, which digitally recorded physiological data online at a sample rate of 1024 samples per second across all channels using J&J Engineering PhysioLab Software. SCL was assessed in micromhos by using a Coulbourn S71-23 isolated coupler, and collected by placing Ag-AgCl electrodes filled with electrode paste to the medial phalanxes of the third and fourth fingers of the nondominant hand. SCL was used because prior studies have shown changes in SCL across a biological challenge (e.g., Bernstein et al. 2009). Any non-readable data (i.e., missing data due to electrode falling off a participant) was eliminated following standard data reduction strategies employed in past biological challenge studies (Zvolensky et al. 1998).
The Diagnostic Sensations Questionnaire (DSQ; Sanderson et al. 1988, 1989) is a widely-used 16-item self-report measure of DSM-IV-TR panic symptom intensity rated on a 9-point Likert scale (0 = not at all noticed to 8 = very strongly felt). The DSQ measures both physical (e.g., “pounding of racing heart”) and cognitive (e.g., “fear of losing control”) symptoms of panic. The DSQ was administered at baseline and post-challenge at both laboratory visits, and the DSQ mean intensity score, as well as physical and cognitive subscales were used as the primary outcomes of self-reported panic response in this study. In the current sample, the internal consistency was excellent: post-challenge DSQ total score (premenstrual α = .94; follicular (α = .94), physical subscale (α = .93; α = .92), and cognitive subscale (α = .88; α = .89).
The Subjective Units of Distress Scale (SUDS; Wolpe 1958) is a self-report visual analogue scale, where participants indicate along a 100-mm line the degree of anxiety they are experiencing from 0 (“no anxiety”) to 100 (“extreme anxiety.”) Participants completed SUDS at baseline and post-challenge.
Procedure
Screening Visit
A medical screening questionnaire and the SCID-NP were administered in order to assess inclusion and exclusion criteria. Participants who met exclusionary psychiatric diagnoses were given referral information. Those meeting final eligibility criteria were administered the provisional PMDD assessment, asked to complete self-report questionnaires, and compensated $10.
Randomization and Scheduling Procedure
The first laboratory visit was scheduled according to random assignment to occur during either the next premenstrual phase (Day −5 to −1) or the next follicular phase (Days 6-12). These day ranges were based on a 28-day cycle and were adjusted for women with other normal cycle lengths (i.e., 25-35 days). First, an estimated first day of bleeding for the next menstrual cycle was calculated at the screening visit based on the beginning date of the last menstrual cycle as a reference point. Then, participants were instructed to call or email the experimenter on their first day of bleeding. At that time, she was either scheduled for her follicular lab visit (if randomized to Follicular-Premenstrual) or scheduled for an ovulation test start date (if randomized to Premenstrual-Follicular). These procedures were completed at the end of the first laboratory visit to schedule their second visit. For participants who were randomized to Premenstrual-Follicular, there were times when a participant would begin bleeding prior to her scheduled premenstrual visit despite use of day count and ovulation testing. In 16% of these cases (n = 9), lab visit 1 was switched to Follicular-Premenstrual in order to increase study retention, resulting in slightly uneven groups for order of testing (63.6% Follicular-Premenstrual vs. 36.4% Premenstrual-Follicular).
Ovulation Testing
At the end of the screening visit, eligible participants were given ovulation detection kits, and instructed to begin testing for their LH peak by urinating midstream on an ovulation test starting on a day specified by the experimenter and to continue testing their urine daily until detection of the LH peak (after which ovulation should occur within 48 hours; Epting and Overman 1998). The start date of testing was determined based on their average menstrual cycle length via the ovulation test kits (i.e., would begin testing on Day 12 if their average cycle length was 28 days). They were scheduled for their premenstrual laboratory visit within 12-14 days following this peak. Participants were compensated $15 for completing these at-home procedures.
Saliva Sampling Procedure and Confirmation of Cycle Phase
There is typically only about 50% agreement between day count and later progesterone assay results (Shirtcliff et al. 2001). Therefore, the use of a more conservative method for assessing cycle phase, including day count, ovulation testing, and a progesterone assay, was used in order to increase confidence that women were measured in the menstrual cycle phase intended. Participants provided a saliva sample at each laboratory visit and progesterone level was examined. Participants rinsed their mouths with water 10 minutes prior to collection. They were instructed to imagine that they were chewing their favorite food, while allowing the saliva to pool in their mouth. Then they passively drooled down a 2-inch piece of a plastic straw into a 2 ml cryovial with their heads tilted forward until 1/3 of the tube was filled. Samples were frozen within 30 minutes at a temperature of −40°C and sent to Salimetrics, LLC within 2 months for assaying of progesterone.
