Abstract
Objective
We conducted a case-control study to examine if short-term glucose control is related to healthcare-associated bloodstream infections (BSI), urinary tract infections (UTI), and pneumonia in hospitalized adults with diabetes.
Setting and Patients
We analyzed 205 BSI, 510 UTI, and 109 pneumonia cases and 989, 2463, and 543 controls matched by age, sex and hospital stay seen at a large healthcare system in Manhattan from 2006 to 2008.
Methods
We examined whether infection risk was associated with serum glucose measured at admission and within 2 days to infection, using conditional logistic regression. Co-morbidities, immunosuppressive medications, prior hospitalizations, and insertion of indwelling devices were considered as potential confounders.
Results
Admission glucose level was not associated with infection. Glucose levels of ≥110mg/dL measured within 2 days to infection were associated with BSI (Odds ratios from 2.04 to 2.67). Glucose level of ≥180mg/dL was associated with pneumonia (Odds ratio = 2.30). Decrease in glucose levels from admission to the infection was greater for controls than for infected cases.
Conclusion
Healthcare-associated BSI and pneumonia were associated with glucose levels prior to infection diagnosis, but not with glucose levels at admission. Persistently high glucose level is could be an indication of an underlying undiagnosed infection.
Keywords: healthcare-associated infections, blood-stream infection, urinary tract infection, pneumonia, glucose control
Introduction
An estimated 366 million people currently live with diabetes mellitus, including 24 million adult U.S. residents; by year 2030, the global estimates are expected to increase to 552 million people. The increasing prevalence of diabetes may exacerbate the burden of healthcare-associated infections (HAIs), which were associated with 99,000 deaths in the U.S. in 2002. Diabetes has been reported to increase the risk of skin and soft tissue infections requiring hospitalizations, as well as healthcare-associated pneumonia.
Controlling glucose levels is a feasible intervention to consider in mitigating the risk of infections in hospitalized diabetes patients, but the potential impact and the target for such an intervention is unclear. Current guidelines for glucose control in inpatients, surgical patients or critically ill patients regardless of diabetes status vary in recommended target glucose levels, with minimum thresholds ranging from 80mg/dL to 140mg/dL and maximum thresholds ranging from 110mg/dL to 200mg/dL. Previous randomized controlled studies on tight glycemic control (80–110 mg/dL) for patients in critical care settings have reported equivocal results on its impact on surgical site infections (SSI) and bloodstream infections (BSI). Prior observational studies found elevated risk of SSI with post-operative glucose levels of 110 mg/dL, and higher levels of post-operative glucose in those with SSI.. However, evidence for an association between glucose level and other types of HAI has been lacking. Further, it is not yet clear whether glucose levels are causally related to infections or merely an indication of critical illness.
We aimed to study how short-term glucose control is associated with the risk of HAIs, specifically BSI, urinary tract infections (UTI), and pneumonia among inpatients with diabetes in a large healthcare system.
Methods
Study setting and data
We undertook a case-control study employing a database of 190,458 de-identified hospital records from patients who were discharged between Jan 1, 2006 and Dec 31, 2008 from three sites of a healthcare system, namely an academic tertiary care adult facility, an acute care pediatric hospital, and a community hospital that collectively serve a diverse population residing in northern Manhattan. Data were extracted from: (1) the Clinical Data Warehouse that integrates information from over 20 clinical electronic sources shared by the three healthcare facilities; (2) the admission, discharge, transfer (ADT) system; and (3) the computerized physician and nursing order entry system (CPOE). For the purpose of the present study, we included laboratory data on blood glucose measurements, microbiologic results for infections and data on central venous and urinary catheterization from the Clinical Data Warehouse. From the ADT system, we included demographic data on age and gender, ICD-9-CM diagnoses and procedure codes on medical conditions including diabetes, renal failure, malignancy, and invasive procedures performed during hospitalization including mechanical ventilation, and feeding tube insertion. We also computed a composite score of illness (Charlson score) based on ICD-9-CM codes for conditions present at admission.
