Abstract
The Sec1p family of proteins is required for vesicle-mediated protein trafficking between various organelles of the endomembrane system. This family includes Vps45p, which is required for transport to the vacuole in yeast (Saccharomyces cerevisiae). We have isolated a cDNA encoding a VPS45 homolog from Arabidopsis thaliana (AtVPS45). The cDNA is able to complement both the temperature-sensitive growth defect and the vacuolar-targeting defect of a yeast vps45 mutant, indicating that the two proteins are functionally related. AtVPS45p is a peripheral membrane protein that associates with microsomal membranes. Sucrose-density gradient fractionation demonstrated that AtVPS45p co-fractionates with AtELP, a potential vacuolar protein sorting receptor, implying that they may reside on the same membrane populations. These results indicate that AtVPS45p is likely to function in the transport of proteins to the vacuole in plants.
Soluble proteins that reside in the plant vacuole are initially translocated into the ER lumen and are then transported through the secretory pathway to the TGN, where vacuolar proteins are sorted from those that are to be secreted by virtue of a vacuolar-targeting signal. These signals are presumably recognized by receptor proteins in the TGN that allow transport to the vacuole. Two types of cleavable targeting signals have been identified: C-terminal propeptides and N-terminal propeptides, both of which are removed during deposition in the vacuole. Different mechanisms may be responsible for the transport of proteins containing different classes of signals (Bassham and Raikhel, 1997; Robinson and Hinz, 1997).
Proteins are transported between organelles of the secretory pathway in vesicles that bud from one compartment and fuse with the next. The mechanism by which transport vesicles containing cargo proteins fuse with a target membrane has begun to be elucidated through a combination of biochemical studies of synaptic vesicle fusion with the presynaptic plasma membrane in mammalian neurons, and in genetic studies of secretion in yeast (Rothman, 1996). Similar sets of proteins were shown to be involved in the highly regulated fusion events in neurotransmission and the constitutive secretory system of yeast.
These studies led to the proposal of the SNARE hypothesis to explain how a transport vesicle could be targeted to and fused with the correct organelle out of the many membrane-bound compartments of the cell. It was proposed that certain membrane proteins on the transport vesicle (v-SNAREs) and the target organelle (t-SNAREs) interact with each other and with several soluble proteins to allow docking of the vesicle with the target membrane and to promote vesicle fusion. For example, at the presynaptic membrane, a complex is formed between the v-SNARE synaptobrevin/vesicle-associated membrane protein and the t-SNAREs syntaxin and SNAP-25 (synaptosome-associated protein of 25 kD), allowing the soluble factors N-ethylmaleimide-sensitive factor and α-SNAP (soluble N-ethylmaleimide-sensitive factor attachment protein) to bind. Hydrolysis of ATP by N-ethylmaleimide-sensitive factor causes disassembly of the complex, leading to membrane fusion. Different target membranes and vesicle populations throughout the cell contain different isoforms of the SNARE proteins, and each v-SNARE can only interact with one or a subset of the t-SNAREs. The specificity of fusion between a vesicle and a target membrane could therefore be controlled at least in part by the isoforms of the v-SNAREs and t-SNAREs present in the membranes (Söllner et al., 1993; Rothman, 1996).
The relevence of the SNARE hypothesis to plant vesicle trafficking was demonstrated by the discovery of several syntaxin-like proteins in Arabidopsis thaliana (Bassham et al., 1995; Lukowitz et al., 1996; Sato et al., 1997). One of these, AtPEP12p, appears to be involved in the transport of proteins to the vacuole. AtPEP12p is a homolog of yeast Pep12p, a syntaxin-like protein required for the correct targeting of several vacuolar hydrolases (Becherer et al., 1996). The ability of AtPEP12 to complement the yeast pep12Δ mutant indicates that the protein may play a role in targeting proteins to the plant vacuole (Bassham et al., 1995), possibly by acting as a receptor for vesicle trafficking from the Golgi complex to a post-Golgi, prevacuolar compartment (Conceição et al., 1997).
In addition to the core components of the SNARE complex, other factors have been identified that may be required to regulate vesicle fusion. These include the rab family of small GTPases, members of which are probably involved in all transport steps, and the Sec1p-like protein family. SEC1, originally identified in a screen for yeast secretory mutants, has been found to interact with the yeast plasma membrane syntaxin homologs and is required for fusion of vesicles with the plasma membrane (Aalto et al., 1993; Halachmi and Lev, 1996). A similar protein, n-Sec1p, was also found to be involved in the regulation of synaptic vesicle transport and to interact with several isoforms of the mammalian plasma membrane syntaxin (Garcia et al., 1994; Pevsner et al., 1994).
Three other Sec1p-like proteins exist in yeast: Sly1p is involved in ER-to-Golgi trafficking, whereas both Vps33p and Vps45p are required for vacuolar protein transport (Halachmi and Lev, 1996). Vps33p is likely to function in endosome-to-vacuole transport (Banta et al., 1990), whereas Vps45p appears to be involved in a Golgi-to-endosome transport step (Cowles et al., 1994; Piper et al., 1994). Vps45p is a 67-kD protein showing 20 to 25% identity with Sec1p-like proteins involved in other transport steps (Cowles et al., 1994; Piper et al., 1994). Genetic and biochemical evidence shows that it interacts with Pep12p (Burd et al., 1997).