For normally menstruating women, mean progesterone level is 136.30 pg/ml (SD = 82.3) in the premenstrual phase and 80.35 pg/ml (SD = 34.8) in the follicular phase (Salimetrics LLC 2010). However, a wide range of normal values were expected, particularly in the premenstrual phase, given that women would be at different rates of progesterone decline. Specifically, progesterone levels begin to decline 8 days prior to menstruation with the steepest drop occurring 2 days prior to menstruation (Rubinow et al. 1988), therefore, premenstrual visit assessment 1 day prior to menstruation would likely reveal lower progesterone levels than assessment 5 days prior to menstruation. Next menstrual cycle start date and LH peak were used as the primary measures of cycle verification, and progesterone level was used as a secondary measure of cycle verification if there was missing information on the primary measures. For the follicular phase laboratory visit, follicular cycle phase was considered verified if: 1) women reported an LH peak following their follicular phase visit, or 2) progesterone level fell within one standard deviation of the follicular phase mean reported above. For the premenstrual phase laboratory visit, premenstrual cycle phase was considered verified if: 1) women reported an LH peak prior to their premenstrual phase visit, 2) women reported a menstrual cycle start date within 5 days following their premenstrual phase visit, or 3) progesterone level fell within one standard deviation of the premenstrual phase mean reported above. Laboratory visits that did not meet verification of current menstrual cycle phase according to the steps outlined above were excluded from the analyses.
Laboratory Visits CO2 Challenge Procedure
Participants were asked to refrain from any type of substance (e.g., caffeine, nicotine, alcohol) on the days of their two laboratory visits to avoid confounding the effect of the challenge with other substances. Upon arriving to the laboratory, participants were seated in a 9 ft × 10 ft room and participants received an overview of the CO2 procedure, including possible side effects of breathing CO2-enriched air (e.g., breathlessness, dizziness, chest pain, and tachycardia; Eifert et al. 1999). Then a positive pressure Downs C-PAP mask and electrodes for measuring skin conductance were attached. The CO2 was stored in a 101 cm cylinder and fed through a 5 cm × 5 cm hole via aerosol tubing from an adjacent control room to the C-PAP mask worn by participants.
The participant was instructed to sit quietly for an adaptation period (i.e., 10-minutes of breathing regular room air through the mask; Forsyth and Eifert 1998), while baseline skin conductance level was recorded. However, she was not told of the onset, timing, dose, or offset of the CO2 inhalation. Prior to CO2 inhalation, participants completed baseline measures of anxiety and panic symptoms (i.e., DSQ and SUDS). Then she underwent a single 3-minute administration of 10% CO2-enriched air (10% CO2, 21%O2, 69% NO2), while skin conductance level was continuously recorded. An automated and well-established apparatus was used for CO2 delivery (Lejuez et al. 1998). Immediately following the biological challenge procedure, participants were instructed to complete post-challenge anxiety (i.e., SUDS) and panic symptom (i.e., DSQ) measures. Following these procedures, participants were debriefed, compensated $20 and $25 for participation in laboratory visit 1 and 2 respectively, and instructed to contact the experimenter on the day they began their next menstrual cycle (if randomized to Premenstrual-Follicular), or given an ovulation test start date and instructions (if randomized to Follicular-Premenstrual).
Data Analytic Strategy
To examine the interactive effects of AS and menstrual cycle phase (premenstrual vs. follicular) on panic-relevant responding, regression equations using Linear Mixed Models (LMM) analysis were conducted in SPSS. Participants with at least one cycle-confirmed timepoint were included. Each model tested had one between-subjects factor (AS) and two within-subjects factors: time during the challenge (baseline vs. post-challenge) and menstrual phase (premenstrual vs. follicular). Each measure of panic response (DSQ mean, DSQ cognitive, DSQ physical, SUDS, and SCL) was modeled as a linear function of these three fixed effects and their interactions in 5 separate LMMs. Participant was included in the model as a random effect to control for correlations between repeated measurements of the same participant. AS was examined continuously rather than as two groups (high, low) as has been done in previous research (Sigmon et al. 1996) in order to more accurately capture the range of AS when examining the interactive effects of AS and menstrual phase.1 Negative affectivity (N-PANAS) was included in the models as a fixed effect covariate, as this variable has been shown to influence challenge response (Vujanovic et al. 2006). Significant interactions were explored graphically in order to characterize the nature of the interaction.
Results
Participant Characteristics
The final sample of participants included 55 women (Mage = 26.18, SD = 8.9). See Tables 1 and 2 for complete demographic information.
Table 1.