Healthcare-associated infections
We examined the association between glucose control and three common types of HAIs: BSI, UTI, and pneumonia. For each infected case we selected up to 5 controls who had been in the hospital the same number of days as when the case was cultured for the infection, and were of the same gender and same age group (<18, 18–39, 40–59, >=60). We used the standard HAI definitions as delineated by Center for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) and modified the definitions where clinical symptoms were indicated, as the use of electronically available data did not permit access to information on symptoms. We therefore defined BSI as cases presenting with a positive blood culture for any bacterial pathogen AND with no positive culture with the same organism at other body sites within 14 days prior to positive blood culture. In the case of a common skin contaminant (e.g., coagulase negative staphylococci), we counted only those cases with two or more blood cultures drawn on separate occasions within two days of each other. We defined UTI as cases presenting with a positive urine culture as follows: 1) either >105 colony forming units per milliliter of urine and no more than one other species of microorganism; or 2) 103–105 colony forming units per milliliter of urine and no more than one other species of microorganism, accompanied by pyuria (≥3 white blood cells per high power field in urine microscopy) within 48 hours of positive culture. We defined pneumonia as cases in which an ICD-9 code from a set of 62 codes was indicative of pneumonia at discharge, and a respiratory culture. Time to infection was determined as time from admission to the day of diagnostic culture collection for each type of infection. Hospital records with an infection within the first 3 days of admission or with shorter than 4 days of hospital stay were excluded from the analysis, as healthcare-associated infections require at least 48 hours of hospital stay by definition and because 48 hours can span over 3 separate dates.
Diabetes and glucose control
We included hospital records from patients who had a discharge diagnosis of diabetes mellitus (ICD-9 250.00–250.99) at any time during the study period. We determined the degree of glucose control based on patient blood glucose values available as part of a panel of metabolic indicators from blood drawn throughout the hospital stay. Glucose level at admission was determined based on the maximum glucose value within the first two days of hospitalization, and short-term glucose control was determined based on the maximum value within a two-day period leading up to the day of culture collection for each respective infection. The two-day period was used to assess glucose levels in a specific time window prior to infection diagnosis, which could be compared to that measured in the first two days. We classified blood glucose levels into four categories: <110, 110–139, 140–179, and ≥180 mg/dL. The categories were determined based on recommended glucose targets from published guidelines for glycemic control in inpatients or patients undergoing surgery. In addition, for each infection type, we examined the change in maximum blood glucose from admission to the time of the infection.
Statistical Analysis
We tested for differences in glucose level at admission, short-term glucose level, and relative change in glucose between cases and controls by Wilcoxon rank sum test. We estimated the relative odds of HAIs by employing conditional logistic regression, taking into account the matching variables: age, sex, and days since admission. Multivariate analyses included glucose levels and any of the following potential confounders that were associated with each outcome at p<0.10: age, sex, Charlson score at admission, malignancy, immunosuppressive medications, renal failure, prior hospitalization, mechanical ventilation, feeding tube, central venous catheterization, and urinary catheterization. Use of immunosuppressive medications, mechanical ventilation, feeding tubes, central venous catheters and urinary catheters were assessed within the same period that short-term glucose was assessed. A trend test was performed in the multivariate model to assess the dose-response of the relationship between increasing level of glucose and risk of infection.
Significance of associations were interpreted at p-value of <0.05. All statistical analyses were performed with SAS Version 9.2 (SAS Institute, Cary, NC).
Results
Study population and infection rates
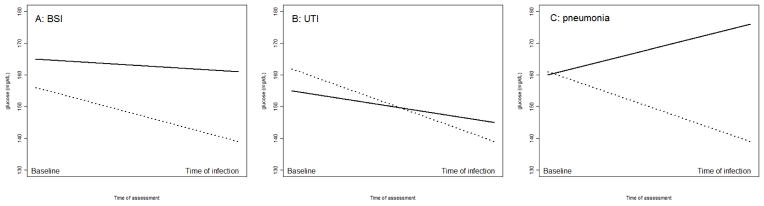
Of 190,458 hospital records available, there were 14,729 records from patients with diabetes after excluding records with infection present at time of admission and those without recorded admission glucose levels. We identified 214 BSI, 576 UTI and 114 pneumonia cases, of these, short- term glucose values were available for 205 BSI, 510 UTI cases and 109 pneumonia cases in people with diabetes. Following our matching protocol, we selected 989 controls for BSI cases, 2,463 controls for UTI cases and 543 controls for pneumonia cases. Demographic and clinical characteristics of the sample of cases and their respective controls by infection type are presented in Table 1. While there were no differences in blood glucose levels at admission by case or control status, median short-term glucose values were higher in BSI and pneumonia cases compared to their controls (BSI: 162 vs. 137 mg/dL, p<0.0001; pneumonia: 175 vs. 139 mg/dL p<0.0001). Median blood glucose levels at admission and two days prior to the infection are plotted in Figure 1. The median decrease in blood glucose levels since admission was smaller in BSI and UTI cases as compared to controls (BSI: −6 vs. −19 mg/dL, p=0.01; UTI: −10 vs. −20 mg/dL, p=0.0008). Glucose levels in pneumonia cases increased slightly from admission to infection, but decreased among the controls (4 vs. −22 mg/dL, p=0.0008). (Table 1)
Table 1.