Recently, mammalian homologs of VPS45 have been isolated from mouse (mVPS45; Tellam et al., 1997), human (h-VPS45; Pevsner et al., 1996), and rat (rVPS45; El-Husseini et al., 1997) that are very closely related to each other and are proposed to be involved in transport to the lysosome. Immunofluorescence microscopy demonstrated that mVps45p colocalizes with the TGN marker p200 (Tellam et al., 1997). Although some in vitro binding of mVps45p to yeast Pep12p and mammalian syntaxin 6 was seen (Bock et al., 1997; Tellam et al., 1997), no binding could be observed between h-Vps45p and the probable mammalian Pep12p homolog syntaxin 7 (Wang et al., 1997). However, a direct comparison using all of these proteins has not been reported. The functional relationships between these yeast and mammalian proteins is therefore unclear, in particular as mVPS45 is unable to complement a yeast vps45Δ mutant (Tellam et al., 1997).
To allow further characterization of vacuolar protein transport in plants, we have isolated a cDNA encoding an Arabidopsis Vps45p-like protein, AtVPS45p. AtVPS45 is able to complement the yeast vps45Δ mutant, and the majority of the protein in Arabidopsis roots is membrane associated. AtVPS45p does not completely cofractionate with AtPEP12p on Suc gradients, but instead co-fractionates with AtELP, a receptor-like protein potentially involved in vacuolar protein sorting (Ahmed et al., 1997).
MATERIALS AND METHODS
cDNA Cloning and Sequencing
A BLAST search (Altschul et al., 1990) of the expressed sequence tag database identified a partial cDNA from Arabidopsis thaliana of approximately 600 bases (GenBank accession no. Z30835) that showed significant similarity to the yeast (Saccharomyces cerevisiae) VPS45 gene. This fragment was radiolabeled using random hexanucleotide primers and was used to screen the PRL2 A. thaliana cDNA library (Newman et al., 1994). After sequential plaque-purification steps and plasmid excision, several cDNAs were obtained, the longest of which was 2.1 kb. This cDNA was sequenced using Taq FS DNA polymerase and fluorescent-dideoxy terminators in a cycle-sequencing method, and the resultant DNA fragments were electrophoresed and analyzed using an automated DNA sequencer (model 373A, Applied Biosystems). Sequencing was performed at the W.M. Keck Foundation (Yale University, New Haven, CT). The sequence was deposited in the GenBank database (accession no. AF036234).
Yeast Complementation
For expression in yeast, the open reading frame of AtVPS45 was inserted into the PvuII/BamHI sites of the 2 μ expression vector pVT100-U (Vernet et al., 1987), which contains an ADH1 constitutive promoter. This plasmid was introduced into the vps45Δ yeast mutant (strain RPY14; Tellam et al., 1997). In addition, this strain was transformed with pVT100-U containing no insert, or plasmid pRS316 (Sikorski and Hieter, 1989) containing yeast VPS45 or yeast VPS33. Plasmids and yeast were generously provided by Drs. Tom Stevens and Rob Piper. CPY activity was assayed using the APE test, as described in Jones (1991).
RNA-Blot Analysis
RNA was isolated from leaves, roots, flowers, and inflorescence stems of A. thaliana (ecotype Columbia) as described previously (Bar-Peled et al., 1995). Equal amounts (30 μg) of total RNA were separated on a 1.2% agarose/6% formaldehyde gel and transferred to nylon membrane. Blots were probed with full-length 32P-labeled antisense RNA synthesized from linearized AtVPS45-containing plasmid using SP6 RNA polymerase (Gibco-BRL).
Antibody Production
An EcoRV fragment (nucleotides 674–1802) of the AtVPS45 cDNA was subcloned into pGEX-5X-3 (Pharmacia) to produce a GST-AtVPS45p fusion plasmid. The fusion protein was synthesized in Escherichia coli according to the manufacturer's protocol, where it accumulated in inclusion bodies. Cells were broken by sonication, and insoluble material was pelleted at 15,000g. The pellet was washed by resuspension in 0.05% (w/v) sarcosyl in PBS, and inclusion bodies were pelleted again by centrifugation at 15,000g. The inclusion bodies were resuspended in 0.2% (w/v) sarcosyl in PBS and incubated at 4°C for 1 h. After centrifugation at 15,000g, proteins in the supernatant were separated by SDS-PAGE, and fusion protein was electroeluted from the gels using an electro-eluter (Bio-Rad) according to the manufacturer's instructions. The eluted protein (250 μg) was used to immunize rabbits.
For affinity purification of antibodies, the solubilized inclusion-body proteins were separated by SDS-PAGE, transferred to nitrocellulose, and the strip containing the fusion protein was cut out after staining with Ponceau S. After blocking in 3% (w/v) dried nonfat milk in PBS, serum was incubated with the strip for 2 h at 4°C to allow binding of the antibodies. The strip was washed with PBS and specific antibodies were eluted using 100 mm Gly, pH 2.5. The eluate was adjusted to pH 7.0 using 2 m Tris-HCl, pH 9.5. These affinity-purified antibodies were used in all further experiments.