Participant Demographics (N = 55)
| Age, Mean (SD) | 26.18 (8.90) |
| Ethnicity, Number (%) | |
| Hispanic/Latino | 4 (7.3%) |
| Non Hispanic | 51 (92.7%) |
| Race, Number (%) | |
| Caucasian | 47 (85.5%) |
| African American | 2 (3.6%) |
| Asian | 2 (3.6%) |
| Native American | 2 (3.6%) |
| Other | 1 (1.8%) |
| Marital Status, Number (%) | |
| Single | 38 (69.1%) |
| Married | 13 (23.6%) |
| Living Together | 3 (5.5%) |
| Divorced | 1 (1.8%) |
| Education, Number (%) | |
| 11th Grade | 1 (1.8%) |
| High School Degree | 1 (1.8%) |
| Currently in College | 30 (54.5%) |
| Freshman | 4 (7.3%) |
| Sophomore | 9 (16.4%) |
| Junior | 6 (10.9%) |
| Senior | 6 (10.9%) |
| Other | 1 (1.8%) |
| Graduated from a 2-yr College | 1 (1.8%) |
| Graduated from a 4-yr College | 19 (34.5%) |
| Graduated with a Master’s Degree | 3 (5.5%) |
Table 2.
Rates of Current and Past Axis I Diagnoses
| Current Axis I Diagnosis, Number (%) |
Past Axis I Diagnosis, Number (%) |
|
|---|---|---|
| Any Diagnoses, Number (%) | 5 (9.1%) | 29 (52.7%) |
| Alcohol Abuse | 0 (0.0%) | 8 (14.4%) |
| Alcohol Dependence | 0 (0.0%) | 3 (5.4%) |
| Anorexia Nervosa | 0 (0.0%) | 4 (7.2%) |
| Anxiety Disorder NOS | 1 (1.8%) | 0 (0.0%) |
| Bulimia Nervosa | 1 (1.8%) | 1 (1.8%) |
| Dysthymia | 3 (5.4%) | 0 (0.0%) |
| Generalized Anxiety Disorder | 0 (0.0%) | 1 (1.8%) |
| Major Depressive Disorder | 3 (5.4%) | 22 (39.6%) |
| Obsessive Compulsive Disorder | 0 (0.0%) | 1 (1.8%) |
| Other Substance Dependence | 0 (0.0%) | 2 (3.6%) |
| Post Traumatic Stress Disorder | 0 (0.0%) | 2 (3.6%) |
| Social Phobia | 0 (0.0%) | 2 (3.6%) |
| Specific Phobia | 0 (0.0%) | 1 (1.8%) |
Menstrual Cycle Phase Differences in Baseline Challenge Variables
Premenstrual baseline SUDS ratings, DSQ total mean scores, DSQ total physical scores, DSQ total cognitive scores, and SCL were not significantly different from follicular baseline scores on these measures (p’s > .05), suggesting that women experienced comparable panic, anxiety, and psychophysiological symptoms in each cycle phase prior to the challenge.
Zero-Order Correlations Among Study Variables
In terms of zero-order correlations among Aim 1 predictor and baseline challenge variables, N-PANAS total scores, and ASI total scores were significantly positively correlated with one another (r = .49, p < .001; sharing approximately 24% of the variance). ASI total scores were significantly positively correlated with follicular baseline DSQ mean (r = .40, p < .01), physical (r = .39, p < .01), and cognitive (r = .32, p < .05) subscale scores and SUDS scores (r = .29, p < .05). Similarly, N-PANAS total scores were significantly positively correlated with follicular baseline DSQ mean (r = .38, p < .01), physical (r = .36, p < .01), and cognitive (r = .34, p < .05) subscale scores and SUDS scores (r = .38, p < .01). Alternatively, neither ASI total scores or N-PANAS total scores were correlated with any of the premenstrual baseline self-report variables or with premenstrual and follicular baseline SCL.
ASI total scores were significantly positively correlated with premenstrual post-challenge DSQ cognitive subscale scores (r = .32, p < .05), but not with any other post-challenge outcome variable (p’s > .05). N-PANAS total scores were significantly positively correlated with follicular post-challenge SUDS scores (r = .31, p < .05), but not with any other post-challenge outcome variable (p’s > .05).
LMM Analyses
Five models were tested examining the main effects of time-invariant or between-subject factors, time-varying or within-subject factors, and their interactions on the five outcome measures (DSQ mean score, DSQ physical, and DSQ cognitive subscale scores, SUDS, and SCL). The final model included N-PANAS, ASI, cycle phase, challenge, ASI × cycle phase, ASI × challenge, cycle phase × challenge, and ASI × cycle phase × challenge. Additionally, after fitting the model with several covariance structures, the unstructured covariance structure provided the best fit based on the AIC and BIC fit statistics (i.e., lower numbers equal better fit). For example, using the unstructured covariance structure, the AIC and BIC fit statistics were 1062.5 and 1114.2, respectively, whereas they were 1207.9 and 1205.9 using the compound symmetry covariance structure.
