Abstract
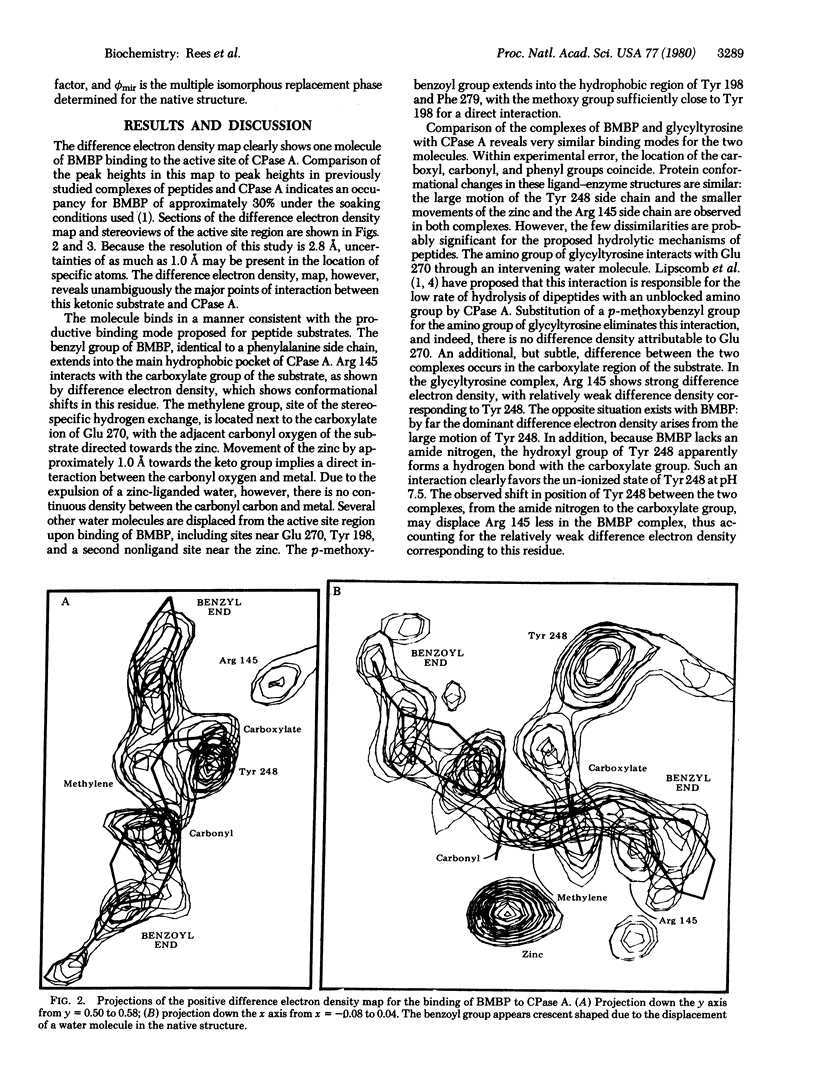
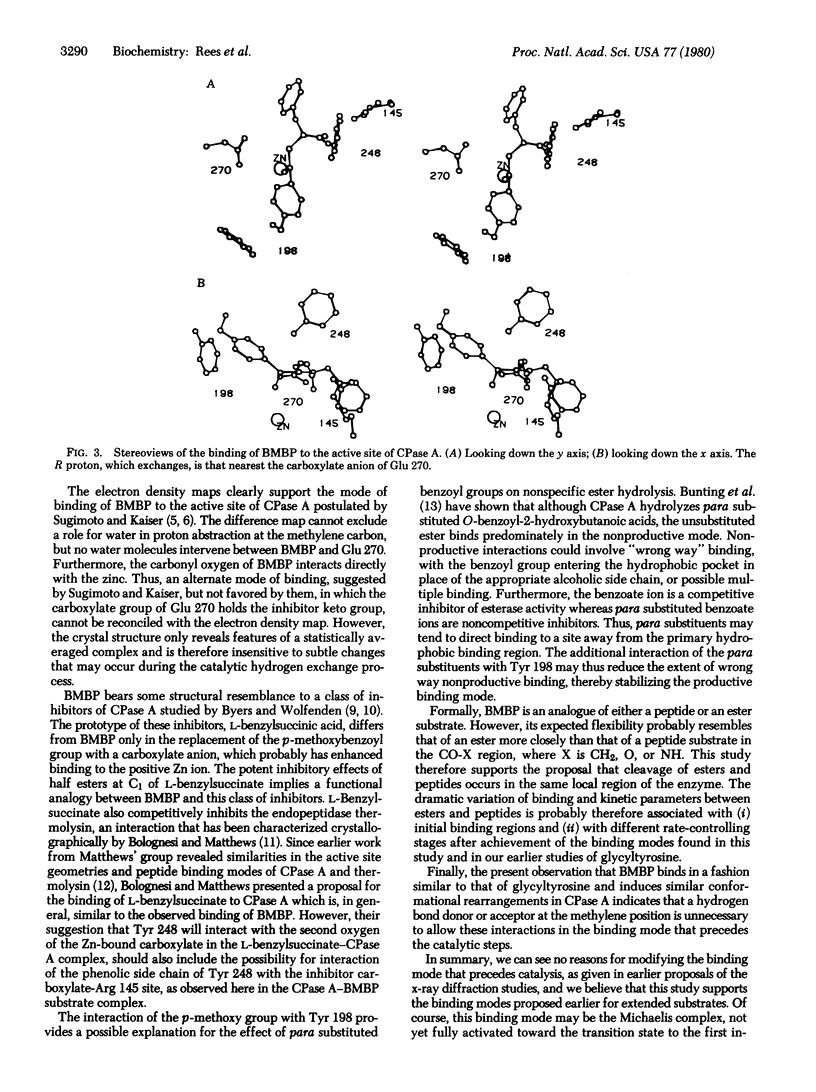
An x-ray diffraction study at 2.8 A resolution has yielded the structure of a complex between bovine carboxypeptidase A (peptidyl-L-amino-acid hydrolase, EC 3.4.17.1) and (-)-2-benzyl-3-p-methoxybenzoylpropionic acid. This substrate is an analogue of N-(p-methoxy)-benzoylphenylalanine, in which the amide NH is replaced by CN2. T. Sugimoto and E T. Kaiser (1979) J. Am. Chem. Soc. 101, 39469--3951] have shown that this complex catalyzes stereospecific exchange of that proton of the CH2 group which is in the R configuration. Our structure of this complex suports the model proposed by Sugimoto and Kaiser and is very similar to the productive peptide binding mode suggested by Lipscomb et al. [Lipscomb, W. N., Hartsuck, J. A., Reeke, G. N., Quiocho, F. A., Bethge, P. A., Ludwig, M. L., Steitz, T. A., Muirhead, H. & Coppola. J. C. (1968) Brookhaven Symp. Biol. 21, 24--90]. The proposed roles of glutamic acid 270 in the proton exchange and the interaction of zinc with the carbonyl group of the substrate are consistent with the observed structure.