Demographic and clinical characteristics of cases and controls
| Variables | Controls for bloodstream infection (n=989) | Bloodstream infections (n=205) | Controls for urinary tract infection (n=2463) | Urinary tract infections (n=510) | Controls for pneumonia (n=543) | Pneumonia (n=109) |
|---|---|---|---|---|---|---|
| Continuous variables, median (Interquartile Range) | ||||||
| Maximum blood glucose at admission (mg/dL) | 156 (121, 225) | 165 (121, 224) | 162 (124, 227) | 155 (124, 216) | 161 (122, 227) | 160 (118, 224) |
| Maximum blood glucose at infection (mg/dL) | 139 (105, 186) | 161 (121, 210) | 139 (105, 189) | 145 (112, 189) | 139 (104, 185) | 176 (127, 227) |
| Change in blood glucose from admission to infection (mg/dL) | −19 (−61, 15) | −6 (−50, 31) | −20 (−68, 12) | −10 (−52, 15) | −22 (−77, 14) | 4 (−37, 63) |
| Age | 63 (54, 75) | 63 (52, 72) | 69 (60, 80) | 71 (59, 79) | 66 (58, 76) | 68 (58, 76) |
| Length of total hospital stay (days) | 16 (9, 34) | 35 (14, 44) | 12 (7, 20) | 16 (10, 27) | 16 (9, 31) | 34 (22, 49) |
| Charlson Score | 2 (0, 3) | 2 (1, 3) | 2 (0, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) |
| Days to infection | N/A | 10 (6, 23) | N/A | 7 (5, 13) | N/A | 11 (7, 19) |
| Categorical variables, n (%) | ||||||
| Male sex | 608 (61%) | 126 (61%) | 841 (34%) | 174 (34%) | 310 (57%) | 62 (57%) |
| Malignancy | 150 (15%) | 41(20%) | 327 (13%) | 66 (13%) | 90 (17%) | 24 (22%) |
| Renal Failure | 457 (46%) | 113(55%) | 922 (37%) | 254 (50%) | 228 (42%) | 67(61%) |
| Immunosuppressive meds | 256 (26%) | 68(33%) | 561 (23%) | 137(27%) | 146 (27%) | 45 (41%) |
| Prior hospitalization | 564 (57%) | 119(58%) | 1431 (58%) | 308 (60%) | 297 (55%) | 66 (61%) |
| Invasive procedures (prior to infection) | ||||||
| Mechanical ventilation | 91 (9.2%) | 32 (16%) | 129 (5.2%) | 47 (9.2%) | 53 (9.8%) | 51 (47%) |
| Feeding tube | 34 (3.4%) | 13 (6.3%) | 38 (1.5%) | 19 (3.7%) | 25 (4.6%) | 9 (8.3%) |
| Central venous catheter | 131 (13%) | 39(19%) | 211 (8.6%) | 32 (6.3%) | 63 (12%) | 42 (39%) |
| Urinary catheter | 333 (34%) | 83(40%) | 736 (30%) | 247 (48%) | 158 (29%) | 64 (59%) |
N/A - not applicable
Figure 1.
Blood glucose levels at admission and at infection (Footnote: Solid lines show change in glucose levels for infection cases, and dotted lines show change in glucose levels of controls)
Associations between glucose control and healthcare-associated infections
In people with diabetes, glucose at admission was not associated with BSI, UTI or pneumonia at any level of glucose in the initial age, sex and hospital stay-matched analysis. (Table 2) On the other hand, BSI was associated with short-term glucose levels of 110mg/dL or higher: OR = 2.23 (95% confidence interval [CI]:1.24, 3.67) for 110–139 mg/dL, OR = 2.20 (95%CI: 1.33, 3.65) for 140–179 mg/dL, OR = 2.70 (95%CI: 1.69, 4.31) for ≥180 mg/dL. Those with short-term glucose levels of 110 mg/dL or higher were also more likely to develop pneumonia: OR = 2.25 (95%CI 1.07, 4.71) for 110–139 mg/dL, OR = 2.24 (95%CI 1.07, 4.70) for 140–179 mg/dL, OR = 3.38 (95%CI: 1.75, 6.54) for ≥180 mg/dL. There was no association between short-term glucose levels and UTI. (Table 2)
Table 2.