Time at challenge significantly predicted DSQ mean total scores (F(1, 43.9) = 31.23, p < .001), physical (F(1, 45.7) = 35.23, p < .001), and cognitive (F(1, 43.8) = 11.46, p < .01) subscale scores, SUDS scores (F(1, 44.9) = 29.54, p < .001), and SCL (F(1, 41.7) = 15.89, p < .001), whereby scores were lower at baseline as compared to post-challenge. There was a trend for N-PANAS on DSQ mean total scores (F(1, 46.2) = 3.78, p = .06), DSQ physical subscale scores (F(1, 46.9) = 4.04, p = .05), and SUDS (F(1, 44.6) = 3.78, p = .06) such that higher N-PANAS scores predicted higher scores regardless of the time at challenge and cycle phase of assessment.
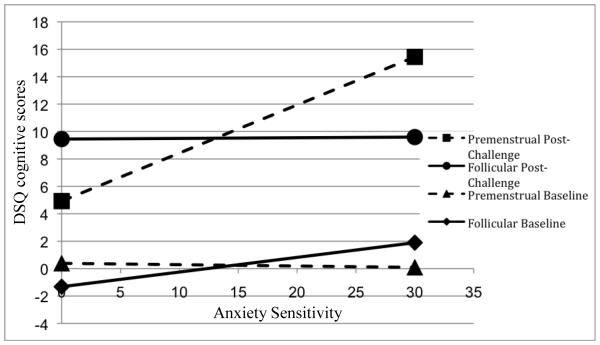
There was a three-way interaction between ASI × cycle phase × challenge was significant (F(1, 37.9) = 4.96, p < .05), suggesting that DSQ cognitive scores in each phase (premenstrual and follicular) and time of challenge (baseline and post-challenge) depended on the level of AS. This interaction was examined by graphing the predicted values of the premenstrual baseline, premenstrual post-challenge, follicular baseline, and follicular post-challenge DSQ cognitive scores as a function of ASI score, allowing examination of the slope of ASI for each cycle phase/time of challenge group; see Figure 1 for a graphic representation of the observed interaction. Women higher on AS measured in the premenstrual cycle phase reported the greatest DSQ cognitive subscale scores as compared to women higher on AS measured in the follicular cycle phase and women lower on AS measured in either cycle phase. See Table 3 for LMM results of DSQ cognitive panic symptoms. There were no other significant main or interaction effects on DSQ outcomes (p’s > .05).
Figure 1.
ASI × Cycle Phase × Challenge Interaction on DSQ Cognitive Symptoms
Table 3.
Linear Mixed Modeling Results for DSQ Cognitive Symptoms
| b | SE | t | p | |
|---|---|---|---|---|
| N-PANAS | .04 | .04 | 1.06 | .29 |
| ASI | .00 | .18 | .03 | .98 |
| Phase | −4.52 | 3.27 | −1.38 | .18 |
| Challenge | −10.77 | 2.71 | −3.98 | .001 |
| Phase × ASI | .35 | .20 | 1.69 | .10 |
| Challenge × ASI | .10 | .17 | .59 | .55 |
| Phase × Challenge | 6.22 | 3.33 | 1.87 | .07 |
| Phase × Challenge × ASI | −.46 | .21 | −2.23 | .03 |
Note: DSQ = Diagnostic Sensations Questionnaire (Sanderson et al. 1988, 1989); N-PANAS = Positive and Negative Affect Scale – Negative Affect Scale (Watson et al. 1988); ASI Total = Anxiety Sensitivity Index Total Score (Reiss and McNally 1985; Reiss et al. 1986); phase = premenstrual vs. follicular cycle; challenge = baseline vs. postchallenge
Analyses were reconducted examining the three ASI subscales (physical, cognitive, and social concerns). The only significant three-way interaction occurred between ASI physical subscale scores × cycle phase × challenge (F(4.4, 37.0) = 4.41, p < .05) predicting DSQ cognitive scores. This finding suggests the possibility that the three-way interaction observed using the total ASI score is driven by the physical items (e.g., “it scares me when my heart beats rapidly”).
Discussion
There was a significant three-way interaction between anxiety sensitivity (AS), time at challenge, and menstrual cycle phase in predicting cognitive panic symptoms following the carbon dioxide challenge. Specifically, during the premenstrual phase, post-challenge scores evidenced the greatest increase as a function of AS level, whereby women with higher AS scores reported higher Diagnostic Sensations Questionnaire (DSQ) cognitive symptom scores during the premenstrual phase than during their follicular phase and as compared to women with lower AS during either phase.