Full text
PDF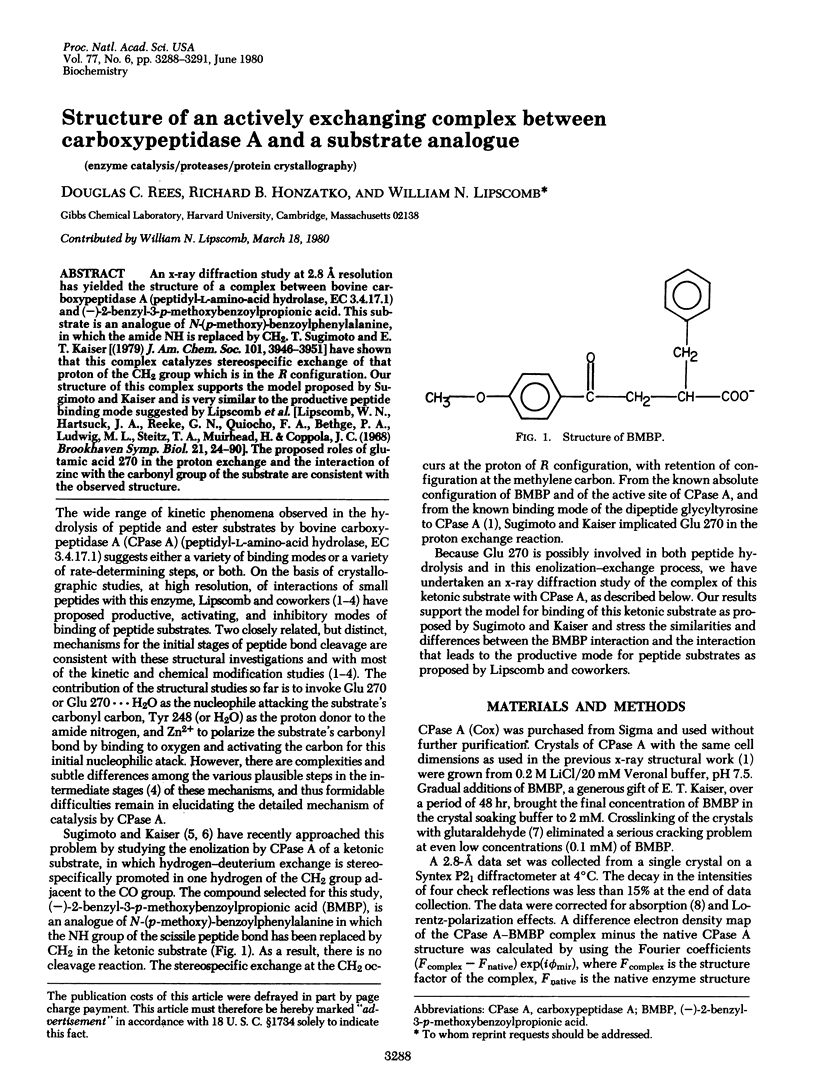

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi M. C., Matthews B. W. Binding of the biproduct analog L-benzylsuccinic acid to thermolysin determined by X-ray crystallography. J Biol Chem. 1979 Feb 10;254(3):634–639. [PubMed] [Google Scholar]
- Byers L. D., Wolfenden R. A potent reversible inhibitor of carboxypeptidase A. J Biol Chem. 1972 Jan 25;247(2):606–608. [PubMed] [Google Scholar]
- Byers L. D., Wolfenden R. Binding of the by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry. 1973 May 22;12(11):2070–2078. doi: 10.1021/bi00735a008. [DOI] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Comparison of the structures of carboxypeptidase A and thermolysin. J Biol Chem. 1977 Nov 10;252(21):7704–7710. [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Ludwig M. L., Quiocho F. A., Lipscomb W. N. The structure of carboxypepidase A. V. Studies of enzyme-substrate and enzyme-inhibitor complexes at 6 A resolution. J Biol Chem. 1967 Oct 25;242(20):4662–4668. [PubMed] [Google Scholar]