Age, sex, and hospital stay-matched association between glucose control and blood stream infections, urinary tract infections and pneumonia in people with diabetes
| Blood stream infections | Urinary tract infections | Pneumonia | ||||
|---|---|---|---|---|---|---|
| Variables | OR (95%CI) | p-value | OR (95%CI) | p-value | OR (95%CI) | p-value |
| Maximum glucose at admission | ||||||
| < 110 mg/dL | Ref | Ref | Ref | Ref | ||
| 110–139 mg/dL | 1.03 (0.62, 1.69) | 0.80 | 1.32 (0.96, 1.80) | 0.14 | 0.88 (0.45, 1.72) | 0.70 |
| 140–179 mg/dL | 1.02 (0.62, 1.68) | 0.95 | 0.97 (0.70, 1.34) | 0.77 | 1.16 (0.61, 2.21) | 0.67 |
| >= 180 mg/dL | 1.12 (0.72, 1.73) | 0.59 | 0.96 (0.72, 1.29) | 0.60 | 0.78 (0.43, 1.43) | 0.41 |
| Maximum short-term glucose | ||||||
| < 110 mg/dL | Ref | Ref | Ref | Ref | ||
| 110–139 mg/dL | 2.23 (1.24, 3.67) | 0.002 | 1.23 (0.93, 1.63) | 0.12 | 2.25 (1.07, 4.71) | 0.03 |
| 140–179 mg/dL | 2.20 (1.33, 3.65) | 0.002 | 1.29 (0.98, 1.71) | 0.05 | 2.24 (1.07, 4.70) | 0.03 |
| >= 180 mg/dL | 2.70 (1.69, 4.31) | <0.0001 | 1.17 (0.89, 1.54) | 0.25 | 3.38 (1.75, 6.54) | 0.0002 |
| Charlson Score at Admission | 1.06 (1.00, 1.13) | 0.06 | 1.05 (1.01, 1.09) | 0.03 | 1.01 (0.92, 1.10) | 0.93 |
| Malignancy | 1.42 (0.96, 2.17) | 0.09 | 1.00 (0.75, 1.32) | 0.84 | 1.43 (0.86, 2.40) | 0.17 |
| Immunosuppressive meds | 1.45 (1.03, 2.04) | 0.03 | 1.24 (1.00, 1.55) | 0.05 | 1.97 (1.27, 3.07) | 0.003 |
| Renal Failure | 1.44 (1.05, 1.96) | 0.02 | 1.68 (1.37, 2.05) | <0.0001 | 2.31 (1.49, 3.57) | 0.0002 |
| Prior hospitalization | 1.06 (0.78, 1.43) | 0.79 | 1.10 (0.90, 1.33) | 0.34 | 1.26 (0.83, 1.91) | 0.26 |
| Invasive procedures prior to infection | ||||||
| Mechvent | 1.97 (1.21, 3.23) | 0.007 | 1.68 (1.15, 2.45) | 0.0006 | 9.42 (5.54, 16.0) | <0.0001 |
| Feeding tube | 1.98 (0.95, 4.12) | 0.06 | 2.20 (1.18, 4.09) | 0.002 | 2.01 (0.86, 4.68) | 0.12 |
| Central venous catheter | 1.56 (1.04, 2.35) | 0.03 | 0.63 (0.43, 0.95) | 0.09 | 5.37 (3.21, 8.97) | <0.0001 |
| Urinary catheter | 1.34 (0.98, 1.83) | 0.06 | 2.20 (1.80, 2.68) | <0.0001 | 3.54 (2.29, 5.46) | <0.0001 |
In multivariate analysis additionally adjusting for variables found to be associated with BSI in the univariate analysis at p<0.10, namely Charlson score, malignancy, immunosuppressive medication, renal failure, mechanical ventilation, feeding tube administration, central venous catheterization and urinary catheterization, the association between BSI and short-term glucose levels of ≥ 110 mg/dL persisted with odds ratios ranging from 2.10 (95%CI: 1.27, 3.49) to 2.44 (95% CI: 1.52, 3.91). (Table 3) There was also evidence of a dose-response, with P-value of 0.0003 for increasing glucose levels. In multivariate analysis adjusting for renal failure, immunosuppressive medication, mechanical ventilation, central venous catheterization and urinary catheterization, the association between pneumonia and short-term glucose levels of 110 to 179 mg/dL was attenuated to a non-significant relationship; however, there was still a significant association between glucose level of 180 mg/dL and pneumonia (OR = 2.30; 95% CI: 1.09, 4.84). (Table 3)
Table 3.