Contrary to prediction, there were no significant main or interactive effects of AS and cycle phase in predicting DSQ mean scores, DSQ physical panic attack symptoms, subjective anxiety (SUDS), and SCL. The null findings for subjective anxiety and physical panic symptoms are inconsistent with a prior study that found increased subjective anxiety and physical panic symptoms in the menstrual vs. mid-luteal phase following a CO2 challenge in Panic Disorder (PD) patients vs. controls (Perna et al. 1995). However, that study differed from the current study in the menstrual cycle phases assessed (menstrual vs. luteal) and in the sample used (PD patients vs. controls). The current findings also are inconsistent with prior research that reported increases in SCL in the premenstrual vs. intermenstrual phase in PD patients vs. controls (Sigmon et al. 2000) and in nonclinical participants with high vs. low AS following an induced external stressor (Sigmon et al. 1996). However, in contrast to those studies, the current study considered AS as a continuous variable rather than as a dichotomous grouping variable (high vs. low AS), and employed a different panic provocation paradigm. Thus, methodological differences may have influenced, in part, consistency in reported findings.
The significant finding for DSQ cognitive, but not physical, symptoms is interesting and provides a platform for theory refinement and future research in at least two substantive ways. First, the present findings suggest that the increased vulnerability to panic-relevant responding may be operative in the cognitive mode of responding, specifically (e.g., misinterpretation and catastrophizing of physical symptoms) among high AS women in the premenstrual phase, rather than in the physiological mode of responding (e.g., intensity of physical symptoms). Therefore, high AS women may experience similar levels of physical symptoms and psychophysiological arousal in response to a biological challenge across the menstrual cycle, but may be more likely to misinterpret the elicited symptoms as ‘more personally dangerous or threatening’ in the premenstrual than in the follicular phase. Given that the CO2 challenge is a potent physiological stressor involving induction of intense physical symptoms (Zvolensky and Eifert 2000), variability in physical panic symptoms and in psychophysiological reactions (i.e., SCL) across repeated measures may be attenuated within subjects, particularly in those without PD.
Second, catastrophic misinterpretations in the meaning, as opposed to the intensity, of physical symptoms may involve other moderating processes that influence the interpretation of physical symptoms. One potential moderator is coping behaviors. Women high on AS engage in more avoidant-oriented coping, including mental and behavioral disengagement, alcohol/drug use, and denial, and engage in less acceptance coping strategies as compared to women low on AS (Sigmon et al. 2004). In nonclinical samples, increased avoidant-oriented coping predicts increased physical and cognitive panic symptoms and subjective anxiety following CO2 challenge procedures (Karekla et al. 2004; Spira et al. 2004). Additionally, women with PD prospectively reported using more mental and behavioral disengagement and less acceptance coping strategies in their premenstrual phase as compared to their intermenstrual phase (Sigmon et al. 2004). Collectively, this literature suggests that at-risk women (e.g., high AS women or PD patients) may use more avoidant-oriented coping during the premenstrual phase as compared to other cycle phases. Therefore, it is possible that at-risk women engage in more maladaptive coping (i.e., avoidant) in response to physical symptoms during the premenstrual phase. Subsequent repeated practice with this type of coping may, in turn, strengthen catastrophic misinterpretations of physical symptoms, as is typical in models of panic etiology and maintenance (Barlow 2002). Longitudinal designs are needed to delineate the progression of coping and panic symptoms, and the current study cannot answer this question, which remains an important area for future research.
The current study adds unique insight into how experience and interpretation of physical sensations varies across the menstrual cycle among the same women without PD. Clinically, this work may inform prevention and intervention efforts for women. For example, prevention or treatment for panic psychopathology could be modified to include intensification of treatment, particularly cognitive restructuring, in the premenstrual phase as well as assessment of and psychoeducation around menstrual cycle-related exacerbations of panic symptoms in both outpatient psychological and primary care settings. Additionally, current AS reduction prevention programs (e.g., Schmidt et al. 2007) may benefit from inclusion of several of the modifications listed above for women. These findings also suggest that menstrual cycle phase should be assessed and potentially controlled for in biological challenge research.
Although the study methodology made several improvements beyond previous studies, several limitations should be noted. First, the sample consisted of a relatively homogenous group of highly educated primarily Caucasian women. Future research would benefit from examining more diverse samples in order to increase generalizeability of the findings. Second, the sample was a relatively healthy group of women with lower variability of AS scores as compared to other biological challenge studies using a community sample (e.g., Nillni et al. 2010). Specifically, only 9% of the sample had an ASI score of ≥ 25, which is often used to denote high AS group status (e.g., Sigmon et al. 1996) and as an inclusion criteria for prevention programs aimed at decreasing AS in at-risk groups (e.g., Schmidt et al. 2007). The null findings regarding the main effects of AS on panic-relevant responding are inconsistent with literature that widely supports the association between AS and anxiety and panic symptoms following a CO2 challenge (see Zvolensky and Eifert 2000, for a review), and may have been a result of lowered variability of AS in this sample.