Immunoprecipitation of an in Vitro Translation Product
mRNA encoding AtVPS45p was synthesized in vitro from the cDNA using T7 RNA polymerase (Gibco-BRL). The mRNA was translated in a rabbit reticulocyte lysate translation system (Promega) in the presence of [35S]Met to produce radiolabeled AtVPS45p protein. The translation mixture was incubated with anti-AtVPS45p antibodies or preimmune serum for 2 h at 4°C, followed by protein A-Sepharose (Pharmacia) for 1 h. Beads were pelleted by centrifugation, washed four times in PBS, and bound protein was eluted in SDS sample buffer. Samples were analyzed by SDS-PAGE and fluorography.
Preparation of Microsomal Fractions
To generate large quantities of root tissue, A. thaliana was grown in liquid culture as described in Bar-Peled et al. (1995). Roots were ground in extraction buffer (100 mm Tris-HCl, pH 7.5, 400 mm Suc, 1 mm EDTA, and 0.1 mm PMSF) using a mortar and pestle, and debris were pelleted by centrifugation at 1,000g for 5 min. The supernatant was centrifuged at 8,000g for 15 min to produce an 8,000g membrane pellet (P8). The 8,000g supernatant was re-centrifuged at 150,000g for 1 h to produce a 150,000g membrane pellet (P150) and a soluble fraction (S150). Pellets were resuspended in extraction buffer, and fractions were analyzed by SDS-PAGE and immunoblotting. Blots were probed using AtVPS45p antibodies, followed by secondary antibodies conjugated to alkaline phosphatase.
Extraction of AtVPS45p from Membranes
P150 membrane pellets were resuspended in 200 μL of extraction buffer, 0.1 m Na2CO3, or extraction buffer containing 1 m NaCl, 2 m urea, or 1% (v/v) Triton X-100, and incubated for 2 h on ice. Insoluble material was repelleted at 150,000g and pellets were resuspended in SDS sample buffer. Supernatants were precipitated using TCA, and protein pellets were washed in acetone and resuspended in SDS sample buffer. Samples were analyzed by SDS-PAGE and immunoblotting. Blots were probed using AtVPS45p antibodies followed by secondary antibodies conjugated to alkaline phosphatase.
Suc Gradients
Roots grown in liquid culture were homogenized in HKE buffer (50 mm Hepes-KOH, pH 7.5, 10 mm potassium acetate, and 1 mm EDTA) containing 400 mm Suc, 1 mm dithiothreitol, and 0.1 mm phenylmethylsulphonyl fluoride, and centrifuged at 1,000g for 5 min to generate a postnuclear supernatant. Three milliliters of this supernatant was loaded onto a Suc-step gradient consisting of 1.0 mL of 54%, 2.7 mL of 40%, 2.2 mL of 33%, 2.0 mL of 24%, and 1.5 mL of 15% Suc (w/v) in HKE buffer. Gradients were centrifuged at 150,000g in a swinging-bucket rotor at 4°C for 3 h. Fractions (500 μL) were collected from the top of the gradient, and the Suc concentration in each was determined using a refractometer. Protein in each fraction was precipitated using TCA, and was analyzed by SDS-PAGE and immunoblotting. Blots were probed using AtVPS45p antibodies, AtPEP12p antibodies (Conceição et al., 1997), or AtELP antibodies (Ahmed et al., 1997), followed by secondary antibodies conjugated to alkaline phosphatase or horseradish peroxidase. The blots were digitized on a flat-bed scanner, and protein amounts were quantitated by densitometry using NIH Image software (National Institutes of Health, Bethesda, MD). Values were normalized to the background and plotted as a percentage of each protein in the gradient.
RESULTS
Isolation of a cDNA Encoding AtVPS45
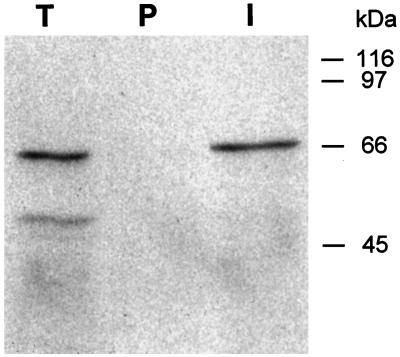
The Arabidopsis Expressed Sequence Tag Database was searched for sequences showing similarity to the S. cerevisiae VPS45 gene using the BLAST algorithm (Altschul et al., 1990). A cDNA was identified that was a good candidate for an Arabidopsis VPS45 homolog and was used to screen an Arabidopsis cDNA library to obtain a full-length cDNA. Several cDNAs were isolated, the longest of which had an insert of 2.1 kb containing a 569-amino acid open reading frame. The putative encoded protein (AtVPS45p) has a predicted molecular mass of approximately 67 kD. Hydropathy plots indicated that AtVPS45p is a hydrophilic protein with no predicted membrane-spanning domains (data not shown). The open reading frame was confirmed by transcription and translation of the cDNA in vitro. Analysis of the translation product by SDS-PAGE and fluorography indicated that the product migrates at 67 kD, as expected from the predicted sequence (see Fig. 4, lane T).
Figure 4.
Immunoprecipitation of AtVPS45p. Radiolabeled AtVPS45p was generated by in vitro transcription and translation from the cDNA. The translation product (T) was subjected to immunoprecipitation using AtVPS45p antibodies (I) or preimmune serum (P). Samples were analyzed by SDS-PAGE and fluorography.