Multivariate association between glucose control and blood stream infections, urinary tract infections and pneumonia in people with diabetes
| Blood stream infections | Urinary tract infections | Pneumonia | ||||
|---|---|---|---|---|---|---|
| Variables | OR (95%CI)* | p-value | OR (95%CI)** | p-value | OR (95%CI)*** | p-value |
| Maximum short-term glucose | ||||||
| < 110 mg/dL | Ref | Ref | Ref | |||
| 110–139 mg/dL | 2.10 (1.27, 3.49) | 0.003 | 1.24 (0.93, 1.66) | 0.13 | 2.04 (0.90, 4.62) | 0.16 |
| 140–179 mg/dL | 1.98 (1.19, 3.30) | 0.007 | 1.29 (0.97, 1.72) | 0.07 | 1.67 (0.73, 3.82) | 0.47 |
| >= 180 mg/dL | 2.44 (1.52, 3.91) | 0.0001 | 1.09 (0.61, 1.44) | 0.61 | 2.30 (1.09, 4.84) | 0.02 |
Adjusted for age, sex, hospital stay, Charlson score, malignancy, immunosuppressive medication, renal failure, mechanical ventilation, feeding tube, central venous catheterization, urinary cathetherization
Adjusted for age, sex, hospital stay, Charlson score, immunosuppressive medication, renal failure, mechanical ventilation, feeding tube, central venous catheterization urinary cathetherization
Adjusted for age, sex, hospital stay, renal failure, immunosuppressive medication, mechanical ventilation, central venous catheterization, urinary catheterization
Discussion
In this study, short-term glucose levels of ≥110mg/dL were independently associated with BSI and short-term glucose levels of ≥180 mg/dL were independently associated with pneumonia in people with diabetes controlling for other risk factors. The strength of association increased with higher glucose levels for BSI. An important question to ask is whether the association with hyperglycemia represents a causal relationship between poor glucose control and risk of BSI and pneumonia. The lack of association with glucose at admission, and the more modest decline in glucose levels after admission in the BSI cases and increase in glucose levels in pneumonia cases as compared to precipitous decrease in glucose levels in controls suggest that persistent hyperglycemia could be an indication of an infection prior to diagnosis (i.e., reverse causation).
This is biologically plausible, given that infection results in inflammation and activated innate immunity, which have been implicated in the development of insulin resistance. For example, elevated levels of inflammatory cytokines may lead to phosphorylation of serine residues on the insulin receptor substrate, which prevents its interaction with insulin receptors, inhibiting insulin action, leading to rising glucose levels. Previous study on viral pneumonia has also found that blood glucose levels predict pneumonia risk and are correlated with various indicators of liver, kidney and myocardium function, which when affected by infection may collectively influence glucose metabolism.
On the other hand, the positive association we observed may also reflect uncontrolled glucose levels increases the risk of HAIs. It is known that hyperglycemia could affect vulnerability to infection through compromising the immune system, by way of decreasing chemotaxis of neutrophils, decreasing the ratio of T helper type 1 immune profile to type 2, and making glucose more available to microorganisms, especially in the respiratory airway. As such, previous studies suggest the possibility that hyperglycemia may be a causal risk factor of HAIs. For instance, Ata et al. and Seghal et al. attributed the increased risk of SSI in patients to high post-operative glucose levels. However, a systematic review of trials on strict intra-operative, post-operative glucose control found no significant reduction in risk of SSI. Similarly, another systematic review of trials of tight glycemic control in the ICU reported no reduction in incidence of bloodstream infections. Yet another meta-analysis of trials of intensive insulin therapy was conducted with a broader inclusion of hospitalized patients in various settings. While this meta-analysis found a 21% reduction in incidence of sepsis the authors found the quality and the results of the included studies to be too heterogeneous for a conclusive evidence. In light of the lack of evidence for a causal relationship between glucose control and infection, we believe that previously observed association between HAIs and hyperglycemia could reflect glucose variation caused by a physiological response to infections.