Third, although the CO2 challenge provides an opportunity to observe panic-relevant responding in “real time,” future research could also benefit from prospective, longitudinal studies that examine how potential risk factors and their interactions relate to the development of naturally-occurring panic psychopathology using ambulatory monitoring. Fourth, the current study methodology assumed the same degree of hormonal cyclical change for every woman at the time of the premenstrual assessment. Gonadal hormone fluctuations, particularly the decline of progesterone and its neurosteroid metabolite allopregnanolone (THP) in the late luteal phase, have been theorized to underlie the experience of premenstrual symptoms (e.g., Halbreich 2003) and anxiety response (e.g., Smith et al. 2006). Therefore, the rate of progesterone decline in the premenstrual phase may be a more important predictor of anxiety response than simply being in the premenstrual phase (Days −5 to −1), and an increased rate of progesterone decline may differentiate women at risk for anxiety response during the premenstrual phase and represent a better marker of risk. Future studies would benefit from inclusion of more frequent hormonal assessments to examine the interaction between a psychological vulnerability (i.e., AS) and the slope of progesterone decline in furthering understanding of panic response across the menstrual cycle.
Acknowledgements
This research was supported by a National Institute of Mental Health Dissertation grant awarded to Yael I. Nillni (1R36MH086170-01A1). We would like to thank Dr. Alessandra Rellini, Dr. Keith Burt, and Dr. Magdalena Naylor for their comments on a prior version of this manuscript.
Footnotes
Additional models were run including a variable indicating which menstrual phase was tested first to examine whether the ordering influenced response, and including a variable indicating whether or not the participant completed one or both laboratory visits. These factors did not predict any of the outcomes and were not included in any of the final models. Additionally, a total of 3 participants (5.5%) met criteria for PMDD, and all analyses were run including PMDD diagnostic status as a covariate. Results did not vary as a function of PMDD diagnosis, therefore, final models presented in this manuscript do not include PMDD diagnosis as a covariate.
There are no conflicts of interest.
Contributor Information
Yael I. Nillni, Department of Psychology, University of Vermont, Burlington, VT 05401 and Department of Psychiatry and Human Behavior, University of Mississippi Medical Center, Jackson, MS 39216.
Kelly J. Rohan, Department of Psychology, University of Vermont, Burlington, VT 05401
Michael J. Zvolensky, Department of Psychology, University of Houston, Houston, TX 77204
References
- Asso D. The Real Menstrual Cycle. John Wiley & Sons; New York: 1983. [Google Scholar]
- AuBuchon PG, Calhoun KS. Menstrual cycle symptomatology: The role of social expectancy and experimental demand characteristics. Psychosomatic Medicine. 1985;47:35–45. doi: 10.1097/00006842-198501000-00004. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. second ed Guilford Press; New York: 2002. [Google Scholar]
- Barlow DH, Brown TA, Craske MG. Definitions of panic attaks and panic disorder in the DSM-IV: Implications for research. J Abnorm Psychol. 1994;103:553–564. doi: 10.1037//0021-843x.103.3.553. [DOI] [PubMed] [Google Scholar]
- Barnard K, Frayne SM, Skinner KM, Sullivan LM. Health status among women with menstrual symptoms. J Womens Health. 2003;12:911–919. doi: 10.1089/154099903770948140. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Marshall EC, Schmidt NB. Laboratory test of a novel structural model of anxiety sensitivity and panic vulnerability. Behavior Therapy. 2009;40:171–180. doi: 10.1016/j.beth.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Rubinow DR. Premenstrual syndrome: evidence for symptom stability across cycles. Am J Psychiatry. 1997;154:1741–1746. doi: 10.1176/ajp.154.12.1741. [DOI] [PubMed] [Google Scholar]
- Borenstein JE, Dean BB, Leifke E, Korner P, Yonkers KA. Differences in symptom scores and health outcomes in premenstrual syndrome. J Womens Health. 2007;16:1139–1144. doi: 10.1089/jwh.2006.0230. [DOI] [PubMed] [Google Scholar]
- Breier A, Charney DS, Heninger GR. Agoraphobia with panic attacks. Arch Gen Psychiatry. 1986;43:1029–1036. doi: 10.1001/archpsyc.1986.01800110015003. [DOI] [PubMed] [Google Scholar]