A BLAST search of the GenBank database revealed that AtVPS45p shows significant similarity to members of the Sec1p family of proteins involved in vesicular transport through the secretory pathway, in particular to Vps45p homologs from various species (Fig. 1). AtVPS45p is most related to the mammalian Vps45p-like proteins mVps45p (Tellam et al., 1997), hVps45p (Pevsner et al., 1996), and rVps45p (El-Husseini et al., 1997), showing 47% identity and 59% similarity (allowing for conservative substitutions) over the full length of the proteins. In addition, AtVPS45p is 35% identical and 45% similar to the S. cerevisiae Vps45p protein. A lower level of identity (between 20 and 25%) is seen with other members of the Sec1p protein family.
Figure 1.
Sequence comparison of Vps45p-like proteins. Sequences of Vps45p-like proteins from Arabidopsis (AtVPS45p), human (h-Vps45p; GenBank accession no. U35246), and yeast (Vps45p; GenBank accession no. U07972) were compared using single-letter amino acid abbreviations. The alignment was generated using the MegAlign program (DNASTAR, Inc., Madison, WI). Amino acids identical in two or more sequences are shaded.
AtVPS45 Can Complement a Yeast vps45 Mutant
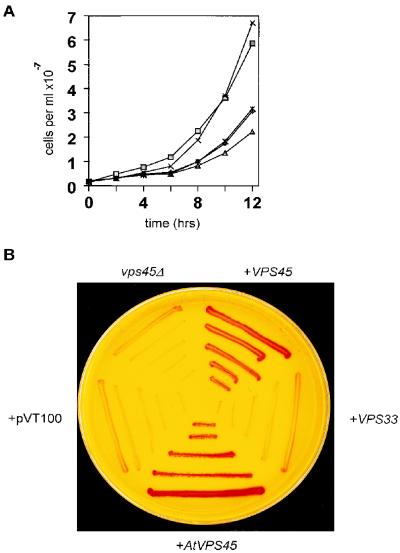
The sequence similarity of AtVPS45p to S. cerevisiae Vps45p indicates that it may have a similar function. To determine whether AtVPS45p is able to replace the requirement for Vps45p in vesicle trafficking to the yeast vacuole, a yeast vps45 deletion mutant was transformed with AtVPS45 in a multicopy yeast expression vector. The yeast mutant has two separable phenotypes: impaired growth at 37°C and missorting of the vacuolar hydrolase CPY to the outside of the cell (Cowles et al., 1994; Piper et al., 1994). The mutant expressing yeast VPS45 was able to grow at 37°C, whereas the mutant alone or the mutant transformed with the yeast VPS33 gene or the vector alone as controls grew very slowly at this temperature. Expression of AtVPS45 in the vps45Δ mutant was able to restore growth at 37°C to a rate comparable to that of the mutant expressing the yeast gene (Fig. 2A). All strains grew normally at 28°C. The Arabidopsis AtVPS45 gene is therefore able to complement the growth defect of the yeast vps45Δ deletion mutant.
Figure 2.
Complementation of a yeast vps45Δ mutant using AtVPS45. A, Yeast cultures of the vps45Δ mutant (♦), or mutant transformed with yeast VPS45 (□), yeast VPS33 (▴), AtVPS45 (×), or pVT100-U (✽) were grown at 37°C, the temperature at which vps45Δ displays a growth defect. Samples were taken at the times indicated and the number of cells was counted using a hemocytometer. B, The vps45Δ mutant and transformants as described in A were assayed for CPY activity using the APE overlay test. Red colonies indicate CPY activity; colonies lacking activity remain yellow.
In vps45Δ mutants approximately 75% of the CPY is secreted and the remaining intracellular CPY is unprocessed. vps45Δ mutants therefore lack detectable CPY activity, as processing in the vacuole to the mature form is required for enzyme activity. An APE plate assay was used to determine whether AtVPS45 can also complement the CPY-sorting defect of the yeast mutant. This assay produces red colonies when CPY activity is present, whereas colonies remain yellow in the absence of CPY (Jones, 1991). The vps45Δ mutant transformed with AtVPS45 produced red colonies in the APE assay, similar to the mutant transformed with yeast VPS45, indicating that the yeast cells contain CPY activity and, therefore, that CPY has reached the vacuole in these cells (Fig. 2B). Yeast VPS33 or vector alone were unable to restore CPY activity to the mutant. AtVPS45 is therefore not only able to complement the growth defect but also the CPY-sorting defect of the vps45Δ mutant. AtVPS45p may therefore play a similar role in vacuolar targeting in plant cells.
Expression of AtVPS45 RNA
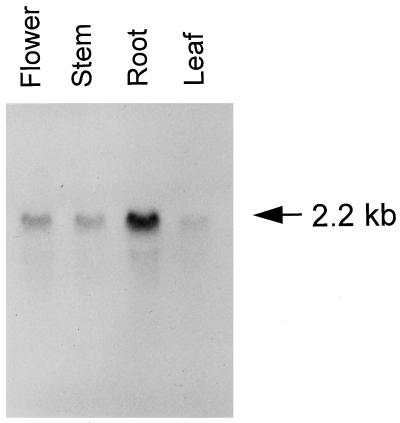
The pattern of expression of AtVPS45 RNA in different tissues of Arabidopsis was examined by RNA-blot analysis. Total RNA isolated from roots, leaves, flowers, or inflorescence stems was separated on an agarose/formaldehyde gel and transferred to nylon membrane. The membrane was probed with radiolabeled antisense RNA synthesized by in vitro transcription from the AtVPS45 cDNA. A single band of approximately 2.2 kb was detected in all tissues tested (Fig. 3). The amount of AtVPS45 mRNA was highest in roots, with much lower levels present in other tissues.