Surprisingly, we did not find increased risk of UTI in those with hyperglycemia, which has previously been thought to be risk factor. Other studies have found that maintaining a glucose level of <140mg/dL was associated with reduced rates of UTI. Discordant results may be due to potential confounders in the latter studies.
The present study makes an important contribution to the medical literature by examining how varying levels of glucose are associated with several types of HAI in those with diabetes, as well as how glucose levels change from admission to the time of infection. However, the observational nature of the study presents some limitations. It is possible that there were unmeasured confounders associated with both changing glucose levels and HAIs, such as other stress-causing events or interventions. Additionally, we were limited by the necessity of using only electronically-available data to define HAIs. We may have misclassified the time of onset of infection, because the time of infection was defined as the day the specimen was collected for culture. Also, pneumonia cases could not be confirmed by chest X-ray; however, we believe the requirement of respiratory culture for pneumonia allowed for a specific identification of bacterial infections in our study. Lastly, we lacked data on insulin and capillary blood glucose levels routinely collected for hospitalized patients with diabetes. The availability of these data would have allowed a more accurate classification of glucose levels and as well as an evaluation of the role of insulin therapy in infection outcomes.
In summary, we found that short-term glucose levels were associated with increased risk of BSI and pneumonia in people with diabetes. Our results suggest that persistently high glucose level could be an indication of an underlying undiagnosed infection.
Acknowledgments
Source of Support: Funding for this project was obtained from NIH-funded grant, “Distribution of the Costs of Antimicrobial Resistant Infections” (5R01NR10822).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010;8:260–71. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–64. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Philips BJ. Hyperglycaemia and pulmonary infection. Proc Nutr Soc. 2006;65:227–35. doi: 10.1079/pns2006499. [DOI] [PubMed] [Google Scholar]
- Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009;182:S51–6. doi: 10.1016/j.juro.2009.07.090. [DOI] [PubMed] [Google Scholar]
- Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic medicine : a journal of the British Diabetic Association. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Frykberg RG. An evidence-based approach to diabetic foot infections. Am J Surg. 2003;186:44S–54S. doi: 10.1016/j.amjsurg.2003.10.008. discussion 61S–64S. [DOI] [PubMed] [Google Scholar]
- Gale SC, Sicoutris C, Reilly PM, Schwab CW, Gracias VH. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73:454–60. doi: 10.1177/000313480707300507. [DOI] [PubMed] [Google Scholar]
- Hemmila MR, Taddonio MA, Arbabi S, Maggio PM, Wahl WL. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144:629–35. doi: 10.1016/j.surg.2008.07.001. discussion 635–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Annals of internal medicine. 2011;154:268–82. doi: 10.7326/0003-4819-154-4-201102150-00008. [DOI] [PubMed] [Google Scholar]
- Kao LS, Knight MT, Lally KP, Mercer DW. The impact of diabetes in patients with necrotizing soft tissue infections. Surg Infect (Larchmt) 2005;6:427–38. doi: 10.1089/sur.2005.6.427. [DOI] [PubMed] [Google Scholar]
- Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev. 2009:CD006806. doi: 10.1002/14651858.CD006806.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. The Annals of thoracic surgery. 2009;87:663–9. doi: 10.1016/j.athoracsur.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest. 2010;137:544–51. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28:810–5. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2011;154:260–7. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–91. doi: 10.1097/SLA.0b013e31818990d1. [DOI] [PubMed] [Google Scholar]
- Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- Sehgal R, Berg A, Figueroa R, Poritz LS, McKenna KJ, Stewart DB, Koltun WA. Risk factors for surgical site infections after colorectal resection in diabetic patients. J Am Coll Surg. 2011;212:29–34. doi: 10.1016/j.jamcollsurg.2010.09.011. [DOI] [PubMed] [Google Scholar]
- The Joint Commission. Surgical Care Improvement Project SCIP. Oakbrook Terrace, IL: The Joint Commission; 2011. Specifications Manual for National Hospital Inpatient Quality Measures Release, Version 3.3; pp. 1–10. SCIP-Inf-4. [Google Scholar]
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7:357–63. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148:346–53. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen H, Li Q, Qiu B, Wang J, Sun X, Xiang Y, Zhang J. Fasting plasma glucose is an independent predictor for severity of H1N1 pneumonia. BMC Infect Dis. 2011;11:104. doi: 10.1186/1471-2334-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]