- Chrisler JC, Caplan P. The strange case of Dr. Jekyll and Ms. Hyde: how PMS became a cultural phenomenon and a psychiatric disorder. Annual Review of Sex Research. 2002;13:274–306. [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Donnell CD, McNally RJ. Anxiety sensitivity and history of panic as predictors of response to hyperventilation. Behav Res and Ther. 1989;27:325–332. doi: 10.1016/0005-7967(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Eifert GH, Zvolensky MJ, Sorrell JT, Hopke DR, Lejuez CW. Predictors of self-reported anxiety and panic symptoms: an evaluation of anxiety sensitivity, suffocation fear, heart-focused anxiety, and breath-holding duration. Journal of Psychopathology and Behavioral Assessment. 1999;21:293–305. [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Epting LK, Overman WH. Sex-sensitive tasks in men and women: A search for performace fluctuations across the menstrual cycle. Behav Neurosci. 1998;112:1304–1317. doi: 10.1037//0735-7044.112.6.1304. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders-non-patient edition. Biometrics Research Department; New York: 1994. [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABAA receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Foot M, Koszycki D. Gender differences in anxiety-related traits in patients with panic disorder. Depress Anxiety. 2004;20:123–130. doi: 10.1002/da.20031. [DOI] [PubMed] [Google Scholar]
- Forsyth JP, Eifert GH. Response intensity in content-specific fear conditioning comparing 20% versus 13% CO2-enriched air as unconditioned stimuli. J Abnorm Psychol. 1998;107:291–304. doi: 10.1037//0021-843x.107.2.291. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. 2003;28:25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- Gonda X, Telek T, Juhasz G, Lazary J, Vargha A, Bagdy G. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1782–1788. doi: 10.1016/j.pnpbp.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual symptoms. Psychoneuroendocrinology. 2003;28:55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Endicott J, Goldstein S, Nee J. Premenstrual changes and changes in gonadal hormones. Acta Psychiatr Scand. 1986;74:576–586. doi: 10.1111/j.1600-0447.1986.tb06287.x. [DOI] [PubMed] [Google Scholar]
- Harrison WM, Sandberg D, Gorman JM, Fyer M, Nee J, Uy J, Endicott J. Provocation of panic with carbon dioxide inhalation in patients with premenstrual dysphoria. Psychiatry Res. 1989;27:183–192. doi: 10.1016/0165-1781(89)90133-9. [DOI] [PubMed] [Google Scholar]
- Karekla M, Forsyth JP, Kelly MM. Emotional avoidance and panicogenic responding to a biological challenge procedure. Behavior Therapy. 2004;35:725–746. [Google Scholar]
- Kaspi SP, Otto MW, Pollack MH, Eppinger S, Rosenbaum JF. Premenstrual exacerbation of symptoms in women with panic disorder. J Anxiety Disord. 1994;8:131–138. [Google Scholar]
- Kent JM, Papp LA, Martinez JM, Browne ST, Coplan JD, Klein DF, Gorman JM. Specificity of panic response to CO2 inhalation in panic disorder: a comparison with major depression and premenstrual dysphoric disorder. Am J Psychiatry. 2001;158:58–67. doi: 10.1176/appi.ajp.158.1.58. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, Merani S, Koszycki D, et al. Sensitivity to CCK-4 in women with and without premenstrual dysphoric disorder (PMDD) during their follicular and luteal phases. Neuropsychopharmacology. 1999;20:81–91. doi: 10.1016/S0893-133X(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Forsyth JP, Eifert GH. Devices and methods for administering carbon dioxide-enriched air in experimental and clinical settings. J Behav Ther Exp Psychiatry. 1998;29:239–248. doi: 10.1016/s0005-7916(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Li W, Zinbarg RE. Anxiety sensitivity and panic attacks: a 1-year longitudinal study. Behav Modif. 2007;31:145–161. doi: 10.1177/0145445506296969. [DOI] [PubMed] [Google Scholar]
- Logue CM, Moos RH. Perimenstrual symptoms: prevalence and risk factors. Psychosom Med. 1986;48:388–414. doi: 10.1097/00006842-198607000-00002. [DOI] [PubMed] [Google Scholar]
- Maller RG, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. J Anxiety Disord. 1992;6:241–247. [Google Scholar]
- McNally RJ. Cognitive bias in the anxiety disorders. Nebr Symp Motiv. 1996;43:211–250. [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Lorenz M. Anxiety sensitivity in agoraphobics. J Behav Ther Exp Psychiatry. 1987;18:3–11. doi: 10.1016/0005-7916(87)90065-6. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle and panic disorder: a putative neuroendocrine and psychological connection. Clin Psychol Rev. 2011;31:1183–1191. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni YI, Rohan KJ, Bernstein A, Zvolensky MJ. Premenstrual distress predicts panic-relevant responding to a CO2 challenge among young adult females. J Anxiety Disord. 2010;24:416–422. doi: 10.1016/j.janxdis.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein T, Stone AB. Premenstrual syndrome. Psychiatr Clin North Am. 1998;21:577–590. doi: 10.1016/s0193-953x(05)70024-1. [DOI] [PubMed] [Google Scholar]
- Perna G, Brambilla F, Aranio C, Bellodi L. Menstrual cycle-related sensitivity to 35% CO2 in panic patients. Biol Psychiatry. 1995;37:528–532. doi: 10.1016/0006-3223(94)00154-U. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Heilbronner RL. The Anxiety Sensitivity Index: Construct validity and factor analytic structure. J Anxiety Disord. 1987;1:117–121. [Google Scholar]