Figure 3.
Northern analysis of AtVPS45. Thirty micrograms of total RNA from Arabidopsis leaves, roots, flowers, and inflorescence stems was separated on an agarose/formaldehyde gel and transferred to nylon membrane. The membrane was incubated with a 32P-labeled antisense RNA probe corresponding to the AtVPS45 cDNA. The estimated size of the hybridizing band is indicated on the right.
Antibody Production and Characterization
A portion of the AtVPS45 cDNA was subcloned into the E. coli expression vector pGEX-5X-3 to create an in-frame fusion between GST and amino acids 184 to 560 of AtVPS45p. The fusion protein was synthesized in E. coli and used to produce rabbit polyclonal antibodies. Antibodies specific to AtVPS45p were affinity purified against the GST-fusion protein immobilized on nitrocellulose. The affinity-purified antibodies recognized an approximately 67-kD protein on immunoblots of Arabidopsis protein from root membrane preparations (see below) that was not detected by the preimmune serum. A protein of the same size was detected by immunoblotting in a total protein extract of yeast expressing AtVPS45, but not in nontransformed yeast (data not shown). The antibodies therefore can recognize AtVPS45p, but are unable to recognize the related endogenous yeast Vps45p. In addition, in vitro transcription and translation of the AtVPS45 cDNA gave a major 67-kD product on SDS-PAGE that could be immunoprecipitated by the affinity-purified antibodies but not by preimmune serum (Fig. 4). Another higher-mobility band was also detected in the translation reaction but was not immunoprecipitated. The affinity-purified antibodies therefore recognize the AtVPS45 gene product.
Although transcripts could be detected in all tissues examined (see Fig. 3), the AtVPS45p protein could not be detected in total protein preparations from any tissue by immunoblotting (data not shown). Upon immunoblot analysis of membrane preparations from various tissues, AtVPS45p could only be seen in roots (Fig. 5 and data not shown). Immunolocalization of the protein in frozen sections of Arabidopsis tissues indicated that the protein is present in a variety of tissues, but at the highest levels in roots (data not shown). This confirms that the RNA distribution correlates with the presence of the AtVPS45p protein.
Figure 5.
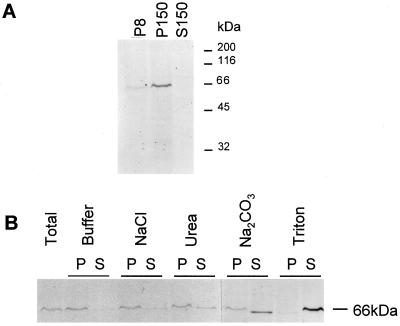
Association of AtVPS45p with membranes. A, AtVPS45p associates with microsomal membranes. An Arabidopsis root extract was separated into an 8,000g membrane fraction (P8), a 150,000g membrane fraction (P150), and a soluble fraction (S150). Equal amounts of protein from each fraction were separated by SDS-PAGE, transferred to nitrocellulose, and probed using AtVPS45p antibodies. The positions of molecular mass standards are indicated on the right in kilodaltons. B, AtVPS45p is a peripheral membrane protein. A 150,000g membrane pellet from an Arabidopsis root extract was resuspended in extraction buffer alone, 1 m NaCl, 0.1 m Na2CO3, 2 m urea, or 1% Triton X-100. Insoluble material was repelleted, and soluble and pellet fractions were analyzed by SDS-PAGE and immunoblotting using AtVPS45p antibodies.
AtVPS45p Is Associated with Microsomal Membranes
Yeast Vps45p is a peripheral membrane protein that associates with a low-density membrane fraction containing Golgi- and endosome-like membranes (Cowles et al., 1994; Piper et al., 1994). The ability of AtVPS45 to complement the yeast vps45Δ mutant implies that the two proteins may have similar properties. Arabidopsis roots grown in liquid culture were used to characterize the AtVPS45p protein because the expression level appears highest in this tissue (see above). Root tissue was homogenized and a postnuclear supernatant was fractionated by differential centrifugation to give an 8,000g membrane fraction (P8), a 150,000g membrane fraction (P150), and a 150,000g soluble supernatant fraction (S150). Fractions were separated by SDS-PAGE, transferred to nitrocellulose, and probed using AtVPS45p antibodies. A single band of approximately 67 kD was detected by the antibodies, corresponding to the predicted size of AtVPS45p (Fig. 5A). Most of the protein was associated with the P150 fraction, which contained microsomal membranes. A small amount of AtVPS45p was found in the P8 fraction, which contains most of the tonoplast and plasma membrane (Ahmed et al., 1997). Upon longer exposures, a small amount of AtVPS45p could also be detected in the soluble fraction (data not shown). Most of the AtVPS45p is therefore membrane associated and fractionates with microsomal membranes.
AtVPS45p Is a Peripheral Membrane Protein
To determine the nature of the interaction of AtVPS45p with membranes, a P150 membrane pellet was prepared from Arabidopsis root tissue. Protein was extracted from the pellet by resuspension in five different media: buffer alone, 1 m NaCl, 0.1 m Na2CO3, 2 m urea, or 1% Triton X-100. Insoluble material was repelleted at 150,000g, and pellets and supernatants were analyzed by immunoblotting using AtVPS45p antibodies (Fig. 5B). The presence of high concentrations of lipids caused AtVPS45p to migrate at a slightly different position in some of the samples. All of the treatments were able to extract a portion of AtVPS45p from the membrane, but to varying extents. Triton X-100 solubilized almost all of the AtVPS45p, and a large proportion could also be solubilized by urea or Na2CO3. NaCl was less efficient at extracting the protein into the supernatant, but was sufficient to release a small amount. AtVPS45p is therefore unlikely to be an integral membrane protein, which confirms the observation that the primary structure contains no predicted transmembrane domain. AtVPS45p appears to be a peripheral membrane protein, presumably binding to the membrane through interaction with other proteins. This is consistent with the biochemical properties of other Sec1p-like proteins, including yeast Vps45p and its mammalian homologs.
Suc-Gradient Fractionation of Arabidopsis Membranes
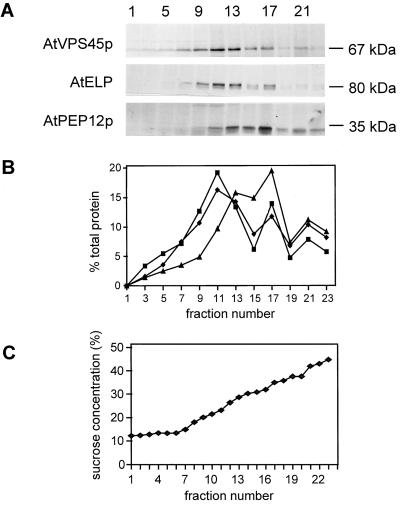
AtVPS45p is associated with a microsomal membrane pellet, and functional complementation of the yeast vps45Δ mutant indicates that it may be involved in transport between the TGN and the prevacuolar compartment. Suc-density gradients were used to further characterize the subcellular location of the protein. A 1000g supernatant of root extract was separated by Suc-density gradient fractionation, and 500-μL fractions were collected. Fractions were then analyzed for the presence of AtVPS45p and various marker proteins by immunoblotting. The distribution of AtVPS45p in the Suc gradient was compared with that of two marker proteins thought to be involved in post-Golgi transport steps (Fig. 6). AtPEP12p, a syntaxin homolog that may be involved in vacuolar protein transport, resides on prevacuolar compartments (Conceição et al., 1997). AtELP (epidermal growth factor receptor-like protein), a potential vacuolar protein sorting receptor, is found in clathrin-coated vesicles and in an additional, uncharacterized compartment (Ahmed et al., 1997). On the Suc gradient, AtVPS45p co-fractionated with AtELP, with a major peak observed at 23% Suc (1.08 g cm−3) and a minor peak at 35% Suc (1.15 g cm−3) for both proteins. In contrast, AtPEP12p could be partially separated from AtELP and AtVPS45p, with no peak present at 23% Suc (1.08 g cm−3) but instead with a major peak at 35% Suc (1.15 g cm−3). AtVPS45p therefore appears to be associated with a heterogeneous population of organelles that are also likely to contain AtELP.
Figure 6.
Suc-density gradient fractionation of AtVPS45p. A postnuclear supernatant of an Arabidopsis root extract was analyzed by fractionation on a Suc-density gradient. A, Fractions were analyzed by SDS-PAGE and immunoblotting. Proteins in each fraction were detected using antibodies against AtVPS45p, AtELP (Ahmed et al., 1997), or AtPEP12p (Conceição et al., 1997). B, Blots from A were analyzed by densitometry to determine the relative amount of AtVPS45p (♦), AtELP (▪), or AtPEP12p (▴) in each fraction. C, The concentration of Suc in each fraction was determined using a refractometer.
DISCUSSION
Soluble plant vacuolar proteins enter the secretory pathway at the ER and are transported through the endomembrane system to the TGN. At the TGN, proteins containing a vacuolar-targeting signal are diverted from the default secretion route and are packaged into vesicles for transport to the vacuole. This transport probably occurs via an intermediate prevacuolar compartment containing the syntaxin homolog AtPEP12p. AtPEP12p is thought to act as a transport-vesicle receptor by interaction with vesicle membrane proteins to allow docking of the vesicle at the prevacuolar compartment (Becherer et al., 1996; Conceição et al., 1997; Fischer von Mollard et al., 1997).
We are interested in characterizing other proteins that may function in the same transport step as AtPEP12p. Vps45p is a Sec1p-like protein that is required for transport between the TGN and the prevacuole/endosome in yeast (Cowles et al., 1994; Piper et al., 1994). We have isolated a cDNA encoding an Arabidopsis VPS45 homolog, AtVPS45, which is able to complement the yeast vps45Δ mutant, indicating that the two proteins are functionally related. AtVPS45p is a peripheral membrane protein that co-fractionates with a potential vacuolar-targeting receptor. We suggest that AtVPS45p may play a role in the transport of proteins to the plant vacuole.