- Reiss S, McNally RJ. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. Academic Press; New York: 1985. pp. 107–122. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res and Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol. 1988;158:5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- Salimetrics . Salivary progesterone enzyme immunoassay kit. State College; PA: 2010. [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. Panic induction via inhalation of 5.5% CO2 enriched air: a single subject analysis of psychological and physiological effects. Behav Res and Ther. 1988;26:333–335. doi: 10.1016/0005-7967(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide-enriched air. Arch Gen Psychiatry. 1989;46:157–162. doi: 10.1001/archpsyc.1989.01810020059010. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Eggleston AM, Woolaway-Bickel K, Fitzpatrick KK, Vasey MW, Richey JA. Anxiety sensitivity amelioration training (ASAT): a longitudinal primary prevention program targeting cognitive vulnerability. J Anxiety Disord. 2007;21:302–319. doi: 10.1016/j.janxdis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Koselka M. Gender differences in patients with panic disorder: Evaluating cognitive mediation of phobic avoidance. Cognitive Therapy and Research. 2000;24:533–550. [Google Scholar]
- Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N Engl J Med. 1991;324:1174–1179. doi: 10.1056/NEJM199104253241705. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: prospective prediction of panic attacks and Axis I pathology. J Psychiatr Res. 2006;40:691–699. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Reavis R, Overman WH, Granger DA. Measurement of gonadal hormones in dried blood spots versus serum: verification of menstrual cycle phase. Horm Behav. 2001;39:258–266. doi: 10.1006/hbeh.2001.1657. [DOI] [PubMed] [Google Scholar]
- Sigmon ST, Dorhofer DM, Rohan KJ, Hotovy LA, Boulard NE, Fink CM. Psychophysiological, somatic, and affective changes across the menstrual cycle in women with panic disorder. J Consult Clin Psychol. 2000;68:425–431. doi: 10.1037//0022-006x.68.3.425. [DOI] [PubMed] [Google Scholar]
- Sigmon ST, Fink CM, Rohan KJ, Hotovy LA. Anxiety sensitivity and menstrual cycle reactivity: Psychophysiological and self-report differences. J Anxiety Disord. 1996;10:393–410. [Google Scholar]
- Sigmon ST, Whitcomb-Smith SR, Rohan KJ, Kendrew JJ. The role of anxiety level, coping styles, and cycle phase in menstrual distress. J Anxiety Disord. 2004;18:177–191. doi: 10.1016/S0887-6185(02)00243-8. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in axiogenic effects of 3α5α-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Zvolensky MJ, Eifert GH, Feldner MT. Avoidance-oriented coping as a predictor of panic-related distress: a test using biological challenge. J Anxiety Disord. 2004;18:309–323. doi: 10.1016/S0887-6185(02)00249-9. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schmidt PJ, Rubinow DR, Uhde TW. Panic disorder and the menstrual cycle: panic disorder patients, healthy control subjects, and patients with premenstrual syndrome. Am J Psychiatry. 1989;146:1299–1303. doi: 10.1176/ajp.146.10.1299. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Taylor S, Baker JM. Gender difference in dimensions of anxiety sensitivity. J Anxiety Disord. 1997;11:179–200. doi: 10.1016/s0887-6185(97)00005-4. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, McNally RJ. How does anxiety sensitivity vary across the anxiety disorders? J Anxiety Disord. 1992;6:249–259. [Google Scholar]
- van Beek N, Griez E. Anxiety sensitivity in first-degree relatives of patients with panic disorder. Behav Res and Ther. 2003;41:949–957. doi: 10.1016/s0005-7967(02)00129-8. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ, Gibson LE, Lynch TR, Leen-Feldner EW, Feldner MT, Bernstein A. Affect intensity: association with anxious and fearful responding to bodily sensations. Journal of Anxiety Disorders. 2006;20:192–206. doi: 10.1016/j.janxdis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: A practical guide using statistical software. Taylor & Francis Group; Boca Raton, FL: 2007. [Google Scholar]
- Wittchen H, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32:119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Stanford University Press; Stanford, CA: 1958. [Google Scholar]
- Zinbarg R, Mohlman J, Hong N. Dimensions of anxiety sensitivity. In: Taylor S, editor. Anxiety sensitivity: Theory, research and treatment of fear anxiety. Erlbaum; Hillsdale, NJ: 1999. [Google Scholar]
- Zvolensky MJ, Eifert GH. A review of psychological factors/processes affecting anxious responding during voluntary hyperventilation and inhalations of carbon dioxide-enriched air. Clin Psychol Rev. 2000;21:375–400. doi: 10.1016/s0272-7358(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Kotov R, Antipova AV, Schmidt NB. Diathesis stress model for panic-related distress: a test in a Russian epidemiological sample. Behav Res and Ther. 2005;43:521–532. doi: 10.1016/j.brat.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Eifert GH. The role of control in anxious responding: an experimental test using repeated administrations of 20% CO2 enriched air. Behavior Therapy. 1998;29:193–209. [Google Scholar]