Subcellular Location of AtVPS45p
Differential centrifugation experiments demonstrated that both yeast and mammalian Vps45 proteins are associated with microsome fractions containing Golgi- and endosome-like membranes (Cowles et al., 1994; Piper et al., 1994; Tellam et al., 1997). In addition, immunofluorescence microscopy studies indicated that mVps45p colocalizes with a TGN marker protein (Tellam et al., 1997). Consistent with these results, the majority of AtVPS45p was also found in a microsomal membrane fraction. Most current models of Golgi-to-vacuole transport in yeast place Vps45p on the endosome together with Pep12p (e.g. Burd et al., 1997) but there is no direct evidence for this. Expression of yeast PEP12 in mammalian cells showed that Pep12p was found at the TGN, along with mVps45p (Tellam et al., 1997). However, in yeast Pep12p is thought to be endosomal (Becherer et al., 1996), indicating that there may be some differences in the targeting of membrane proteins or in the structure of the endomembrane system itself between yeast and mammals.
Characterization of the membrane fraction containing AtVPS45p demonstrated that, surprisingly, AtVPS45p and AtPEP12p do not co-fractionate upon Suc-density gradient analysis. Instead, AtVPS45p appears to cofractionate with AtELP, which is located on a variety of membrane types, including clathrin-coated vesicles and an additional uncharacterized organelle (Ahmed et al., 1997). However, the identity of the organelles in the major peak at 23% Suc (1.08 g/cm−3) is currently unclear. AtVPS45p does not give a single peak on Suc gradients, suggesting that it too may be present on more than one type of organelle. Co-fractionation of AtVPS45p with AtELP throughout the gradient indicates that it is potentially associated with the same membrane types as AtELP.
It is still unclear how Vps45p associates with membranes. AtVPS45p does not have any predicted membrane-spanning domains, but the majority of the protein is associated with a microsomal membrane fraction. It is likely that membrane association occurs via interaction with a protein or protein complex in the membrane. This is supported by membrane-extraction experiments, which indicate that AtVPS45p is a peripheral membrane protein, consistent with results from yeast and mammals. As AtVPS45p and AtPEP12p do not co-fractionate, AtVPS45p is unlikely to associate with membranes via an interaction with AtPEP12p. AtVPS45p probably forms a stable interaction with an as yet unidentified membrane protein(s), which enables it to associate with the membrane.
Function of Vps45-Like Proteins
Although AtVPS45p and mammalian Vps45p homologs are more closely related in sequence to each other than either protein is to yeast Vps45p, AtVPS45 is able to complement the yeast vps45Δ mutant, whereas mVPS45 cannot (Tellam et al., 1997). This is reminiscent of the results observed for another secretory pathway gene, AtERD2, which is involved in the retrieval of resident ER proteins from the Golgi complex (Lee et al., 1993). This indicates that AtVPS45p is functionally related to yeast Vps45p and can substitute for the protein in yeast vacuolar transport. It is also a good indication that AtVPS45p is involved in vacuolar transport in plants. However, this does not demonstrate conclusively that AtVPS45p has an identical function in plant cells. Vacuolar transport in plants is likely to be a more complex process than in yeast. Some plant cells contain two distinct vacuole types, each with a discrete protein composition: a protein-storage vacuole and a lytic vacuole (Paris et al., 1996). Plants must therefore contain mechanisms for directing proteins to the correct vacuole type, which could also involve the trafficking of proteins through different prevacuolar compartments.
In addition, at least two mechanisms are responsible for the transport of soluble proteins to the plant vacuole (Matsuoka et al., 1995; Hohl et al., 1996), with a separate pathway for membrane proteins (Gomez and Chrispeels, 1993), implying that distinct populations of transport vesicles exist for each pathway. This complexity may require proteins involved in vesicle trafficking to acquire a more specialized role in plants, and any one protein may only be involved in a subset of vacuolar transport steps. The existence of several AtPEP12p-like proteins in Arabidopsis (H. Zheng and N. Raikhel, unpublished results) is consistent with the idea that a single protein in yeast may have several isoforms in plants.
Whereas yeast PEP12 and VPS45 have been shown to interact both genetically and biochemically, we have been unable to show binding between the Arabidopsis proteins in an in vitro assay (data not shown). This could be due to technical problems with the assay (such as incorrect folding of the proteins upon synthesis in vitro), although binding also could not be demonstrated between mammalian homologs of these proteins (Wang et al., 1997). It is possible that a different syntaxin homolog is the physiological binding partner of AtVPS45p.
Alternatively, AtPEP12p and AtVPS45p may interact, but only weakly or transiently, such that the interaction is not detected in the suboptimal in vitro assay. The organelles of the secretory pathway are dynamic structures, and AtVPS45p function may require it to be associated with more than one membrane type, or to cycle between different compartments or between membrane-bound and soluble forms. It is also possible that AtVPS45p can associate with more than one t-SNARE, which in turn would be located in different membrane compartments such as the TGN and prevacuole.
Expression Pattern of Plant Secretory Pathway Proteins
Although AtVPS45p could only be detected in membrane fractions from roots by immunoblotting, in situ immunolocalization revealed the presence of the protein in other tissues (data not shown). This confirms the results of the northern analysis, which showed low levels of the mRNA in several tissues, in addition to higher levels in roots. It is likely that AtVPS45p is present in most or all cell types, but that the amount of protein in some is too small to detect using these antibodies. The presence of the RNA and protein in many different cell types implies that it is involved in a fundamental cell process, as would be expected for a protein functioning in vacuolar protein trafficking. However, the amount of protein and mRNA detected varied widely between tissues, indicating that its synthesis may be regulated in a tissue-specific manner. How this regulation is achieved is currently unclear, but is likely to be coordinated for many proteins involved in vesicular trafficking to the vacuole. Indeed, the level of many proteins of the secretory pathway appears to be higher in roots than in most other tissues (Bar-Peled et al., 1995; Ahmed et al., 1997; Conceição et al., 1997). The role of these proteins in general vacuolar targeting in all cell types and in specialized tissues such as developing seeds is currently under investigation.
ACKNOWLEDGMENTS
We thank Dr. Zhenbiao Yang and Yakang Lin for their help with the immunolocalization, and Drs. Tom Stevens and Rob Piper for yeast strains and plasmids. We also thank members of the Raikhel group, in particular Dr. Tony Sanderfoot, for helpful comments and discussion during this work.
Abbreviations:
- APE
N-acetyl-dl-phenylalanine β-naphthyl ester
- CPY
carboxypeptidase Y
- GST
glutathione S-transferase
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TGN
trans-Golgi network
Footnotes
This research was supported by grants from the National Science Foundation (no. MCB-9507030) and the U.S. Department of Energy (no. DE-FG02-91ER-20021) to N.V.R.
LITERATURE CITED
- Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SU, Bar-Peled M, Raikhel NV. Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 1997;114:325–336. doi: 10.1104/pp.114.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Banta LM, Vida TA, Herman PK, Emr SD. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol Cell Biol. 1990;10:4638–4649. doi: 10.1128/mcb.10.9.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M, Conceição AS, Frigerio L, Raikhel NV. Expression and regulation of aERD2, a gene encoding the KDEL receptor homolog in plants, and other genes encoding proteins involved in ER-Golgi vesicular trafficking. Plant Cell. 1995;7:667–676. doi: 10.1105/tpc.7.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Gal S, Conceição AS, Raikhel NV. An Arabidopsis syntaxin homologue isolated by functional complementation of a yeast pep12 mutant. Proc Natl Acad Sci USA. 1995;92:7262–7266. doi: 10.1073/pnas.92.16.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV. Molecular aspects of vacuole biogenesis. In: Leigh RA, Sanders D, editors. Advances in Botanical Research: The Plant Vacuole, Vol 25. San Diego, CA: Academic Press; 1997. pp. 43–58. [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homolog, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceição AS, Marty-Mazars D, Bassham DC, Sanderfoot AA, Marty F, Raikhel NV. The syntaxin homologue AtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell. 1997;9:571–582. [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Emr SC, Horazdovsky BF. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J Cell Sci. 1994;107:3449–3459. doi: 10.1242/jcs.107.12.3449. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Guthrie H, Snutch TP, Vincent SR. Molecular cloning of a mammalian homologue of the yeast vesicular transport protein vps45. Biochim Biophys Acta. 1997;1325:8–12. doi: 10.1016/s0005-2736(97)00014-x. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, Gatti E, Butler M, Burton J, De Camilli P. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc Natl Acad Sci USA. 1994;91:2003–2007. doi: 10.1073/pnas.91.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Chrispeels MJ. Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell. 1993;5:1113–1124. doi: 10.1105/tpc.5.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Hohl I, Robinson DG, Chrispeels MJ, Hinz G. Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J Cell Sci. 1996;109:2539–2550. doi: 10.1242/jcs.109.10.2539. [DOI] [PubMed] [Google Scholar]
- Jones EW (1991) Tackling the protease problem in Saccharomyces cerevisiae. In C Guthrie, GR Fink, eds, Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego, CA, pp 428–452
- Lee H-I, Gal S, Newman TC, Raikhel NV. The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci USA. 1993;90:11433–11437. doi: 10.1073/pnas.90.23.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jürgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell. 1996;84:61–71. doi: 10.1016/s0092-8674(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T, de Bruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M and others. Genes galore. A summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Stanley EM, Jones RL, Rogers JC. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu S-C, Hyde PS, Scheller RH. Mammalian homologues of yeast vacuolar protein sorting (vps) genes implicated in Golgi-to-lysosome trafficking. Gene. 1996;183:7–14. doi: 10.1016/s0378-1119(96)00367-8. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu S-C, Scheller RH. nSec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Robinson DG, Hinz G. Vacuole biogenesis and protein transport to the plant vacuole: a comparison with the yeast vacuole and the mammalian lysosome. Protoplasma. 1997;197:1–25. [Google Scholar]
- Rothman JE. The protein machinery of vesicle budding and fusion. Protein Sci. 1996;5:185–194. doi: 10.1002/pro.5560050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato MH, Nakamura N, Ohsumi Y, Kouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Wada Y. The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J Biol Chem. 1997;272:24530–24535. doi: 10.1074/jbc.272.39.24530. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tellam JT, James DA, Stevens TH, Piper RC. Identification of a mammalian Golgi Sec1p-like protein, mVps45. J Biol Chem. 1997;272:6187–6193. doi: 10.1074/jbc.272.10.6187. [DOI] [PubMed] [Google Scholar]
- Vernet T, Dignard D, Thomas DY. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Frelin L, Pevsner J. Human syntaxin 7: a Pep12p/Vps6p homologue implicated in vesicle trafficking to lysosomes. Gene. 1997;199:39–48. doi: 10.1016/s0378-1119(97)00343-0. [DOI] [PubMed] [Google Scholar]